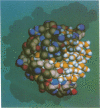
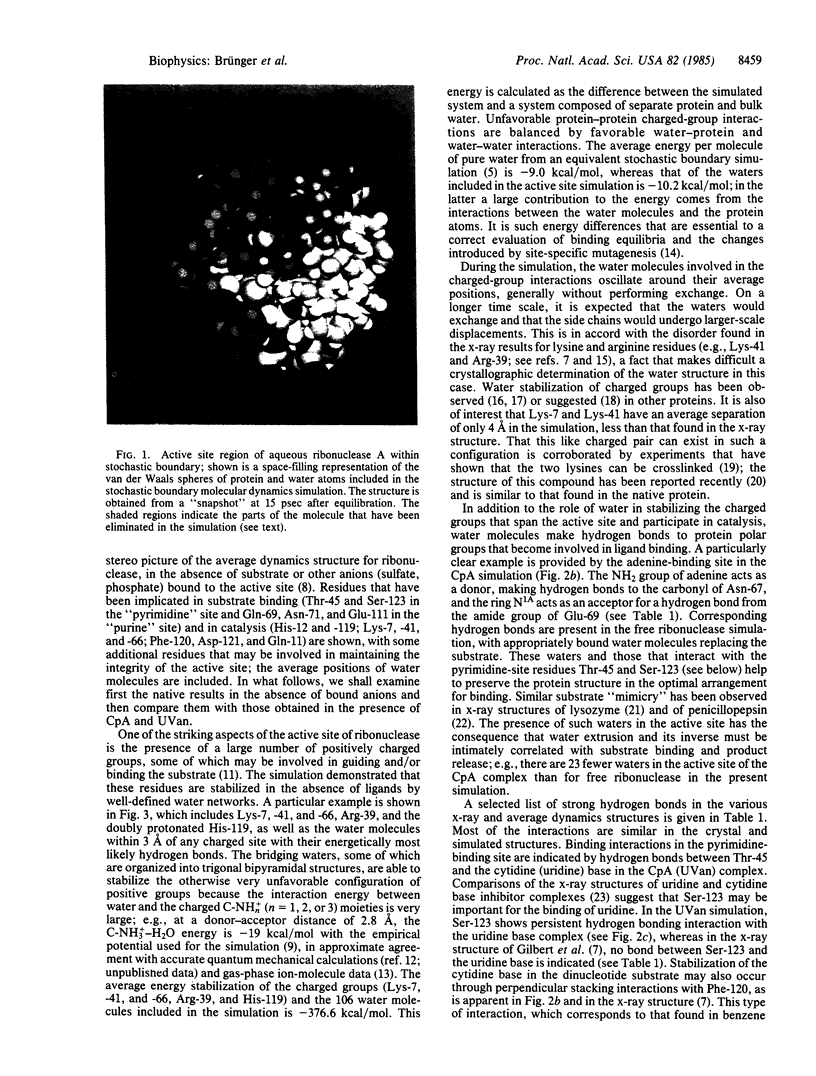
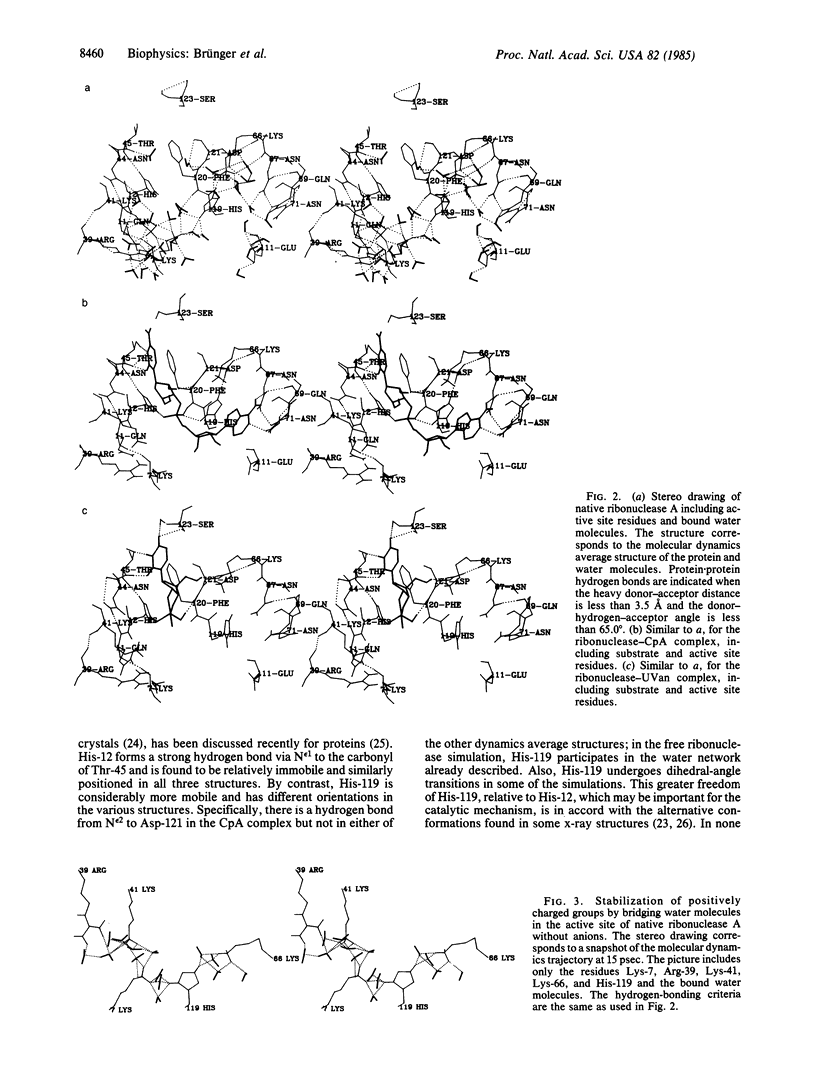
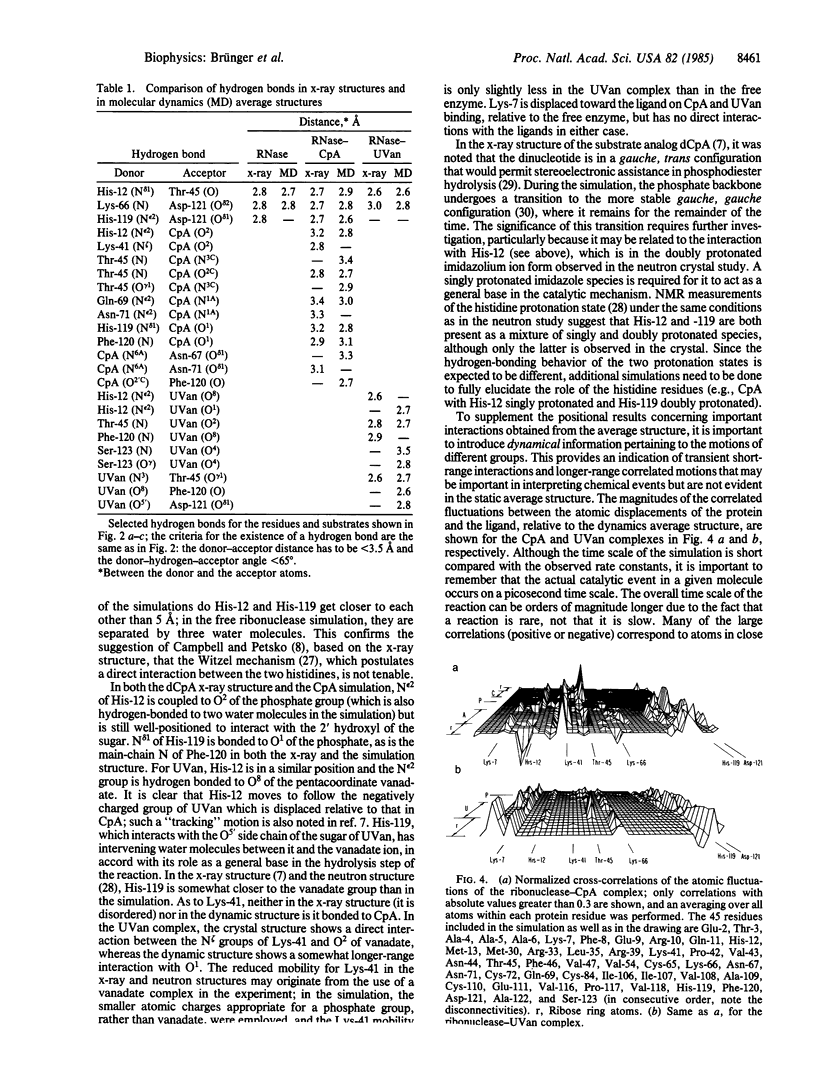
Abstract
The stochastic boundary molecular dynamics method is used to study the structure, dynamics, and energetics of the solvated active site of bovine pancreatic ribonuclease A. Simulations of the native enzyme and of the enzyme complexed with the dinucleotide substrate CpA and the transition-state analog uridine vanadate are compared. Structural features and dynamical couplings for ribonuclease residues found in the simulation are consistent with experimental data. Water molecules, most of which are not observed in crystallographic studies, are shown to play an important role in the active site. Hydrogen bonding of residues with water molecules in the free enzyme is found to mimic the substrate-enzyme interactions of residues involved in binding. Networks of water stabilize the cluster of positively charged active site residues. Correlated fluctuations between the uridine vanadate complex and the distant lysine residues are mediated through water and may indicate a possible role for these residues in stabilizing the transition state.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker E. N., Hubbard R. E. Hydrogen bonding in globular proteins. Prog Biophys Mol Biol. 1984;44(2):97–179. doi: 10.1016/0079-6107(84)90007-5. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Pulford W. C., Artymiuk P. J. X-ray studies of water in crystals of lysozyme. J Mol Biol. 1983 Jul 5;167(3):693–723. doi: 10.1016/s0022-2836(83)80105-3. [DOI] [PubMed] [Google Scholar]
- Borah B., Chen C. W., Egan W., Miller M., Wlodawer A., Cohen J. S. Nuclear magnetic resonance and neutron diffraction studies of the complex of ribonuclease A with uridine vanadate, a transition-state analogue. Biochemistry. 1985 Apr 9;24(8):2058–2067. doi: 10.1021/bi00329a038. [DOI] [PubMed] [Google Scholar]
- Borkakoti N. The active site of ribonuclease A from the crystallographic studies of ribonuclease-A-inhibitor complexes. Eur J Biochem. 1983 Apr 15;132(1):89–94. doi: 10.1111/j.1432-1033.1983.tb07329.x. [DOI] [PubMed] [Google Scholar]
- Brooks C. L., 3rd, Brünger A., Karplus M. Active site dynamics in protein molecules: a stochastic boundary molecular-dynamics approach. Biopolymers. 1985 May;24(5):843–865. doi: 10.1002/bip.360240509. [DOI] [PubMed] [Google Scholar]
- Burley S. K., Petsko G. A. Aromatic-aromatic interaction: a mechanism of protein structure stabilization. Science. 1985 Jul 5;229(4708):23–28. doi: 10.1126/science.3892686. [DOI] [PubMed] [Google Scholar]
- Fersht A. R., Shi J. P., Knill-Jones J., Lowe D. M., Wilkinson A. J., Blow D. M., Brick P., Carter P., Waye M. M., Winter G. Hydrogen bonding and biological specificity analysed by protein engineering. Nature. 1985 Mar 21;314(6008):235–238. doi: 10.1038/314235a0. [DOI] [PubMed] [Google Scholar]
- Gorenstein D. G., Findlay J. B., Luxon B. A., Kar D. Stereoelectronic control in carbon-oxygen and phosphorus-oxygen bond breaking processes. Ab initio calculations and speculations on the mechanism of action of ribonuclease A, staphylococcal nuclease, and lysozyme. J Am Chem Soc. 1977 May 11;99(10):3473–3479. doi: 10.1021/ja00452a047. [DOI] [PubMed] [Google Scholar]
- James M. N., Sielecki A. R. Structure and refinement of penicillopepsin at 1.8 A resolution. J Mol Biol. 1983 Jan 15;163(2):299–361. doi: 10.1016/0022-2836(83)90008-6. [DOI] [PubMed] [Google Scholar]
- MARFEY P. S., UZIEL M., LITTLE J. REACTION OF BOVINE PANCREATIC RIBONUCLEASE A WITH 1,5-DIFLUORO-2,4-DINITROBENZENE. II. STRUCTURE OF AN INTRAMOLECULARLY BRIDGED DERIVATIVE. J Biol Chem. 1965 Aug;240:3270–3275. [PubMed] [Google Scholar]
- Matthew J. B., Richards F. M. Anion binding and pH-dependent electrostatic effects in ribonuclease. Biochemistry. 1982 Sep 28;21(20):4989–4999. doi: 10.1021/bi00263a024. [DOI] [PubMed] [Google Scholar]
- Northrup S. H., Pear M. R., Lee C. Y., McCammon J. A., Karplus M. Dynamical theory of activated processes in globular proteins. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4035–4039. doi: 10.1073/pnas.79.13.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G. C., Dennis E. A., Meadows D. H., Cohen J. S., Jardetzky O. The mechanism of action of ribonuclease. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1151–1158. doi: 10.1073/pnas.62.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer L., James M. N. Carboxyl-carboxylate interactions in proteins. Nature. 1982 Jan 7;295(5844):79–80. doi: 10.1038/295079a0. [DOI] [PubMed] [Google Scholar]
- Weber P. C., Salemme F. R., Lin S. H., Konishi Y., Scheraga H. A. Preliminary crystallographic data for cross-linked (lysine7-lysine41)-ribonuclease A. J Mol Biol. 1985 Feb 5;181(3):453–453. doi: 10.1016/0022-2836(85)90232-3. [DOI] [PubMed] [Google Scholar]