Abstract
HMG-I proteins are DNA-binding proteins thought to affect the formation and function of transcription complexes. Each protein contains three DNA-binding motifs, known as AT-hooks, that bind in the minor groove of AT tracts in DNA. Multiple AT-hooks within a polypeptide chain should contact multiple AT tracts, but the rules governing these interactions have not been defined. In this study, we demonstrate that high-affinity binding uses two or three appropriately spaced AT tracts as a single multivalent binding site. These principles have implications for binding to regulatory elements such as the interferon beta enhancer, TATA boxes, and serum response elements.
Full text
PDF

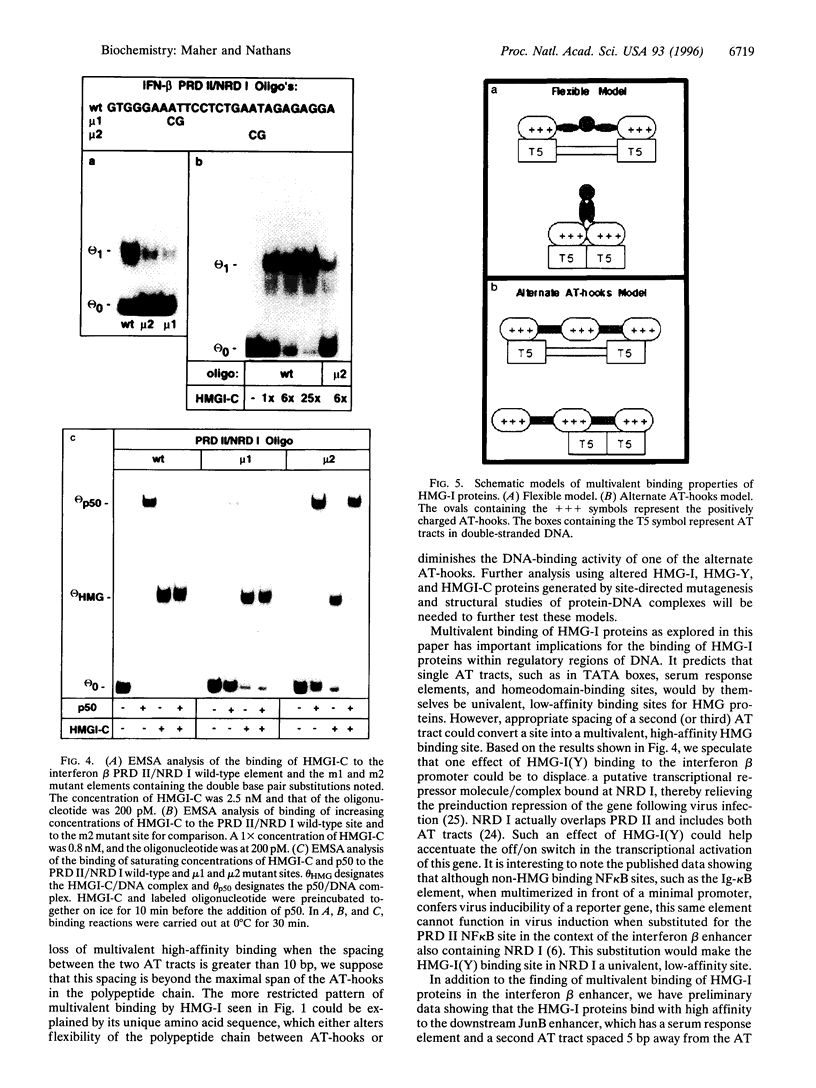

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdulkadir S. A., Krishna S., Thanos D., Maniatis T., Strominger J. L., Ono S. J. Functional roles of the transcription factor Oct-2A and the high mobility group protein I/Y in HLA-DRA gene expression. J Exp Med. 1995 Aug 1;182(2):487–500. doi: 10.1084/jem.182.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashar H. R., Fejzo M. S., Tkachenko A., Zhou X., Fletcher J. A., Weremowicz S., Morton C. C., Chada K. Disruption of the architectural factor HMGI-C: DNA-binding AT hook motifs fused in lipomas to distinct transcriptional regulatory domains. Cell. 1995 Jul 14;82(1):57–65. doi: 10.1016/0092-8674(95)90052-7. [DOI] [PubMed] [Google Scholar]
- Chuvpilo S., Schomberg C., Gerwig R., Heinfling A., Reeves R., Grummt F., Serfling E. Multiple closely-linked NFAT/octamer and HMG I(Y) binding sites are part of the interleukin-4 promoter. Nucleic Acids Res. 1993 Dec 11;21(24):5694–5704. doi: 10.1093/nar/21.24.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W., Maniatis T. The high mobility group protein HMG I(Y) can stimulate or inhibit DNA binding of distinct transcription factor ATF-2 isoforms. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11318–11322. doi: 10.1073/pnas.91.24.11318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W., Thanos D., Maniatis T. Mechanisms of transcriptional synergism between distinct virus-inducible enhancer elements. Cell. 1993 Sep 10;74(5):887–898. doi: 10.1016/0092-8674(93)90468-6. [DOI] [PubMed] [Google Scholar]
- Falvo J. V., Thanos D., Maniatis T. Reversal of intrinsic DNA bends in the IFN beta gene enhancer by transcription factors and the architectural protein HMG I(Y). Cell. 1995 Dec 29;83(7):1101–1111. doi: 10.1016/0092-8674(95)90137-x. [DOI] [PubMed] [Google Scholar]
- Fashena S. J., Reeves R., Ruddle N. H. A poly(dA-dT) upstream activating sequence binds high-mobility group I protein and contributes to lymphotoxin (tumor necrosis factor-beta) gene regulation. Mol Cell Biol. 1992 Feb;12(2):894–903. doi: 10.1128/mcb.12.2.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann M., Holth L. T., Zoghbi H. Y., Reeves R. Organization, inducible-expression and chromosome localization of the human HMG-I(Y) nonhistone protein gene. Nucleic Acids Res. 1993 Sep 11;21(18):4259–4267. doi: 10.1093/nar/21.18.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geierstanger B. H., Volkman B. F., Kremer W., Wemmer D. E. Short peptide fragments derived from HMG-I/Y proteins bind specifically to the minor groove of DNA. Biochemistry. 1994 May 3;33(17):5347–5355. doi: 10.1021/bi00183a043. [DOI] [PubMed] [Google Scholar]
- Goodbourn S., Burstein H., Maniatis T. The human beta-interferon gene enhancer is under negative control. Cell. 1986 May 23;45(4):601–610. doi: 10.1016/0092-8674(86)90292-8. [DOI] [PubMed] [Google Scholar]
- Goodbourn S., Maniatis T. Overlapping positive and negative regulatory domains of the human beta-interferon gene. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1447–1451. doi: 10.1073/pnas.85.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John S., Reeves R. B., Lin J. X., Child R., Leiden J. M., Thompson C. B., Leonard W. J. Regulation of cell-type-specific interleukin-2 receptor alpha-chain gene expression: potential role of physical interactions between Elf-1, HMG-I(Y), and NF-kappa B family proteins. Mol Cell Biol. 1995 Mar;15(3):1786–1796. doi: 10.1128/mcb.15.3.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. R., Lehn D. A., Reeves R. Alternative processing of mRNAs encoding mammalian chromosomal high-mobility-group proteins HMG-I and HMG-Y. Mol Cell Biol. 1989 May;9(5):2114–2123. doi: 10.1128/mcb.9.5.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanahan A., Williams J. B., Sanders L. K., Nathans D. Growth factor-induced delayed early response genes. Mol Cell Biol. 1992 Sep;12(9):3919–3929. doi: 10.1128/mcb.12.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger H., Sock E., Renner K., Grummt F., Wegner M. Functional interaction between the POU domain protein Tst-1/Oct-6 and the high-mobility-group protein HMG-I/Y. Mol Cell Biol. 1995 Jul;15(7):3738–3747. doi: 10.1128/mcb.15.7.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis H., Kaszubska W., DeLamarter J. F., Whelan J. Cooperativity between two NF-kappa B complexes, mediated by high-mobility-group protein I(Y), is essential for cytokine-induced expression of the E-selectin promoter. Mol Cell Biol. 1994 Sep;14(9):5701–5709. doi: 10.1128/mcb.14.9.5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund T., Holtlund J., Fredriksen M., Laland S. G. On the presence of two new high mobility group-like proteins in HeLa S3 cells. FEBS Lett. 1983 Feb 21;152(2):163–167. doi: 10.1016/0014-5793(83)80370-6. [DOI] [PubMed] [Google Scholar]
- Manfioletti G., Giancotti V., Bandiera A., Buratti E., Sautière P., Cary P., Crane-Robinson C., Coles B., Goodwin G. H. cDNA cloning of the HMGI-C phosphoprotein, a nuclear protein associated with neoplastic and undifferentiated phenotypes. Nucleic Acids Res. 1991 Dec 25;19(24):6793–6797. doi: 10.1093/nar/19.24.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neish A. S., Read M. A., Thanos D., Pine R., Maniatis T., Collins T. Endothelial interferon regulatory factor 1 cooperates with NF-kappa B as a transcriptional activator of vascular cell adhesion molecule 1. Mol Cell Biol. 1995 May;15(5):2558–2569. doi: 10.1128/mcb.15.5.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel U. A., Bandiera A., Manfioletti G., Giancotti V., Chau K. Y., Crane-Robinson C. Expression and cDNA cloning of human HMGI-C phosphoprotein. Biochem Biophys Res Commun. 1994 May 30;201(1):63–70. doi: 10.1006/bbrc.1994.1669. [DOI] [PubMed] [Google Scholar]
- Perez-Albuerne E. D., Schatteman G., Sanders L. K., Nathans D. Transcriptional regulatory elements downstream of the JunB gene. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11960–11964. doi: 10.1073/pnas.90.24.11960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R., Nissen M. S. The A.T-DNA-binding domain of mammalian high mobility group I chromosomal proteins. A novel peptide motif for recognizing DNA structure. J Biol Chem. 1990 May 25;265(15):8573–8582. [PubMed] [Google Scholar]
- Solomon M. J., Strauss F., Varshavsky A. A mammalian high mobility group protein recognizes any stretch of six A.T base pairs in duplex DNA. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1276–1280. doi: 10.1073/pnas.83.5.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss F., Varshavsky A. A protein binds to a satellite DNA repeat at three specific sites that would be brought into mutual proximity by DNA folding in the nucleosome. Cell. 1984 Jul;37(3):889–901. doi: 10.1016/0092-8674(84)90424-0. [DOI] [PubMed] [Google Scholar]
- Thanos D., Maniatis T. The high mobility group protein HMG I(Y) is required for NF-kappa B-dependent virus induction of the human IFN-beta gene. Cell. 1992 Nov 27;71(5):777–789. doi: 10.1016/0092-8674(92)90554-p. [DOI] [PubMed] [Google Scholar]
- Thanos D., Maniatis T. Virus induction of human IFN beta gene expression requires the assembly of an enhanceosome. Cell. 1995 Dec 29;83(7):1091–1100. doi: 10.1016/0092-8674(95)90136-1. [DOI] [PubMed] [Google Scholar]
- Treisman R. The serum response element. Trends Biochem Sci. 1992 Oct;17(10):423–426. doi: 10.1016/0968-0004(92)90013-y. [DOI] [PubMed] [Google Scholar]
- Whitley M. Z., Thanos D., Read M. A., Maniatis T., Collins T. A striking similarity in the organization of the E-selectin and beta interferon gene promoters. Mol Cell Biol. 1994 Oct;14(10):6464–6475. doi: 10.1128/mcb.14.10.6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
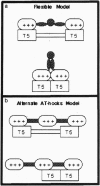
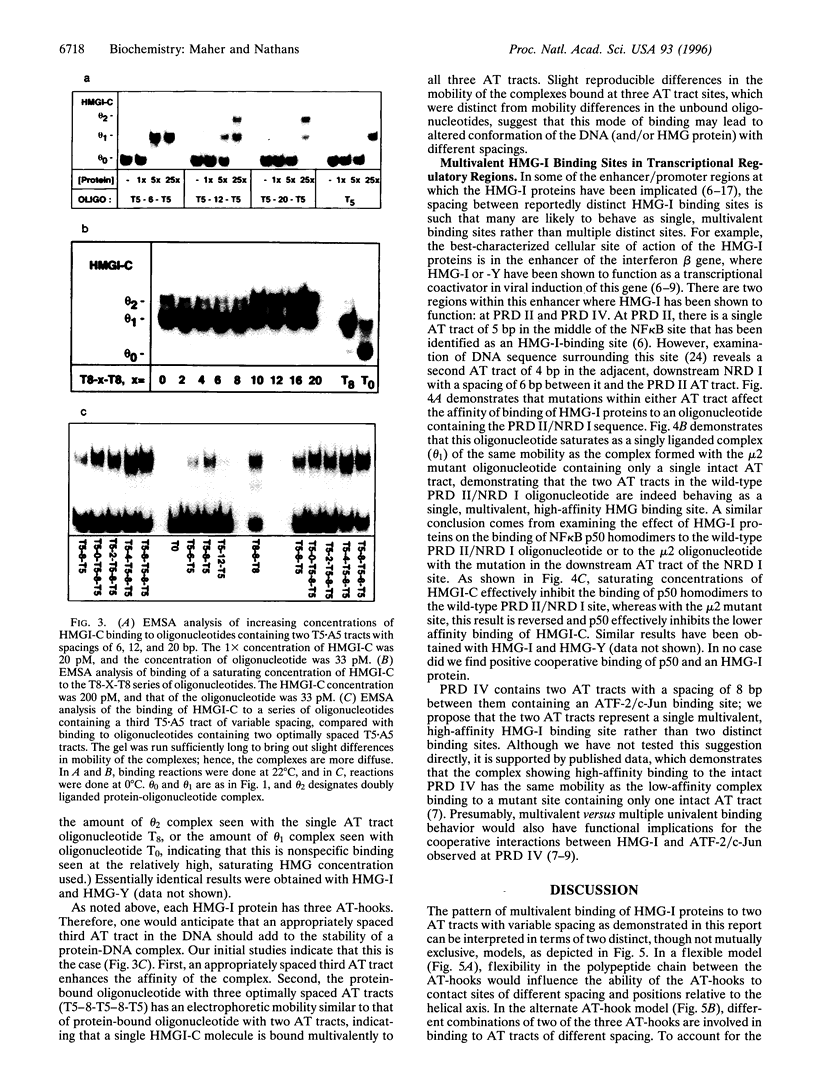
- Zhou X., Benson K. F., Ashar H. R., Chada K. Mutation responsible for the mouse pygmy phenotype in the developmentally regulated factor HMGI-C. Nature. 1995 Aug 31;376(6543):771–774. doi: 10.1038/376771a0. [DOI] [PubMed] [Google Scholar]