Abstract
OBJECTIVE
Among chronic dialysis patients an obesity paradox or reverse epidemiology is known, but it is no clear whether the association of body size and muscle mass with survival is consistent across race, especially in East Asian versus Caucasian and African–American hemodialysis (HD) patients
PATIENTS AND METHODS
Using 20,818 HD patients in South Korea from February 1, 2001 to June 30, 2009 and 20,000 matched HD patients in the United States (10,000 Caucasians and 10,000 African–Americans) from July 1, 2001 to June 30, 2006, we compared mortality associations of baseline body mass index (BMI) and serum creatinine (Cr) level, as likely surrogates of obesity and muscle mass across the three races.
RESULTS
In Korean HD patients higher BMI together with higher serum Cr levels were associated with greater survival, as previously reported from US and European studies. In the matched cohort (n=10,000 in each of the three races), mortality risks were lower across higher BMI and serum Cr levels, and these association were similar in all three races (reference groups: patients with BMI >25.0 kg/m2 or serum Cr >12 mg/dL in each race). Patients with BMI level ≤18.5 kg/m2 (underweight) reported 78%, 79% and 57% higher mortality risk among Caucasian, African–American and Korean, respectively, and so did patients with serum Cr level ≤6.0 mg/dL, who reported 108%, 87% and 78% higher mortality, respectively.
CONCLUSION
Race does not modify the association of higher body size and muscle mass with greater survival in HD patients. Given consistency of the obesity paradox, which may be related to mitigated effect of protein–energy wasting on mortality irrespective of racial disparities, nutritional support to improve survival should be tested across all races of HD patients.
INTRODUCTION
Despite improvements in dialysis technique and patient care during past decades, mortality of dialysis patients has remained extremely high. The mortality of US dialysis patient has remained at 20% per year, mostly attributable to cardiovascular events.1 Obesity is a risk factor for several chronic diseases in the general population, including chronic kidney disease (CKD).2 Previous observational studies, however, have reported a survival benefit of obesity, measured as body mass index (BMI) in CKD and dialysis patients.3–9 High serum creatinine levels as a likely surrogate of muscle mass10–13, not of residual renal function14,15, is also related to better survival in dialysis patients.3,4 These observations suggest that protein–energy wasting (PEW) is a strong and biologically plausible cause for extremely high mortality in dialysis patients.16,17 However, most of the evidences have come from US and European cohorts. Epidemiologic studies including a large number of Asian dialysis patients have been substantially limited. The limited evidence from Asian patients has partially hindered the ability to generalize the PEW–mortality association beyond racial differences. We hypothesized that both BMI and serum creatinine levels may be inversely associated with mortality even in Asian hemodialysis (HD) patients, and the associations may be identical across Caucasian, African–Americas and Asian patients. We evaluated our hypothesis with two large, nationally representative and contemporary cohorts of Korean and the matched US HD patients.
PATIENTS AND METHODS
Patients
We examined data from patients receiving HD treatment in South Korea from February 1, 2001 to June 30, 2009 [End stage renal disease (ESRD) Registry of Korean Society of Nephrology]. The ESRD Registry Committee of the Korean Society of Nephrology has collected data of dialysis centers and patients through an online registry program on the Korean Society of Nephrology Web Site (http://www.ksn.or.kr) since 2001. Information regarding non–responding dialysis centers was collected through mail questionnaires. During the period, 45,864 HD patients were registered, and measures for BMI and serum creatinine at the entry into the cohort were available in 20,818 and 20,408 patients, respectively.
The matched US HD patients was selected from administrative database from July 1, 2001 to June 30, 2006 (for 20 consecutive calendar quarters), who were treated in one of the outpatient dialysis facilities owned by a large US dialysis organization (DaVita Inc., El Segundo, California). To create the matched cohort, we randomly selected 10,000 Korean HD patients who were registered within the same period of the US cohort (July 1, 2001 to June 30, 2006). Then we matched them with the same number of Caucasian (n = 10,000) and African–American (n = 10,000) HD patients using propensity score (one–to–one matching). Flow diagram for patient selection is presented in eFigure 1.
Follow–up time began on the date of entry into the cohort. Patients were censored at the time of death, renal transplantation, or end of the study period (September 30, 2009). Date and cause of death were periodically reported to the registry office by dialysis staff in the Korean cohort, and were obtained from the US Renal Data System (USRDS) in the US cohort.
Demographic and Laboratory Measures
In Korean HD patients, information on dates of birth and the first dialysis treatment, gender, cause of ESRD, presence of diabetes and dialysis modality were obtained at the first entry into the cohort. In the matched US patients, the same variables and race were obtained from DaVita databases and the USRDS. Dialysis vintage was defined as the time between the first day of dialysis treatment and the first day that the patient entered the cohort. Height and post–HD body weight were used to calculate BMI. Data for hemoglobin, serum albumin and creatinine, single–pool Kt/V as a marker of delivered dialysis dose and normalized protein nitrogen appearance (nPNA) reflecting daily dietary protein intake were obtained. All laboratory values were measured for clinical purpose. Blood samples were drawn at the start of HD treatment and laboratory values were measured in each renal unit (Korea) and in the central DaVita Laboratory in Deland, FL (the US). Single–pool Kt/V and nPNA were estimated by 3–point urea kinetic model based on pre–HD and post–HD blood urea nitrogen level at the first session of week, and pre–HD level at next mid–week session. Values at the first entry (baseline) into the cohort were used in the analysis.
Statistical Analyses
Because the study cohorts were non–concurrent cohorts, we defined the value of the first entry into cohort as the baseline value, and constructed fixed survival models. Survival analyses included Cox regression model. The primary outcome was all–cause mortality. The baseline BMI and serum creatinine concentration were main predictors. The entire range of BMI (kg/m2) was divided into 6 a priori categories: ≤18.5, 18.6–20.0, 20.1–21.5, 21.6–23.0, 23.1–25.0, and >25.0 (reference category). Serum creatinine levels (mg/dL, conversion factor to μmol/L: ×88.4) were divided into 5 categories: ≤6.0, 6.1–8.0, 8.1–10.0, 10.1–12.0, and >12.0 (reference category). First, an association of baseline BMI or serum creatinine level with all–cause mortality was evaluated in Korean HD patients. For each analysis, three levels of multivariable adjustments were examined: (1) unadjusted model that only included main predictors (BMI or serum creatinine level) and year of the entry; (2) case–mix model that included age, gender, diabetes mellitus, HD vintage and single–pool Kt/V; (3) model adjusted for case–mix and nutrition, which included all of the covariates in the case–mix model as well as the three following surrogates of nutritional status: hemoglobin, serum albumin and nPNA. Propensity score, which was the probability of having the same characteristics as Korean HD patient, was calculated by logistic regression including all variables in the Cox regression model. After matching, we compared hazard ratios (HR) by BMI and serum creatinine categories across races.
In Korean HD patients, missing covariate data were imputed using the mean of the existing values of BMI or serum creatinine categories, as appropriate. There were no missing values for age, gender, diabetes, and HD vintage. Percentages of missing data for hemoglobin and serum albumin were below 3%. Single–pool Kt/V and nPNA, however, had about 28% and 29% missing, respectively. We undertook sensitivity analyses separately without imputing missing data, and compared with main results. There was no missing in the US data because only patients with all variables were considered as the matched cohort. All analyses were carried out with STATA, version 12.1 (StataCorp LP, College Station, TX). The study was approved by the Harbor–UCLA Medical Center Institutional Review Board with exemption of the requirement for a written consent form.
RESULTS
Associations of BMI and serum creatinine levels with mortality in Korean HD patients
Baseline characteristics of Korean HD patients, stratified by baseline BMI and serum creatinine level are listed in eTable 1 and eTable 2. Patients with higher BMI values were diabetic and had shorter HD vintages. nPNA level tended to increase with BMI value. Patients with higher serum creatinine levels were younger, men, non–diabetic, and had longer dialysis vintages. Serum albumin level and nPNA showed positive relationships to serum creatinine level.
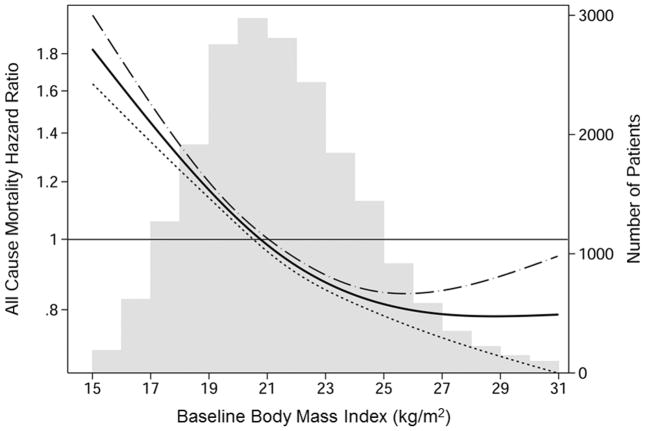
The mean (± standard deviation, SD) duration of follow–up was 5.9 ± 3.4 years, and a total of 5,580 (26.8%) deaths were reported. The crude mortality rate was 41.6 per 1,000 person–years. Kaplan–Meier curves showed a stepwise decrease in all–cause mortality with higher BMI and serum creatinine categories (eFigure 2 and eFigure 3). HR for all–cause mortality decreased linearly with increase in BMI level in 20,818 HD patients (eTable 3). Patients with BMI level ≤18.5 kg/m2 (underweight) reported 78% higher mortality risk compared to patients with BMI level >25.0 kg/m2 (reference group) in the case–mix and nutrition adjusted model [HR, 1.78; 95% confidence interval (CI), 1.60–1.99]. Cox regression using restricted cubic splines showed higher BMI level exhibited an association with greater survival, although the advantage of high BMI was reduced in the range above 27 kg/m2 (Figure 1).
Figure 1.
Association between baseline body mass index and all–cause mortality in 20,818 Korean hemodialysis patients†
†Hazard ratios were estimated with Cox regression using restricted cubic splines. Values of <1 percentile and >99 percentile in body mass index were excluded from the model to minimize an influence from the outlier values. The model was adjusted for case–mix and nutrition covariates including age, gender, diabetes mellitus, dialysis vintage, single–pool Kt/V, hemoglobin, serum albumin and normalized protein nitrogen appearance. Dashed lines are 95% point–wise confidence bands. Number of patients is displayed simultaneously.
Serum creatinine level was linearly and inversely associated with all–cause mortality in 20,408 HD patients (eTable 4 and eFigure 4). Patients with serum creatinine level ≤6.0 mg/dL reported 76% higher mortality risk compared to patients with >12.0 mg/dL (reference group) in the fully adjusted model (HR, 1.76; 95% CI, 1.57–1.97).
Effect of race on associations of baseline BMI and serum creatinine levels with mortality
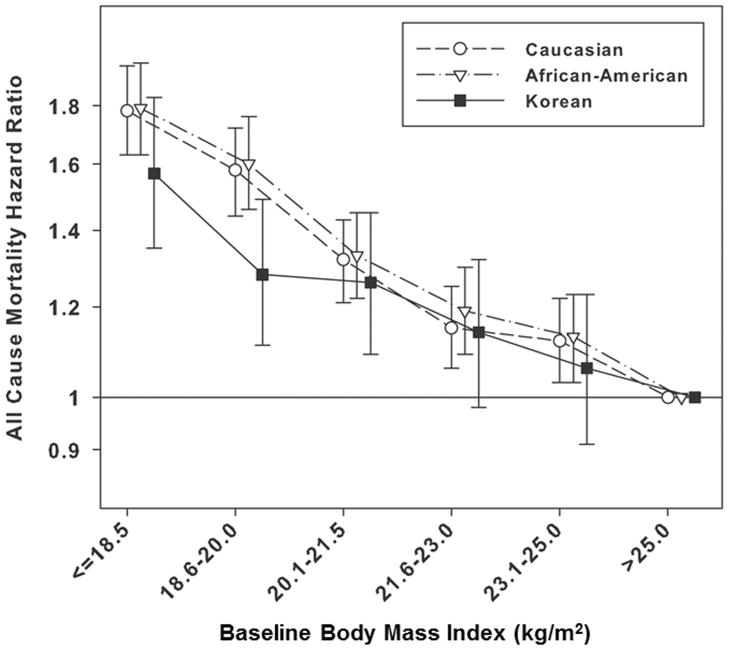
Baseline characteristics among the three different races are presented in Table 1. Even using the propensity score matching method, Caucasian and African–American patients were older, had higher BMI and hemoglobin levels than Korean patients, whereas Korean patients reported higher serum creatinine levels. Within a same range of BMI, HRs for all–cause mortality were comparable across Caucasian, African–American and Korean patients (reference: patients with BMI >25.0 kg/m2 in each race) in the case–mix and nutrition adjusted model (Table 2). Patients with BMI level ≤18.5 kg/m2 (underweight) reported 78%, 79% and 57% higher mortality risk in Caucasian, African–American and Korean patients [HR, 1.78 (95% CI, 1.63–1.95) for Caucasian, HR, 1.79 (95% CI, 1.63–1.96) for African–American, and HR, 1.57 (95% CI, 1.35–1.83) for Korean, respectively]. Patterns of the associations were similar among all races (Figure 2A).
Table 1.
| Total | Caucasian | African–American | Korean | p –valuec | |
|---|---|---|---|---|---|
| N | 30,000 | 10,000 | 10,000 | 10,000 | |
| Propensity score | 0.42±0.31 | 0.27±0.23 | 0.32±0.23 | 0.68±0.30 | |
| Age (yrs) | 56±16 | 59±18 | 55±16 | 53±14 | <0.001 |
| Female (%) | 43 | 42 | 43 | 43 | 0.63 |
| Diabetes (%) | 37 | 38 | 37 | 37 | 0.03 |
| HD vintage (yrs) | 2.4±3.6 | 2.1±3.6 | 2.6±3.6 | 2.6±3.6 | <0.001 |
| BMI (kg/m2) | 22.1±3.7 | 22.5±4.0 | 22.3±3.9 | 21.4±3.0 | <0.001 |
| Creatinine (mg/dL) | 9.2±4.2 | 8.0±3.1 | 9.5±3.8 | 9.9±6.9 | <0.001 |
| Hemoglobin (g/dL) | 10.3±1.4 | 10.7±1.2 | 10.6±1.3 | 9.5±1.4 | <0.001 |
| Albumin (g/dL) | 3.6±0.6 | 3.6±0.5 | 3.6±0.6 | 3.6±0.6 | 0.43 |
| Single pool Kt/V | 1.41±0.31 | 1.44±0.30 | 1.42±0.30 | 1.37±0.33 | <0.001 |
| nPNA (g/kg/day) | 0.93±0.26 | 0.93±0.26 | 0.92±0.26 | 0.93±0.25 | 0.27 |
BMI = body mass index; HD = hemodialysis; nPNA = normalized protein nitrogen appearance
Categorical variables are given as percentage; continuous variables as mean ± standard deviation. Conversion factors for units: hemoglobin and serum albumin in g/dL to g/L, ×10; creatinine in mg/dL to μmol/L, ×88.4
p–value indicates one–way ANOVA for continuous variables and chi–square test for categorical variables.
Table 2.
Case–mix and nutrition adjusted hazard ratios by baseline body mass index and races (n=10,000 in each race)a,b
| BMI (kg/m2) | Caucasian | African–American | Korean | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| n | HR | 95% CI | n | HR | 95% CI | n | HR | 95% CI | |
| ≤18.5 | 1,409 | 1.78 | 1.63–1.95 | 1,479 | 1.79 | 1.63–1.96 | 1,566 | 1.57 | 1.35–1.83 |
| 18.6–20.0 | 1,271 | 1.58 | 1.44–1.72 | 1,352 | 1.60 | 1.46–1.76 | 1,956 | 1.28 | 1.11–1.49 |
| 20.1–21.5 | 1,627 | 1.32 | 1.21–1.43 | 1,700 | 1.33 | 1.22–1.45 | 2,098 | 1.25 | 1.09–1.45 |
| 21.6–23.0 | 1,678 | 1.15 | 1.06–1.25 | 1,682 | 1.19 | 1.09–1.30 | 1,744 | 1.14 | 0.98–1.32 |
| 23.0–25.0 | 1,678 | 1.13 | 1.04–1.22 | 1,656 | 1.13 | 1.03–1.23 | 1,549 | 1.06 | 0.91–1.23 |
| >25.0 | 2,337 | Reference | 2,131 | Reference | 1,087 | Reference | |||
BMI = body mass index; CI = confidence interval; HR = hazard ratio
The model was adjusted for case–mix and nutrition covariates including age, gender, diabetes mellitus, dialysis vintage, single–pool Kt/V, hemoglobin, serum albumin and normalized protein nitrogen appearance.
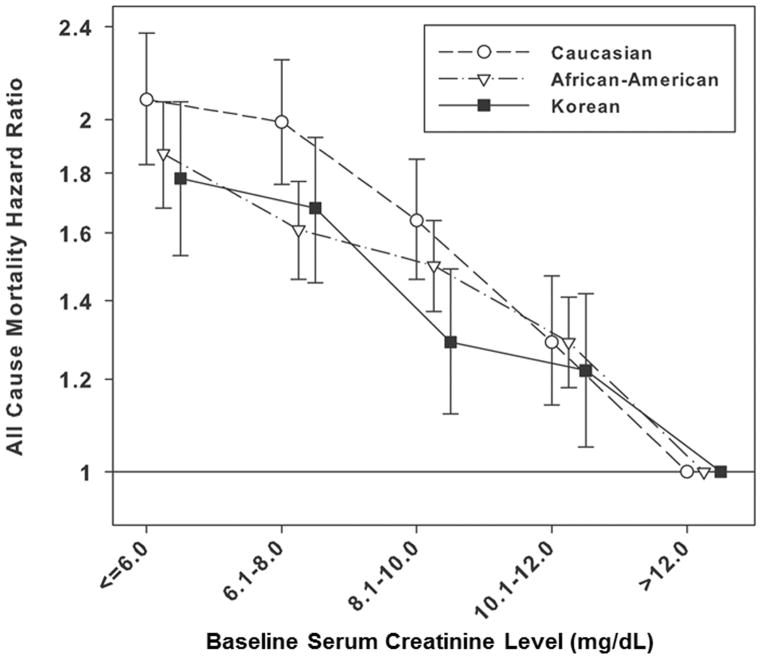
Figure 2.
Adjusted hazard ratios (95% confidence intervals) for all–cause death with baseline body mass index (A) and serum creatinine (B) categories across 3 different races in the matched cohort (n = 10,000 in each race)†
†Patients with BMI >25.0 kg/m2 and serum creatinine level >12.0 mg/dL in each race were reference groups. The model was adjusted for case–mix and nutrition covariates including age, gender, diabetes mellitus, dialysis vintage, single–pool Kt/V, hemoglobin, serum albumin and normalized protein nitrogen appearance. Propensity score which was the probability of having the same characteristics as Korean HD patient was calculated by logistic regression including all variables in the survival analysis. The matched cohort was created by 1:1 matching based on propensity score.
HRs for all–cause mortality decreased linearly with increase in serum creatinine levels similarly among all races. There was no difference in the patterns (Figure 2B). Compared to patients with serum creatinine >12.0 mg/dL in each race, patients with serum creatinine level ≤6.0 mg/dL reported HR 2.08 (95% CI, 1.83–2.37) for Caucasian, HR 1.87 (95% CI, 1.68–2.07) for African–American, and HR 1.78 (95% CI, 1.53–2.07) for Korean patients in the fully adjusted model, respectively (Table 3).
Table 3.
Case–mix and nutrition adjusted hazard ratios by baseline serum creatinine levels and races (n=10,000 in each race)a,b
| Creatinine (mg/dL) | Caucasian | African–American | Korean | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| n | HR | 95% CI | n | HR | 95% CI | n | HR | 95% CI | |
| ≤6.0 | 2,814 | 2.08 | 1.83–2.37 | 1,784 | 1.87 | 1.68–2.07 | 1,860 | 1.78 | 1.53–2.07 |
| 6.1–8.0 | 2,516 | 1.99 | 1.76–2.25 | 2,045 | 1.61 | 1.46–1.77 | 2,204 | 1.68 | 1.45–1.93 |
| 8.1–10.0 | 2,217 | 1.64 | 1.46–1.85 | 2,111 | 1.50 | 1.37–1.64 | 2,302 | 1.29 | 1.12–1.49 |
| 10.1–12.0 | 1,371 | 1.29 | 1.14–1.47 | 1,668 | 1.29 | 1.18–1.41 | 1,772 | 1.22 | 1.05–1.42 |
| >12.0 | 1,082 | Reference | 2,392 | Reference | 1,862 | Reference | |||
BMI = body mass index; CI = confidence interval; HR = hazard ratio
The model was adjusted for case–mix and nutrition covariates including age, gender, diabetes mellitus, dialysis vintage, single–pool Kt/V, hemoglobin, serum albumin and normalized protein nitrogen appearance.
Sensitivity analysis
Separate analyses without imputing missing covariates in Korean HD patients also showed comparable results with those after imputation (eTable 5). After dividing patients by dialysis vintage < 1 versus ≥ 1 year, mortality predictability by BMI and serum creatinine levels were examined. No significant difference was observed between two groups (eFigure 5).
DISCUSSION
In this comparison of two large cohorts of Korean and US HD patients, we observed valid findings. Higher baseline BMI and serum creatinine levels predicted greater survival even in the Asian HD population. In addition, racial disparity did not modify the associations of BMI and serum creatinine levels with all–cause mortality.
PEW appears to be responsible for high mortality rate in dialysis patients.16,17 Accordingly, better nutritional status may confer survival benefits. Recent large observational studies from the US have confirmed the association of higher BMI with greater survival in HD patients.3,4,18,19 The data for Asian patients, however, have been substantially limited. In 1999, Wong et al. compared Asian–American (n = 4,471) with Caucasian dialysis patients using the USRDS data. In the analysis, the Caucasian reported mortality decreased as the BMI increased, but for Asian, the relationship was U–shaped, which suggested the effect of BMI on survival differed by race.20 A three–year longitudinal observational study from Taiwan reported that patients with BMI below 18.5 kg/m2 had a 123% higher risk of death compared to patients with BMI over 25.0 kg/m2 in 959 maintenance HD patients (HR, 2.23; 95% CI, 1.22–4.05).21 Our study validated the association of higher BMI with better survival in a large cohort of Asian HD patients, and furthermore, the association was similar with those of the Caucasian and African–American.
Higher serum creatinine levels were also associated with greater survival across all races similarly, which suggests race does not modify the association. Serum creatinine is originated from creatine of which almost 95% pool is stored in muscle.22,23 Considering it, serum creatinine levels could be used as a surrogate of muscle mass especially in ESRD patients who have very low renal function.4,10–12,24–26 If serum creatinine levels is influenced dominantly by residual renal function, higher serum creatinine levels (ie. lower residual renal function) would relate to higher mortality,27,28 whereas if serum creatinine levels reflect muscle mass dominantly, higher serum creatinine levels (ie. larger muscle mass) would relate to lower mortality.3,4,29,30 Previous studies from US dialysis patients reported high serum creatinine levels as a likely surrogate of muscle mass, not of residual renal function, were related to better survival.3,4,29,30 In a study among them, serum creatinine levels below 8.0 mg/dL predicted higher mortality, whereas those above 10 mg/dL were associated with greater survival compared to the level of 8–10 mg/dL.4 Our study may extend a significance of muscle mass for survival into Asian HD patients beyond racial disparity.
PEW and mortality association have been also evaluated using other surrogates of body composition. A study with 1,709 patients included in the Hemodialysis (HEMO) Study reported lower quartiles of triceps skin–fold thickness as a measure of fat mass, mid–arm muscle circumference as a surrogate of muscle mass, and BMI were all significantly associated with higher all–cause mortality.31 Survival advantages with both higher mid–arm muscle circumference and greater triceps skin fold were observed in other studies.31,32 A study in 535 HD patients showed that lower body fat or a decline in body fat percentage over time measured by near infra–red interactance method was associated with an increased risk of death.33 In Asian studies, increased fat and lean mass were also associated with better outcomes.34,35 A study from Japan showed the independent associations between a higher fat mass index measured by dual–energy X–ray absortiometry and a lower risk of non–cardiovascular death (HR, 0.85; 95% CI, 0.81–0.90), as well as between a higher lean mass index and a lower risk of cardiovascular death (HR, 0.87; 95% CI, 0.81–0.94) in 808 maintenance HD patients.34 These previous studies support our observations indirectly.
Despite substantial evidences from observational studies, biologically plausible mechanisms to explain the PEW–mortality association are not clearly known. The underlying mechanisms appear complex, and no single, adequately inclusive pathophysiologic mechanism has been set forth to explain the entire range of observed outcomes. First, because inflammation may be associated with both anorexia and increased net protein catabolism36, inflammation has been proposed as a missing link between PEW and mortality. Inflammation may induce endothelial cell damage and dysfunction, predisposing to atherosclerotic plaque formation.37 Second, muscle wasting in the skeletal and respiratory system may compromise the vital functions of the organs.17 Increase in circulating actin by the muscle breakdown and consequently consuming gelsolin which has salutary and protective action may be a possible mechanism underlying a muscle wasting and poor outcomes.38 Third, gradual loss of body fat could be also important, resulting in lower production of certain anti–inflammatory cytokines and adiponectin39 and decreased sequestration of uremic toxins.40 In addition, deficiencies of multiple micronutrients may have some effects.
Despite considerable strengths, our study has several limitations. First, this is a retrospective observational study. Hence control for confounders is limited to those that are recognized and measured. The Korean database had limited variables for adjustments. There were no data for cardiovascular co–morbidities and inflammatory markers such as C–reactive protein. Patients with higher BMI or serum creatinine levels are generally healthier, have better appetite and higher protein or energy intake. The difference in health may not have been captured adequately by the available information. Second, residual renal function could not be included in the analyses. However, it is notable that a greater residual renal function is generally related to a better survival, the confounding effect from the lack of this variable might influence to the direction in which a higher serum creatinine level was related to a higher mortality, attenuating our results. We undertook the sensitivity analysis stratified by dialysis vintage to evaluate the confounding from residual renal function. In the Korean HD patients, the associations were similar between patients with vintage of < 1 versus ≥1 year, suggesting the effect from residual renal function might not influence our results significantly. Third, crude mortality rate was 42 per 1,000 person–years in the Korean cohort, which was quite lower than 224 per 1,000 person–years in the US cohort (matched patients only). Even though a difference in mortality among races would exist20, the magnitude of difference between the two cohorts is unreasonable. The difference in mortality rate must be due to underreporting of death in the Korean cohort because the reporting was voluntary but not mandatory. Because of the limitation, we could not directly compare mortality rate across races, instead we compared HRs using patients with BMI >25 kg/m2 or serum creatinine >12.0 mg/dL in each race as reference groups. Conceptually, the underreporting might not significantly influence to estimate HRs for mortality if it occurred randomly. Fourth, although BMI is often used as an indicator of obesity, BMI cannot differentiate skeletal muscle mass or body water from fat mass.41,42 In addition, serum creatinine level could be influenced by recent intake of meats.43 We did not have other surrogates of muscle mass and body fat, such as mid–arm muscle circumference and triceps skin–fold thickness.
CONCLUSION
Larger body size and muscle mass represented by higher BMI and serum creatinine levels were associated with greater survivals even in Asian HD patients. Furthermore, race did not significantly modify these associations. It suggests that PEW is a strong and modifiable risk factor beyond racial disparity. Patients with low BMI and serum creatinine level may be in higher risk, and need careful examinations for body composition and dietary intake to assess presence of PEW. If PEW is suspicious, individualized nutritional intervention would be helpful. Efficient strategies to prevent and improve PEW are needed across all races of HD patients.
Supplementary Material
Supplemental Figure 1. Flow diagram for patient selection.
Supplemental Figure 2. Kaplan–Meier curves to all–cause mortality by BMI categories in Korean HD patients (n = 20,818).
Supplemental Figure 3. Kaplan–Meier curves to all–cause mortality by serum creatinine categories in Korean HD patients (n = 20,408).
Supplemental Figure 4. Association between baseline serum creatinine level and all–cause mortality in 20,408 Korean hemodialysis patients.†
†Hazard ratios were estimated with Cox regression using restricted cubic splines. Values of <1 percentile and >99 percentile in serum creatinine levels were excluded from the model to minimize an influence from the outlier values. The model was adjusted for case–mix and nutrition covariates including age, gender, diabetes mellitus, dialysis vintage, single–pool Kt/V, hemoglobin, serum albumin and normalized protein nitrogen appearance. Dashed lines are 95% point–wise confidence bands. Number of patients is displayed simultaneously.
Supplemental Figure 5. Adjusted hazard ratios (95% confidence interval) for all–cause death in Korean patients with vintage <1 versus ≥ 1 year: A) baseline body mass index, B) baseline serum creatinine level.†
†Patients with BMI >25.0 kg/m2 and serum creatinine level >12.0 mg/dL were reference groups. The model was adjusted for case–mix and nutrition covariates including age, gender, diabetes mellitus, dialysis vintage, single–pool Kt/V, hemoglobin, serum albumin and normalized protein nitrogen appearance.
Acknowledgments
Financial support and disclosure: The work is supported by Dr. Kalantar–Zadeh’s NIH (NIDDK) grants K24–DK091419, R01–DK078106 and a philanthropic grant from Mr. Harold Simmons. Dr. Molnar is recipient of the Hungarian Eötvös Scholarship (MÖB/77–2/2012). Dr. Kalantar–Zadeh was medical director of DaVita Harbor–UCLA Long Beach during 2007–2012. Dr. Nissenson is employee of DaVita. Other authors have not declared any conflict of interest.
We greatly appreciate the ESRD Registry Committee in Korean Society of Nephrology and DaVita Clinical Research for providing the clinical data and review for this research project.
ABBREVIATIONS
- BMI
body mass index
- CI
confidence interval
- CKD
chronic kidney disease
- ESRD
end stage renal disease
- HD
hemodialysis
- HR
hazard ratio
- nPNA
normalized protein nitrogen appearance
- PEW
protein–energy wasting
- USRDS
United States Renal Data System
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.USRDS. [Accessed April 10, 2012];Annual Data Report: Atlas of End-Stage Renal Disease in the United States. 2011 doi: 10.1053/j.ajkd.2011.11.015. http://www.usrds.org/atlas.asps. [DOI] [PubMed]
- 2.Chertow GM, Hsu CY, Johansen KL. The enlarging body of evidence: obesity and chronic kidney disease. J Am Soc Nephrol. 2006 Jun;17(6):1501–1502. doi: 10.1681/ASN.2006040327. [DOI] [PubMed] [Google Scholar]
- 3.Kalantar-Zadeh K, Streja E, Molnar MZ, et al. Mortality Prediction by Surrogates of Body Composition: An Examination of the Obesity Paradox in Hemodialysis Patients Using Composite Ranking Score Analysis. Am J Epidemiol. 2012 Mar 16; doi: 10.1093/aje/kwr384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalantar-Zadeh K, Streja E, Kovesdy CP, et al. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin Proc. 2010 Nov;85(11):991–1001. doi: 10.4065/mcp.2010.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Paradoxical association between body mass index and mortality in men with CKD not yet on dialysis. Am J Kidney Dis. 2007 May;49(5):581–591. doi: 10.1053/j.ajkd.2007.02.277. [DOI] [PubMed] [Google Scholar]
- 6.Kalantar-Zadeh K, Kopple JD, Kilpatrick RD, et al. Association of morbid obesity and weight change over time with cardiovascular survival in hemodialysis population. Am J Kidney Dis. 2005 Sep;46(3):489–500. doi: 10.1053/j.ajkd.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 7.Abbott KC, Glanton CW, Trespalacios FC, et al. Body mass index, dialysis modality, and survival: analysis of the United States Renal Data System Dialysis Morbidity and Mortality Wave II Study. Kidney Int. 2004 Feb;65(2):597–605. doi: 10.1111/j.1523-1755.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 8.Chazot C, Gassia JP, Di Benedetto A, Cesare S, Ponce P, Marcelli D. Is there any survival advantage of obesity in Southern European haemodialysis patients? Nephrol Dial Transplant. 2009 Sep;24(9):2871–2876. doi: 10.1093/ndt/gfp168. [DOI] [PubMed] [Google Scholar]
- 9.Beddhu S, Pappas LM, Ramkumar N, Samore M. Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol. 2003 Sep;14(9):2366–2372. doi: 10.1097/01.asn.0000083905.72794.e6. [DOI] [PubMed] [Google Scholar]
- 10.Patel SS, Molnar MZ, Tayek JA, et al. Serum creatinine as a marker of muscle mass in chronic kidney disease: results of a cross-sectional study and review of literature. J Cachexia Sarcopenia Muscle. 2012 Jul 10; doi: 10.1007/s13539-012-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noori N, Kovesdy CP, Bross R, et al. Novel equations to estimate lean body mass in maintenance hemodialysis patients. Am J Kidney Dis. 2011 Jan;57(1):130–139. doi: 10.1053/j.ajkd.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008 Feb;73(4):391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 13.Kovesdy CP, Kalantar-Zadeh K. Accuracy and limitations of the diagnosis of malnutrition in dialysis patients. Semin Dial. 2012 Jul;25(4):423–427. doi: 10.1111/j.1525-139X.2012.01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Traynor JP, Simpson K, Geddes CC, Deighan CJ, Fox JG. Early initiation of dialysis fails to prolong survival in patients with end-stage renal failure. J Am Soc Nephrol. 2002 Aug;13(8):2125–2132. doi: 10.1097/01.asn.0000025294.40179.e8. [DOI] [PubMed] [Google Scholar]
- 15.Stel VS, Dekker FW, Ansell D, et al. Residual renal function at the start of dialysis and clinical outcomes. Nephrol Dial Transplant. 2009 Oct;24(10):3175–3182. doi: 10.1093/ndt/gfp264. [DOI] [PubMed] [Google Scholar]
- 16.Kalantar-Zadeh K, Norris KC. Is the malnutrition-inflammation complex the secret behind greater survival of African-American dialysis patients? J Am Soc Nephrol. 2011 Dec;22(12):2150–2152. doi: 10.1681/ASN.2011101002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovesdy CP, Kalantar-Zadeh K. Why is protein-energy wasting associated with mortality in chronic kidney disease? Semin Nephrol. 2009 Jan;29(1):3–14. doi: 10.1016/j.semnephrol.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noori N, Kovesdy CP, Dukkipati R, et al. Racial and ethnic differences in mortality of hemodialysis patients: role of dietary and nutritional status and inflammation. Am J Nephrol. 2011;33(2):157–167. doi: 10.1159/000323972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ricks J, Molnar MZ, Kovesdy CP, et al. Racial and ethnic differences in the association of body mass index and survival in maintenance hemodialysis patients. Am J Kidney Dis. 2011 Oct;58(4):574–582. doi: 10.1053/j.ajkd.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong JS, Port FK, Hulbert-Shearon TE, et al. Survival advantage in Asian American end-stage renal disease patients. Kidney Int. 1999 Jun;55(6):2515–2523. doi: 10.1046/j.1523-1755.1999.00464.x. [DOI] [PubMed] [Google Scholar]
- 21.Yen TH, Lin JL, Lin-Tan DT, Hsu CW. Association between body mass and mortality in maintenance hemodialysis patients. Ther Apher Dial. 2010 Aug 1;14(4):400–408. doi: 10.1111/j.1744-9987.2010.00818.x. [DOI] [PubMed] [Google Scholar]
- 22.Flugel-Link RM, Salusky IB, Jones MR, Kopple JD. Enhanced muscle protein degradation and amino acid release from the hemicorpus of acutely uremic rats. Adv Exp Med Biol. 1984;167:545–555. doi: 10.1007/978-1-4615-9355-3_48. [DOI] [PubMed] [Google Scholar]
- 23.Andrews R, Greenhaff P, Curtis S, Perry A, Cowley AJ. The effect of dietary creatine supplementation on skeletal muscle metabolism in congestive heart failure. Eur Heart J Apr. 1998;19(4):617–622. doi: 10.1053/euhj.1997.0767. [DOI] [PubMed] [Google Scholar]
- 24.Kaizu Y, Ohkawa S, Kumagai H. Muscle mass index in haemodialysis patients: a comparison of indices obtained by routine clinical examinations. Nephrol Dial Transplant. 2002 Mar;17(3):442–448. doi: 10.1093/ndt/17.3.442. [DOI] [PubMed] [Google Scholar]
- 25.Moreau-Gaudry X, Guebre-Egziabher F, Jean G, et al. Serum creatinine improves body mass index survival prediction in hemodialysis patients: a 1-year prospective cohort analysis from the ARNOS study. J Ren Nutr. 2011 Sep;21(5):369–375. doi: 10.1053/j.jrn.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Walther CP, Carter CW, Low CL, et al. Interdialytic creatinine change versus predialysis creatinine as indicators of nutritional status in maintenance hemodialysis. Nephrol Dial Transplant. 2012 Feb;27(2):771–776. doi: 10.1093/ndt/gfr389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004 Sep 23;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 28.Ryan TP, Fisher SG, Elder JL, et al. Increased cardiovascular risk associated with reduced kidney function. Am J Nephrol. 2009;29(6):620–625. doi: 10.1159/000194455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Streja E, Molnar MZ, Kovesdy CP, et al. Associations of pretransplant weight and muscle mass with mortality in renal transplant recipients. Clin J Am Soc Nephrol. 2011 Jun;6(6):1463–1473. doi: 10.2215/CJN.09131010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molnar MZ, Streja E, Kovesdy CP, et al. Associations of body mass index and weight loss with mortality in transplant-waitlisted maintenance hemodialysis patients. Am J Transplant. 2011 Apr;11(4):725–736. doi: 10.1111/j.1600-6143.2011.03468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang CX, Tighiouart H, Beddhu S, et al. Both low muscle mass and low fat are associated with higher all-cause mortality in hemodialysis patients. Kidney Int. 2010 Apr;77(7):624–629. doi: 10.1038/ki.2009.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noori N, Kopple JD, Kovesdy CP, et al. Mid-arm muscle circumference and quality of life and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2010 Dec;5(12):2258–2268. doi: 10.2215/CJN.02080310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalantar-Zadeh K, Kuwae N, Wu DY, et al. Associations of body fat and its changes over time with quality of life and prospective mortality in hemodialysis patients. Am J Clin Nutr. 2006 Feb;83(2):202–210. doi: 10.1093/ajcn/83.2.202. [DOI] [PubMed] [Google Scholar]
- 34.Kakiya R, Shoji T, Tsujimoto Y, et al. Body fat mass and lean mass as predictors of survival in hemodialysis patients. Kidney Int. 2006 Aug;70(3):549–556. doi: 10.1038/sj.ki.5000331. [DOI] [PubMed] [Google Scholar]
- 35.Nishizawa Y, Shoji T, Ishimura E. Body composition and cardiovascular risk in hemodialysis patients. J Ren Nutr. 2006 Jul;16(3):241–244. doi: 10.1053/j.jrn.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 36.Kalantar-Zadeh K, Block G, McAllister CJ, Humphreys MH, Kopple JD. Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am J Clin Nutr. 2004 Aug;80(2):299–307. doi: 10.1093/ajcn/80.2.299. [DOI] [PubMed] [Google Scholar]
- 37.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999 Jan 14;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 38.Lee PS, Sampath K, Karumanchi SA, et al. Plasma gelsolin and circulating actin correlate with hemodialysis mortality. J Am Soc Nephrol. 2009 May;20(5):1140–1148. doi: 10.1681/ASN.2008091008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohamed-Ali V, Goodrick S, Bulmer K, Holly JM, Yudkin JS, Coppack SW. Production of soluble tumor necrosis factor receptors by human subcutaneous adipose tissue in vivo. Am J Physiol. 1999 Dec;277(6 Pt 1):E971–975. doi: 10.1152/ajpendo.1999.277.6.E971. [DOI] [PubMed] [Google Scholar]
- 40.Jandacek RJ, Anderson N, Liu M, Zheng S, Yang Q, Tso P. Effects of yo-yo diet, caloric restriction, and olestra on tissue distribution of hexachlorobenzene. Am J Physiol Gastrointest Liver Physiol. 2005 Feb;288(2):G292–299. doi: 10.1152/ajpgi.00285.2004. [DOI] [PubMed] [Google Scholar]
- 41.Sarkar SR, Kuhlmann MK, Kotanko P, et al. Metabolic consequences of body size and body composition in hemodialysis patients. Kidney Int. 2006 Nov;70(10):1832–1839. doi: 10.1038/sj.ki.5001895. [DOI] [PubMed] [Google Scholar]
- 42.Oreopoulos A, Ezekowitz JA, McAlister FA, et al. Association between direct measures of body composition and prognostic factors in chronic heart failure. Mayo Clin Proc. 2010 Jul;85(7):609–617. doi: 10.4065/mcp.2010.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kopple JD, Greene T, Chumlea WC, et al. Relationship between nutritional status and the glomerular filtration rate: results from the MDRD study. Kidney Int. 2000 Apr;57(4):1688–1703. doi: 10.1046/j.1523-1755.2000.00014.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Flow diagram for patient selection.
Supplemental Figure 2. Kaplan–Meier curves to all–cause mortality by BMI categories in Korean HD patients (n = 20,818).
Supplemental Figure 3. Kaplan–Meier curves to all–cause mortality by serum creatinine categories in Korean HD patients (n = 20,408).
Supplemental Figure 4. Association between baseline serum creatinine level and all–cause mortality in 20,408 Korean hemodialysis patients.†
†Hazard ratios were estimated with Cox regression using restricted cubic splines. Values of <1 percentile and >99 percentile in serum creatinine levels were excluded from the model to minimize an influence from the outlier values. The model was adjusted for case–mix and nutrition covariates including age, gender, diabetes mellitus, dialysis vintage, single–pool Kt/V, hemoglobin, serum albumin and normalized protein nitrogen appearance. Dashed lines are 95% point–wise confidence bands. Number of patients is displayed simultaneously.
Supplemental Figure 5. Adjusted hazard ratios (95% confidence interval) for all–cause death in Korean patients with vintage <1 versus ≥ 1 year: A) baseline body mass index, B) baseline serum creatinine level.†
†Patients with BMI >25.0 kg/m2 and serum creatinine level >12.0 mg/dL were reference groups. The model was adjusted for case–mix and nutrition covariates including age, gender, diabetes mellitus, dialysis vintage, single–pool Kt/V, hemoglobin, serum albumin and normalized protein nitrogen appearance.