Abstract
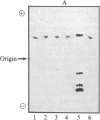
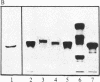
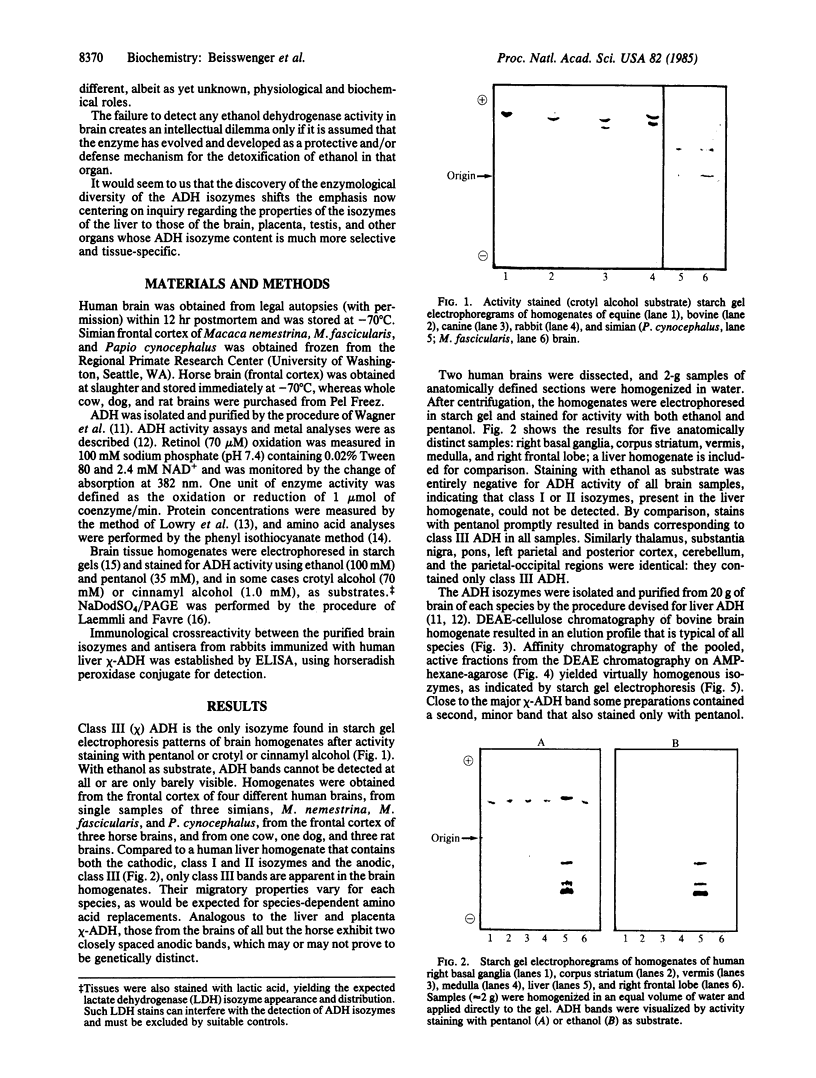
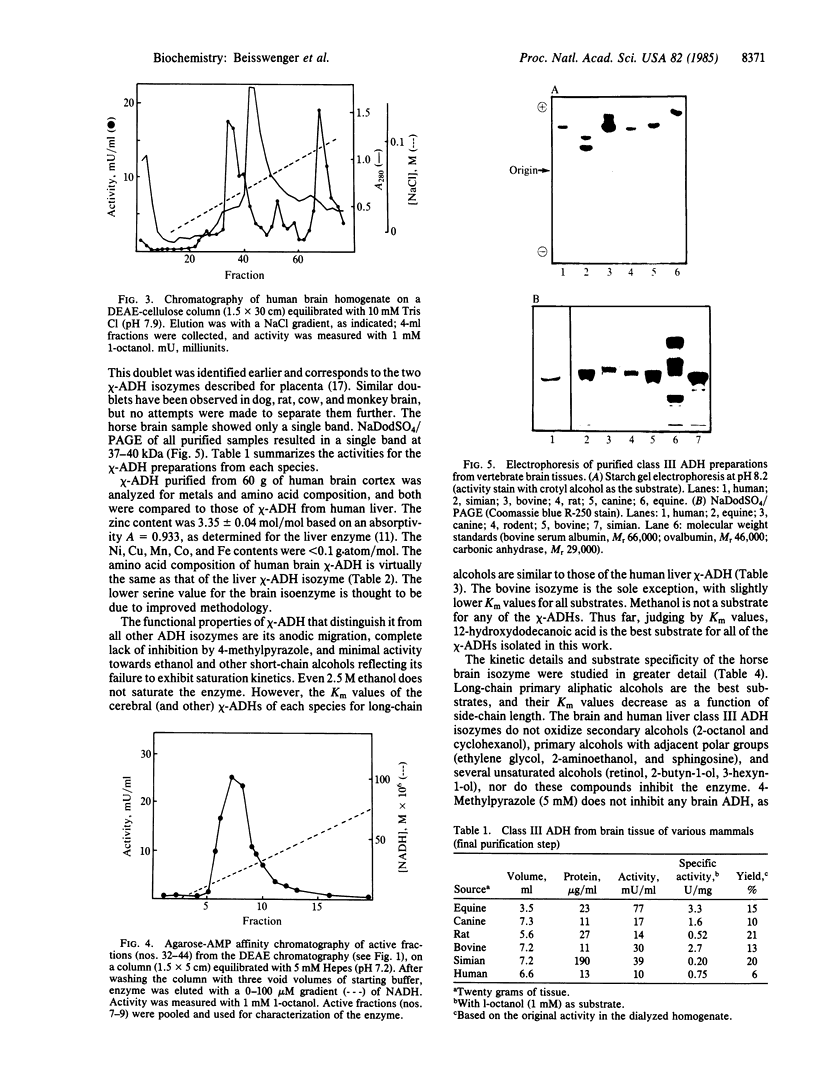
Class III (chi) is the only alcohol dehydrogenase (ADH) in human, equine, bovine, simian, canine, and rodent brain and is the first to be identified, purified, and characterized from the brain of humans or other vertebrates. Like the corresponding isozymes from human placenta and liver, the chi-ADH isozymes purified from mammalian brain are neither inhibited by nor do they bind to immobilized pyrazole, and they oxidize ethanol only very poorly (Km greater than 2.5 M). Indeed, it would be incorrect to classify them as "ethanol dehydrogenases." They contain 4 g.atom of zinc/mol, bind 2 moles of NAD, and readily oxidize long-chain aliphatic and aromatic primary alcohols. These findings appear to exclude the possibilities that ADH protects the brain of these vertebrates against ethanol or its metabolic products and that the brain can generate energy for cerebral function from ADH-monitored ethanol metabolism. Thus chi-ADH must serve a totally different but as yet unknown role. The failure to detect any ethanol dehydrogenase activity in brain creates an intellectual dilemma only if it is assumed that such an enzyme has evolved and developed as a protective mechanism for ethanol detoxification in that organ, as has been assumed. Tissue and substrate specificities of ADH isozymes are likely to give new insight regarding their physiological roles.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Blair A. H., Vallee B. L. Some catalytic properties of human liver alcohol dehydrogenase. Biochemistry. 1966 Jun;5(6):2026–2034. doi: 10.1021/bi00870a034. [DOI] [PubMed] [Google Scholar]
- Bosron W. F., Li T. K., Dafeldecker W. P., Vallee B. L. Human liver pi-alcohol dehydrogenase: kinetic and molecular properties. Biochemistry. 1979 Mar 20;18(6):1101–1105. doi: 10.1021/bi00573a026. [DOI] [PubMed] [Google Scholar]
- Bühler R., Pestalozzi D., Hess M., Von Wartburg J. P. Immunohistochemical localization of alcohol dehydrogenase in human kidney, endocrine organs and brain. Pharmacol Biochem Behav. 1983;18 (Suppl 1):55–59. doi: 10.1016/0091-3057(83)90147-8. [DOI] [PubMed] [Google Scholar]
- Dafeldecker W. P., Parés X., Vallee B. L., Bosron W. F., Li T. K. Simian liver alcohol dehydrogenase: isolation and characterization of isoenzymes from Saimiri sciureus. Biochemistry. 1981 Feb 17;20(4):856–861. doi: 10.1021/bi00507a031. [DOI] [PubMed] [Google Scholar]
- Deetz J. S., Luehr C. A., Vallee B. L. Human liver alcohol dehydrogenase isozymes: reduction of aldehydes and ketones. Biochemistry. 1984 Dec 18;23(26):6822–6828. doi: 10.1021/bi00321a084. [DOI] [PubMed] [Google Scholar]
- Ditlow C. C., Holmquist B., Morelock M. M., Vallee B. L. Physical and enzymatic properties of a class II alcohol dehydrogenase isozyme of human liver: pi-ADH. Biochemistry. 1984 Dec 18;23(26):6363–6368. doi: 10.1021/bi00321a012. [DOI] [PubMed] [Google Scholar]
- Hempel J. D., Pietruszko R. Human stomach alcohol dehydrogenase: isoenzyme composition and catalytic properties. Alcohol Clin Exp Res. 1979 Apr;3(2):95–98. doi: 10.1111/j.1530-0277.1979.tb05280.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Mukherji B., Kashiki Y., Ohyanagi H., Sloviter H. A. Metabolism of ethanol and acetaldehyde by the isolated perfused rat brain. J Neurochem. 1975 Apr;24(4):841–843. [PubMed] [Google Scholar]
- Parés X., Farrés J., Vallee B. L. Organ specific alcohol metabolism: placental chi-ADH. Biochem Biophys Res Commun. 1984 Mar 30;119(3):1047–1055. doi: 10.1016/0006-291x(84)90880-5. [DOI] [PubMed] [Google Scholar]
- Parés X., Vallee B. L. New human liver alcohol dehydrogenase forms with unique kinetic characteristics. Biochem Biophys Res Commun. 1981 Jan 15;98(1):122–130. doi: 10.1016/0006-291x(81)91878-7. [DOI] [PubMed] [Google Scholar]
- Raskin N. H., Sokoloff L. Alcohol dehydrogenase activity in rat brain and liver. J Neurochem. 1970 Dec;17(12):1677–1687. doi: 10.1111/j.1471-4159.1970.tb11392.x. [DOI] [PubMed] [Google Scholar]
- Smith M., Hopkinson D. A., Harris H. Studies on the properties of the human alcohol dehydrogenase isozymes determined by the different loci ADH1, ADH2, ADH3. Ann Hum Genet. 1973 Jul;37(1):49–67. doi: 10.1111/j.1469-1809.1973.tb01814.x. [DOI] [PubMed] [Google Scholar]
- Strydom D. J., Vallee B. L. Characterization of human alcohol dehydrogenase isoenzymes by high-performance liquid chromatographic peptide mapping. Anal Biochem. 1982 Jul 1;123(2):422–429. doi: 10.1016/0003-2697(82)90467-5. [DOI] [PubMed] [Google Scholar]
- Taberner P. V. Alcohol dehydrogenase activity in rat brain: evidence for the metabolism of succinic semialdehyde to gamma-hydroxybutyrate. Biochem Pharmacol. 1974 Apr 1;23(7):1219–1220. doi: 10.1016/0006-2952(74)90297-4. [DOI] [PubMed] [Google Scholar]
- VONWARTBURG J. P., BETHUNE J. L., VALLEE B. L. HUMAN LIVER--ALCOHOL DEHYDROGENASE. KINETIC AND PHYSICOCHEMICAL PROPERTIES. Biochemistry. 1964 Nov;3:1775–1782. doi: 10.1021/bi00899a033. [DOI] [PubMed] [Google Scholar]
- Wagner F. W., Burger A. R., Vallee B. L. Kinetic properties of human liver alcohol dehydrogenase: oxidation of alcohols by class I isoenzymes. Biochemistry. 1983 Apr 12;22(8):1857–1863. doi: 10.1021/bi00277a018. [DOI] [PubMed] [Google Scholar]
- Wagner F. W., Parés X., Holmquist B., Vallee B. L. Physical and enzymatic properties of a class III isozyme of human liver alcohol dehydrogenase: chi-ADH. Biochemistry. 1984 May 8;23(10):2193–2199. doi: 10.1021/bi00305a014. [DOI] [PubMed] [Google Scholar]