Abstract
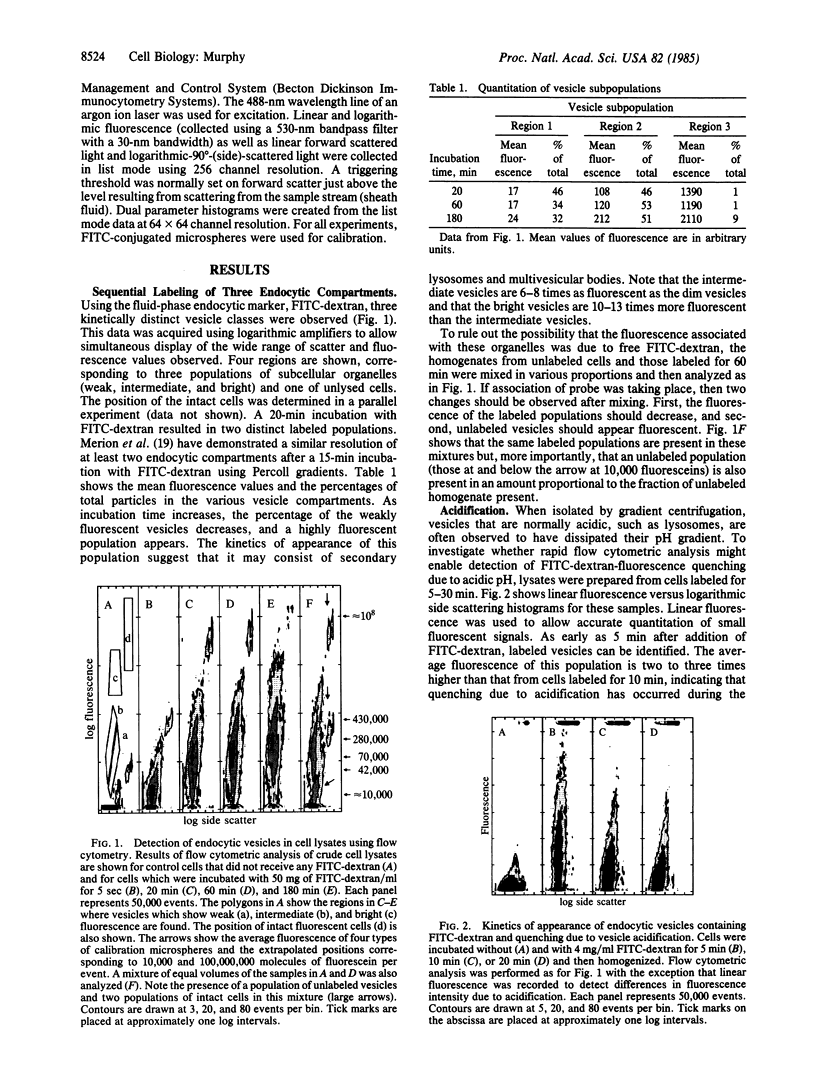
The existence of three distinct classes of endocytic vesicles that are part of the pathway of fluid-phase endocytosis has been demonstrated by flow cytometry. Amounts of fluorescent and scattered light were measured on a particle-by-particle basis for unfractionated whole cell lysates from cells incubated with fluorescein isothiocyanate-dextran. After 20 min two different fluorescent populations were observed, and after a 180-min incubation a third highly fluorescent population was found. Since the fluorescein isothiocyanate-dextran per fluid-phase vesicle should be a function solely of the external fluorescein isothiocyanate-dextran concentration, the existence of endocytic compartments with widely different amounts of fluorescence could result from a wide range of sizes of initial endocytic vesicles. However, the kinetics of appearance of the intermediate and highly fluorescent vesicles suggest that these compartments become labeled through fusion with the smaller primary endocytic vesicles.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. K., Dennison D. K., Schmitz K. S., Morrisett J. D. Direct observation of the distribution of fluorescent probes in phosphatidylcholine/cholesterol vesicles using flow microfluorometry. Anal Biochem. 1984 Aug 1;140(2):409–416. doi: 10.1016/0003-2697(84)90186-6. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Anderson R. G., Goldstein J. L. Recycling receptors: the round-trip itinerary of migrant membrane proteins. Cell. 1983 Mar;32(3):663–667. doi: 10.1016/0092-8674(83)90052-1. [DOI] [PubMed] [Google Scholar]
- Dolbeare F. A., Smith R. E. Flow cytometric measurement of peptidases with use of 5-nitrosalicylaldehyde and 4-methoxy-beta-naphthylamine derivatives. Clin Chem. 1977 Aug;23(8):1485–1491. [PubMed] [Google Scholar]
- Dolbeare F., Vanderlaan M. A fluorescent assay of proteinases in cultured mammalian cells. J Histochem Cytochem. 1979 Nov;27(11):1493–1495. doi: 10.1177/27.11.512330. [DOI] [PubMed] [Google Scholar]
- Finney D. A., Sklar L. A. Ligand/receptor internalization: a kinetic, flow cytometric analysis of the internalization of N-formyl peptides by human neutrophils. Cytometry. 1983 Jul;4(1):54–60. doi: 10.1002/cyto.990040108. [DOI] [PubMed] [Google Scholar]
- Galloway C. J., Dean G. E., Marsh M., Rudnick G., Mellman I. Acidification of macrophage and fibroblast endocytic vesicles in vitro. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3334–3338. doi: 10.1073/pnas.80.11.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Peppard J., von Figura K., Hasilik A., Schwartz A. L. Intracellular receptor sorting during endocytosis: comparative immunoelectron microscopy of multiple receptors in rat liver. Cell. 1984 May;37(1):195–204. doi: 10.1016/0092-8674(84)90315-5. [DOI] [PubMed] [Google Scholar]
- Glickman J., Croen K., Kelly S., Al-Awqati Q. Golgi membranes contain an electrogenic H+ pump in parallel to a chloride conductance. J Cell Biol. 1983 Oct;97(4):1303–1308. doi: 10.1083/jcb.97.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Bolzau E., Helenius A. Penetration of Semliki Forest virus from acidic prelysosomal vacuoles. Cell. 1983 Mar;32(3):931–940. doi: 10.1016/0092-8674(83)90078-8. [DOI] [PubMed] [Google Scholar]
- Merion M., Schlesinger P., Brooks R. M., Moehring J. M., Moehring T. J., Sly W. S. Defective acidification of endosomes in Chinese hamster ovary cell mutants "cross-resistant" to toxins and viruses. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5315–5319. doi: 10.1073/pnas.80.17.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merion M., Sly W. S. The role of intermediate vesicles in the adsorptive endocytosis and transport of ligand to lysosomes by human fibroblasts. J Cell Biol. 1983 Mar;96(3):644–650. doi: 10.1083/jcb.96.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R. F. Automated identification of subpopulations in flow cytometric list mode data using cluster analysis. Cytometry. 1985 Jul;6(4):302–309. doi: 10.1002/cyto.990060405. [DOI] [PubMed] [Google Scholar]
- Murphy R. F., Jorgensen E. D., Cantor C. R. Kinetics of histone endocytosis in Chinese hamster ovary cells. A flow cytofluorometric analysis. J Biol Chem. 1982 Feb 25;257(4):1695–1701. [PubMed] [Google Scholar]
- Murphy R. F., Powers S., Cantor C. R. Endosome pH measured in single cells by dual fluorescence flow cytometry: rapid acidification of insulin to pH 6. J Cell Biol. 1984 May;98(5):1757–1762. doi: 10.1083/jcb.98.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R. F., Powers S., Verderame M., Cantor C. R., Pollack R. Flow cytofluorometric analysis of insulin binding and internalization by Swiss 3T3 cells. Cytometry. 1982 May;2(6):402–406. doi: 10.1002/cyto.990020608. [DOI] [PubMed] [Google Scholar]
- Murphy R. F., Tse D. B., Cantor C. R., Pernis B. Acidification of internalized class I major histocompatibility complex antigen by T lymphoblasts. Cell Immunol. 1984 Oct 15;88(2):336–342. doi: 10.1016/0008-8749(84)90166-7. [DOI] [PubMed] [Google Scholar]
- Quintart J., Courtoy P. J., Baudhuin P. Receptor-mediated endocytosis in rat liver: purification and enzymic characterization of low density organelles involved in uptake of galactose-exposing proteins. J Cell Biol. 1984 Mar;98(3):877–884. doi: 10.1083/jcb.98.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrie B., Pool R. R., Jr, Sachdeva M., Maurey K. M., Oliver C. Evidence for both prelysosomal and lysosomal intermediates in endocytic pathways. J Cell Biol. 1984 Jan;98(1):108–115. doi: 10.1083/jcb.98.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Hanover J. A., Dickson R. B., Pastan I. Morphologic characterization of the pathway of transferrin endocytosis and recycling in human KB cells. Proc Natl Acad Sci U S A. 1984 Jan;81(1):175–179. doi: 10.1073/pnas.81.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]


