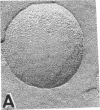
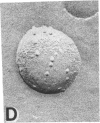
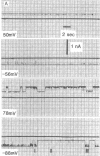
Abstract
Junctions isolated from bovine lenses were solubilized with the detergent octyl glucoside, and their protein(s) was reconstituted in unilamellar vesicles. The protein(s) appears as annular-shaped intramembrane particles approximately equal to 10 nm in diameter on the vesicles' fracture faces. The addition of the vesicle-containing junctional protein(s) to both sides of preformed lipid films induced voltage-dependent channels. The channels have a conductance of 200 pS in 0.1 M salt solutions and are thus large enough to account for the electrical coupling observed between intact lens fibers; they turn off when the magnitude of the voltage is increased and in the presence of octanol. Although the identity of the reconstituted channels as the communicating pathway between lens fibers remains to be proven, it is most likely that the reconstituted channels are formed by MIP-26, the major protein component of the isolated lens junctions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bok D., Dockstader J., Horwitz J. Immunocytochemical localization of the lens main intrinsic polypeptide (MIP26) in communicating junctions. J Cell Biol. 1982 Jan;92(1):213–220. doi: 10.1083/jcb.92.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekhuyse R. M., Kuhlmann E. D., Winkens H. J. Lens membranes VII. MIP is an immunologically specific component of lens fiber membranes and is identical with 26K band protein. Exp Eye Res. 1979 Sep;29(3):303–313. doi: 10.1016/0014-4835(79)90009-5. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P. G., Bok D., Horwitz J. Immunocytochemical localization of the main intrinsic polypeptide (MIP) in ultrathin frozen sections of rat lens. J Cell Biol. 1983 Nov;97(5 Pt 1):1491–1499. doi: 10.1083/jcb.97.5.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girsch S. J., Peracchia C. Lens cell-to-cell channel protein: II. Conformational change in the presence of calmodulin. J Membr Biol. 1985;83(3):227–233. doi: 10.1007/BF01868697. [DOI] [PubMed] [Google Scholar]
- Gorin M. B., Yancey S. B., Cline J., Revel J. P., Horwitz J. The major intrinsic protein (MIP) of the bovine lens fiber membrane: characterization and structure based on cDNA cloning. Cell. 1984 Nov;39(1):49–59. doi: 10.1016/0092-8674(84)90190-9. [DOI] [PubMed] [Google Scholar]
- Hall J. E. Voltage-dependent lipid flip-flop induced by alamethicin. Biophys J. 1981 Mar;33(3):373–381. doi: 10.1016/S0006-3495(81)84901-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg E. L., Anderson D. J., Friedlander M., Gilula N. B. Comparative analysis of the major polypeptides from liver gap junctions and lens fiber junctions. J Cell Biol. 1982 Jan;92(1):53–59. doi: 10.1083/jcb.92.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M. F., Simon S. A., Ramón F. Interaction of anaesthetics with electrical synapses. Nature. 1980 Jul 31;286(5772):498–500. doi: 10.1038/286498a0. [DOI] [PubMed] [Google Scholar]
- Kuszak J., Maisel H., Harding C. V. Gap junctions of chick lens fiber cells. Exp Eye Res. 1978 Oct;27(4):495–498. doi: 10.1016/0014-4835(78)90026-x. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R., Kanno Y., Socolar S. J. Quantum jumps of conductance during formation of membrane channels at cell-cell junction. Nature. 1978 Jul 13;274(5667):133–136. doi: 10.1038/274133a0. [DOI] [PubMed] [Google Scholar]
- Mimms L. T., Zampighi G., Nozaki Y., Tanford C., Reynolds J. A. Phospholipid vesicle formation and transmembrane protein incorporation using octyl glucoside. Biochemistry. 1981 Feb 17;20(4):833–840. doi: 10.1021/bi00507a028. [DOI] [PubMed] [Google Scholar]
- Neyton J., Trautmann A. Single-channel currents of an intercellular junction. 1985 Sep 26-Oct 2Nature. 317(6035):331–335. doi: 10.1038/317331a0. [DOI] [PubMed] [Google Scholar]
- Nicholson B. J., Takemoto L. J., Hunkapiller M. W., Hood L. E., Revel J. P. Differences between liver gap junction protein and lens MIP 26 from rat: implications for tissue specificity of gap junctions. Cell. 1983 Mar;32(3):967–978. doi: 10.1016/0092-8674(83)90081-8. [DOI] [PubMed] [Google Scholar]
- Obaid A. L., Socolar S. J., Rose B. Cell-to-cell channels with two independently regulated gates in series: analysis of junctional conductance modulation by membrane potential, calcium, and pH. J Membr Biol. 1983;73(1):69–89. doi: 10.1007/BF01870342. [DOI] [PubMed] [Google Scholar]
- Rae J. L., Levis R. A. Patch Clamp Recordings from the Epithelium of the Lens Obtained using Glasses Selected for Low Noise and Improved Sealing Properties. Biophys J. 1984 Jan;45(1):144–146. doi: 10.1016/S0006-3495(84)84142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. A., Zampighi G., McIntosh T. J., Costello M. J., Ting-beall H. P., Robertson J. D. The structure of junctions between lens fiber cells. Biosci Rep. 1982 May;2(5):333–341. doi: 10.1007/BF01115119. [DOI] [PubMed] [Google Scholar]
- Spray D. C., White R. L., de Carvalho A. C., Harris A. L., Bennett M. V. Gating of gap junction channels. Biophys J. 1984 Jan;45(1):219–230. doi: 10.1016/S0006-3495(84)84150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampighi G., Simon S. A., Robertson J. D., McIntosh T. J., Costello M. J. On the structural organization of isolated bovine lens fiber junctions. J Cell Biol. 1982 Apr;93(1):175–189. doi: 10.1083/jcb.93.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]