Abstract
An imbalance between pro-angiogenic (vascular endothelial growth factor, VEGF) and anti-angiogenic (soluble fms-like tyrosine kinase-1, sFlt-1) factors plays an important role in hypertension associated with reduced utero-placental perfusion (RUPP). Exercise has been shown to stimulate pro-angiogenic factors such as VEGF in both the pregnant and non-pregnant state, thus we hypothesized exercise training would attenuate both angiogenic imbalance and hypertension due to RUPP. Four groups of animals were studied: RUPP and normal pregnant (NP) controls and NP and RUPP + exercise training (NP or RUPP+EX). Exercise training attenuated RUPP-induced: hypertension (P<0.05); increased sFlt-1(P<0.05); decreased VEGF (P<0.05), and elevated sFlt-1:VEGF ratio. The positive effects of exercise on angiogenic balance in the RUPP rats were confirmed by restoration (P<0.05) of the RUPP-induced decrease in endothelial tube formation in HUVECs treated with serum from each of the experimental groups. Placental prolyl hydroxylase-1 (PHD1) was increased (P<0.05) in RUPP+Ex rats. Decreased trolox equivalent antioxidant capacity in the placenta, amniotic fluid and kidney of the RUPP rats was reversed by exercise. RUPP induced increase in renal TBARS was attenuated by exercise. The present data show exercise training before and during pregnancy attenuates placental ischemia-induced hypertension, angiogenic imbalance and oxidative stress in the RUPP rat and reveals that increased PHD1 is associated with decreased sFlt-1 thus revealing several potential pathways for exercise training to mitigate the effects of placental ischemia-induced hypertension. Lastly, the present study demonstrates exercise training may be a useful approach to attenuate the development of placental ischemia-induced hypertension during pregnancy.
Keywords: Preeclampsia, pregnancy, VEGF, blood pressure
INTRODUCTION
Hypertensive disorders during pregnancy, such as preeclampsia (PE), occur in approximately 5–8% of pregnancies. Due to its prevalence, PE is a substantial source of maternal and fetal morbidity and mortality, as well as premature delivery. Despite recent advances that have identified and begun to optimize use of several new biomarkers of preeclampsia, current treatment options remain limited (1). While the exact mechanisms of the pathogenesis of preeclampsia remain unclear, recent studies continue to support the hypothesis that a poorly perfused, ischemic placenta leading to an imbalance in hypoxia inducible factor (HIF) regulated angiogenic factors (e.g. sFlt-1, VEGF, PlGF, etc) along with increased renal and placental oxidative stress (2) and widespread endothelial dysfunction is central to the development of the disease (3;4).
Previous work from clinical studies has shown that exercise during pregnancy promotes placental growth (5) and angiogenic balance (6). Further, there is growing interest in the possibility that exercise during pregnancy may hold therapeutic potential to mitigate the effects of placental insufficiency or the angiogenic imbalance associated with preeclampsia. Several reports suggest exercise during pregnancy may positively influence fetal growth and later developmental milestones (7–9). Recent evidence suggests 10 weeks of exercise during pregnancy lowers diastolic blood pressure in women with a predisposition to pregnancy-induced hypertension (10) and decrease cardiovascular risk profile (7). Other studies have recently shown that exercise during pregnancy may promote a pro-angiogenic state by increasing placental growth factor (PlGF) in pregnant women (6). Similar findings have been reported recently by our group and others in studies using rodent models (11;12). Thus, it has been proposed that stimulating pro-angiogenic factors such as PlGF and vascular endothelial growth factor (VEGF) may be beneficial in mitigating the development of hypertensive disorders of pregnancy such as preeclampsia.
In addition to the recent findings regarding exercise and angiogenic factors, exercise training has long been observed to stimulate the expression of a number of cytoprotective molecules such as antioxidant enzymes (13–16) and data suggests that oxygen sensing molecules such as hypoxia inducible factor-1α and prolyl hydroxylases (PHDs) are also influenced by exercise (17). Taken together, these findings suggest exercise may mitigate the effects of placental ischemia that are thought to initiate the development of pregnancy-induced hypertension and preeclampsia. Despite the potential for positive outcomes associated with exercise during pregnancy, it has remained contraindicated in pregnancies complicated with high blood pressure (18). To this end, we sought to test the hypothesis that voluntary exercise before and during pregnancy in the rat would decrease blood pressure and oxidative stress in the placenta and kidney, as well as restore angiogenic balance in pregnant rats with hypertension that develops from chronic reductions in uterine perfusion pressure (RUPP).
METHODS
Animals
Studies were performed in age-matched female Sprague Dawley rats purchased from Charles River (Portage, MI) at three weeks of age. Animals were housed in a temperature-controlled room (23°C) with a 12:12 light:dark cycle. Rats were assigned to one of the following experimental groups: normal pregnant (NP, n=8), NP+exercise (NP+Ex, n=6), reduced uterine perfusion pressure (RUPP, n=10) or RUPP+exercise (RUPP+Ex, n=7). All experimental procedures executed in this study were in accordance with National Institutes of Health guidelines for use and care of animals. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Minnesota.
Exercise wheels, quantification of exercise and breeding
Standard rodent wire activity wheels were fitted with a cyclocomputer and running distance was measured weekly, as described previously (12). Voluntary wheel running was chosen for this study to minimize potentially deleterious effects that have been previously reported with treadmill running in Sprague-Dawley rats (19). Further, we have previously reported this period of exercise is sufficient to stimulate increases in mitochondrial markers of exercise adaptation PGC1-α and ATP synthase (12).
After six-weeks of exercise on the activity wheels or no exercise in the control group, breeding pairs were placed in a wire bottom cage and the presence of two or more vaginal plugs was observed to confirm mating. That day was designated as gestation day 1. Females were returned to individual cages with exercise wheels or without wheels until the morning of gestation day 19.
Reduced Uterine Perfusion Pressure (RUPP) Procedure
The RUPP procedure is a well-established model for studying the link between placental ischemia and hypertension in the pregnant rat and has been described in detail previously (20;21). In brief, silver clips were placed on the lower abdominal aorta (0.203-mm inner diameter (ID)) above the iliac bifurcation and also on branches (0.100-mm ID) of both the right and left ovarian arteries supplying the uterus on day 14 of pregnancy (term = 21). Normal pregnant rats all underwent a sham surgery, which included the midline incision and suture.
Measurement of Mean Arterial Pressure in Chronically Instrumented Conscious Rats
Animals were instrumented on day 17 of gestation and arterial pressure was determined in conscious rats at day 19 of gestation using an indwelling arterial catheter placed in the carotid artery as described previously (12;21;22).
Conceptus measurements & tissue collection
After the measurement of blood pressure, the dams were placed under isoflurane anesthesia and a midline ventral incision was made to isolate the abdominal aorta for plasma and serum collection as reported previously (23). Amniotic fluid was collected by aspirating samples into a syringe with a 27 gauge needle. Pups and placentas were excised blotted dry and weighed. Tissues were snap frozen in liquid nitrogen and stored at −80°C until further analyses were performed.
Plasma/Serum/Assays
Blood was collected for subsequent assays into Corvac® sterile serum separator tubes (Sherwood Davis, St. Louis, MO) and plasma into BD Vacutainer® EDTA containing tubes. Circulating VEGF and sFlt-1 (R & D systems Minneapolis, MN) concentrations were measured using commercial enzyme linked immunosorbant assay (ELISA) kits available from R&D systems (Quantikine®; Minneapolis, MN) according to the manufacturer’s directions as described previously (23).
Oxidative stress was assessed by measuring total antioxidant capacity and thiobarbituric acid reactive species (TBARS) assay which measures malondialdehyde (MDA). Total antioxidant capacity was assessed in amniotic fluid, placenta and renal tissue by measuring Trolox-equivalent antioxidant capacity assay kit (Cayman Chemical Company, Ann Arbor, MI) according to the manufacturer’s directions as previously described (22;24). Additionally, MDA was measured in kidney tissue by using a TBARS assay (Cayman Chemical Company, Ann Arbor, MI) according to the manufacturer’s directions as previously described (22).
Protein Extraction and Quantitation
As described previously (12;23), total soluble protein was extracted from whole placentas and whole kidneys in radioimmunoprecipitation assay (RIPA) lysis buffer containing phenylmethanesulphonylfluoride (PMSF) in dimethyl sulfoxide (DMSO), sodium orthovanadate and a protease inhibitor cocktail (SantaCruz Biotechnology, Inc.). Total soluble cellular protein concentration was determined using the bicinchoninic acid (BCA) method (Pierce Biotechnology).
Western blot
Western immunoblots were performed in placental, skeletal muscle and renal tissue as described previously using 50 µg of total protein per lane separated on a Bis-Tris polyacrylamide gel (Invitrogen) and transferred to nitrocellulose membrane (13;24). Blots were probed for superoxide dismutase 2 (SOD2; Abcam, ab13533; 1:5000), PHD1 (Abcam, 86980; 1:1000), β-actin (Abcam, ab8226; 1:5000) and alpha tubulin (Cell Signaling; 9099, 1:1000).
Endothelial tube formation assay
Angiogenic balance was also assessed in the serum of pregnant rats in vitro as we have published previously (25). Briefly, Phenol-red free growth factor reduced Matrigel™ (BD Biosciences, San Jose, CA) was pipetted into each well of a 24-well cell culture-treated plate, and incubated at 37°C for a minimum of 30 minutes to solidify. Primary human umbilical vascular endothelial cells (HUVEC) (ATCC, Manassas, VA) were washed twice with serum-less vascular cell growth media (ATCC) and plated at 50,000 cells per ml of serum-less media. The cells were then treated with a 5% serum from the respective rat treatment groups and incubated for 8 hours. Tube formations per frame was assessed at 40× optical zoom with a digital inverted compound microscope and ImageJ analysis software (National Institutes of Health, Bethesda, MD) by at least two individual investigators that were blinded to the identity of the experimental groups. Values from each observer were averaged to obtain final counts.
Statistical Analysis and Calculations
All data are presented as mean ± SEM and statistical significance was accepted when P<0.05. sFlt-1/VEGF and sFlt-1 data were square root transformed prior to statistical analysis. Conceptus data were calculated as mean per pregnancy. Comparisons between experimental groups were two-way analysis of variance and post-hoc tests were employed when indicated. Statistical calculations were made with GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA USA).
RESULTS
Exercise amount
There was no difference in the amount of wheel running per week before pregnancy in the animals from the NP and RUPP groups (30.4 vs. 30.0 km/wk). Likewise, there was no difference in the amount of wheel running per week during pregnancy between the NP and RUPP rats (4.5 vs. 4.8 km/wk).
Blood pressure
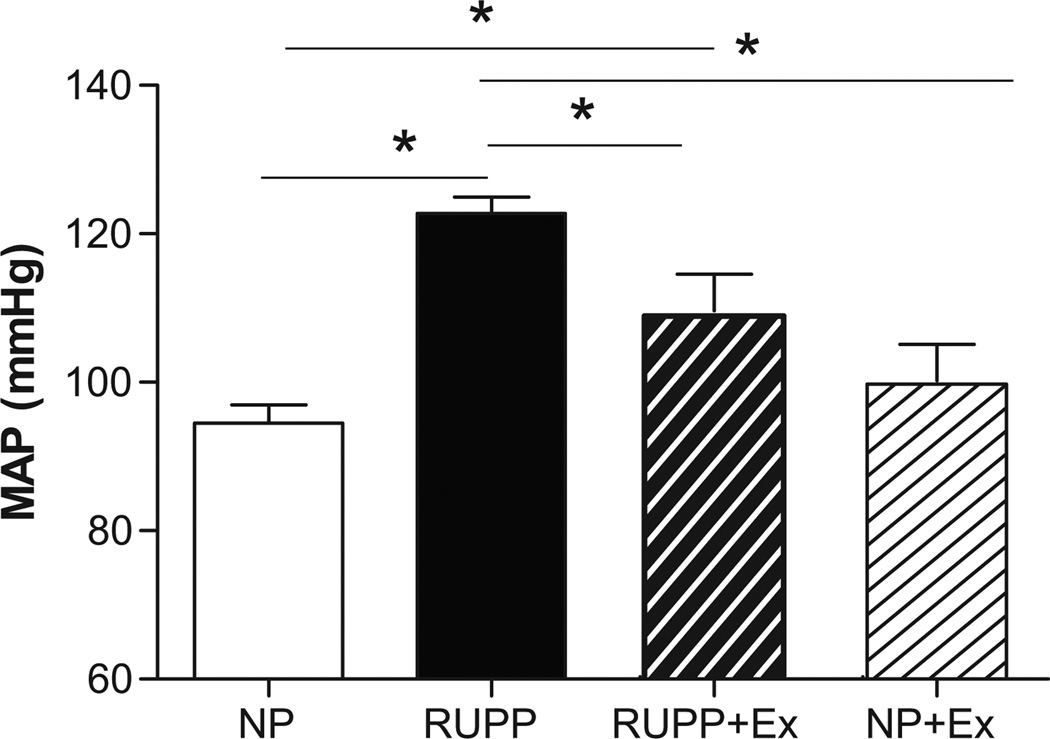
Figure 1 illustrates that RUPP-induced hypertension was decreased (P<0.05) by wheel running before and during pregnancy but was still greater than the NP control rats. Wheel running did not alter blood pressure in the NP+Ex group.
Figure 1. Effects of RUPP and Exercise on Mean Arterial Pressure (MAP).
MAP was increased by RUPP and was attenuated by exercise before and during pregnancy. Data are expressed as mean ± SEM. Statistical significance for comparisons that are different by post-hoc testing are indicated by lines above the bars and * P<0.05.
Conceptus morphometrics
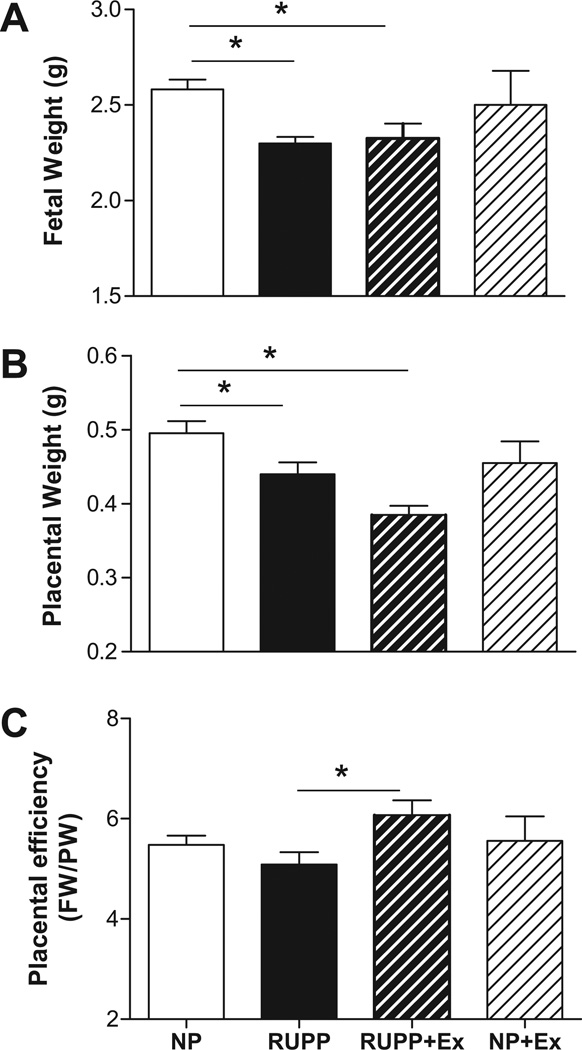
Figure 2A illustrates that exercise before and during pregnancy had no effect on fetal weight in either the RUPP or NP groups. Figure 2B demonstrates that placental weight, which was decreased by RUPP when compared to NP rats, was further decreased by exercise in the RUPP group. Figure 2C shows that placental efficiency was not altered between the NP and RUPP rats in the present cohort but was increased in the RUPP+Ex group.
Figure 2. Effects of exercise on fetal (panel A), placental (panel B) weight and placental efficiency (Fetal weight/placental weight; panel C).
Fetal and placental weight was decreased in the reduced uterine perfusion pressure (RUPP) compared to normal pregnant (NP) rats. Exercise had no effect on fetal weight in either the RUPP or NP rats. Exercise decreased placental weight in the RUPP rats, but had no effect in the NP rats. Placental efficiency was increased (P<0.05) in the RUPP+Ex group compared to the RUPP group. Data are expressed as mean ± SEM. Statistical significance for comparisons that are different by post-hoc testing are indicated by lines above the bars and * P<0.05.
Angiogenic and endocrine factors
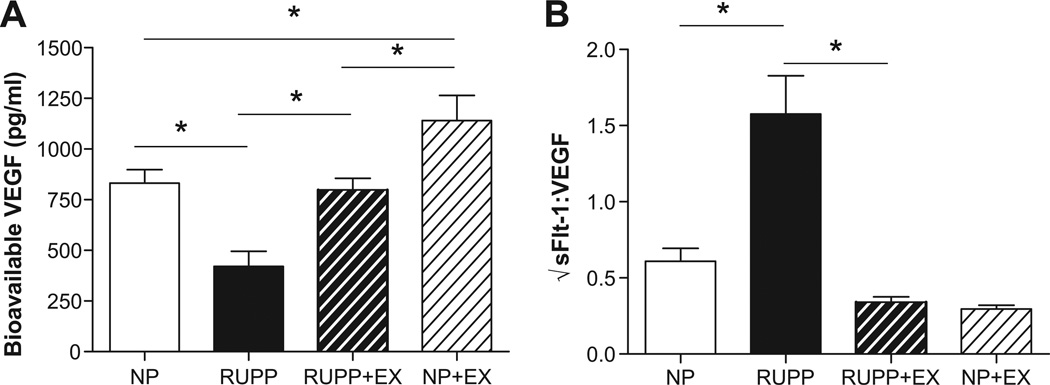
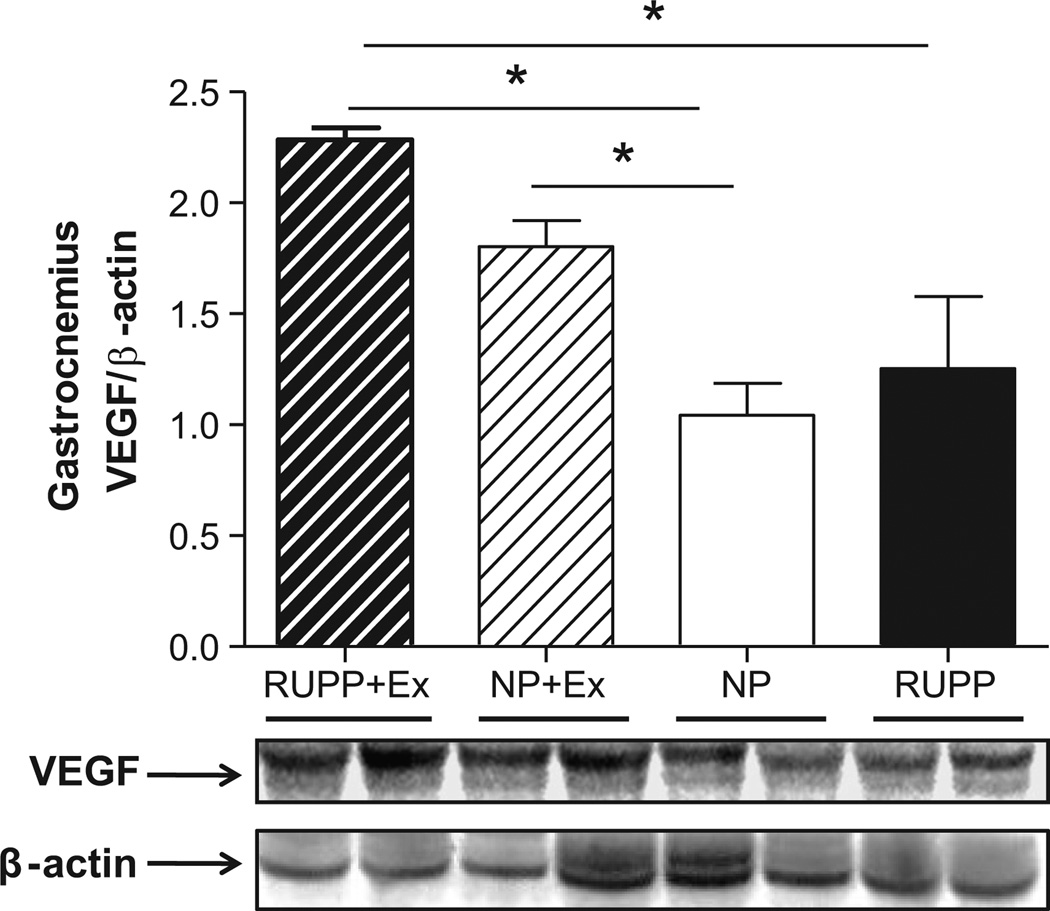
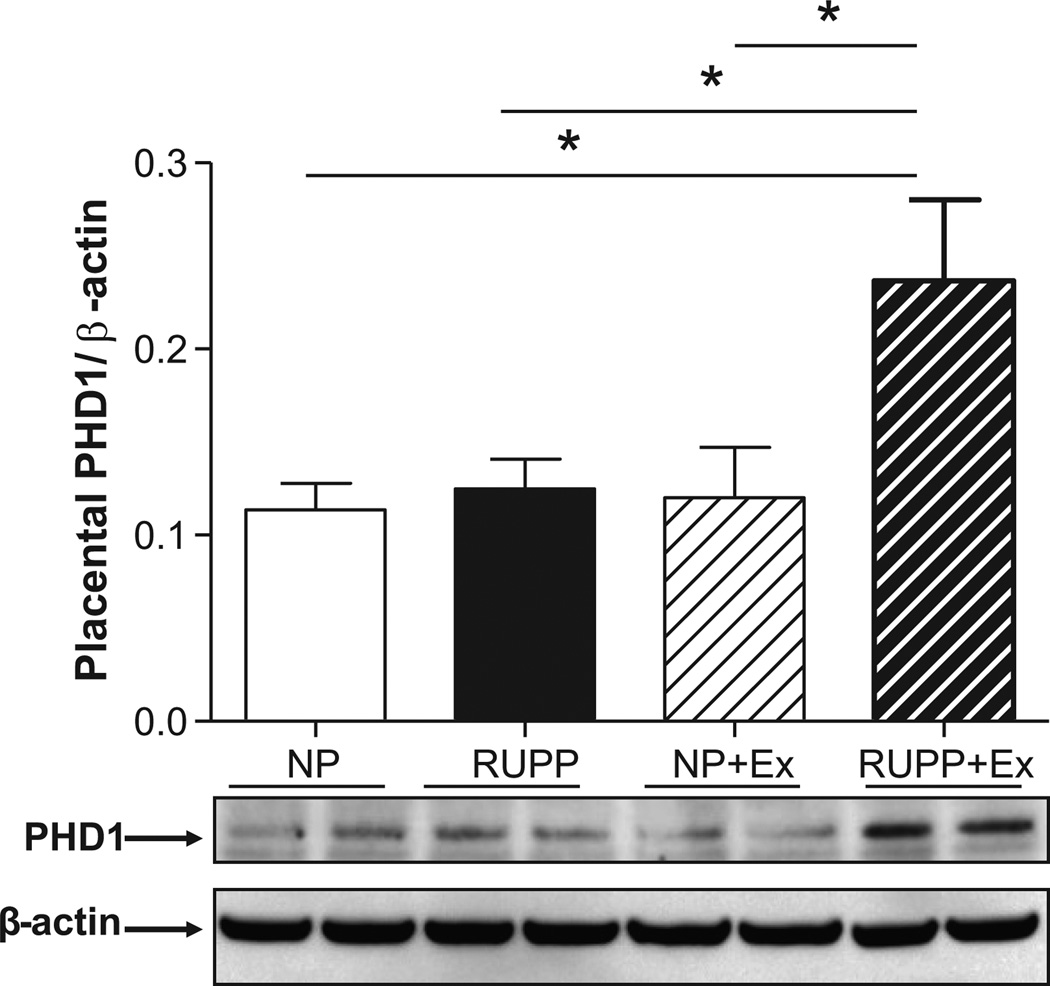
Figure 3 (panel A) shows that circulating free levels of VEGF which were decreased (P<0.05) in RUPP where increased by wheel running. Likewise, Figure 3B illustrates that the √sFlt-1/VEGF ratio which was increased by RUPP was decreased in wheel running before and during pregnancy. In addition, circulating levels of sFlt-1 were increased (P<0.05) in the RUPP rats when compared to the NP, NP+Ex, and RUPP+Ex rats (1300.0 ± 345.3 vs. 334.3 ±104.8 vs. 110.0 ± 16.76 vs. 101.4 ± 22.0 pg/ml). Figure 4 shows that VEGF expression was increased (P<0.05) in the skeletal muscle of the RUPP +Ex rats compared to the NP and RUPP groups. Figure 5 illustrates that PHD1 was increased (P<0.05) in the placentas of the RUPP+Ex rats compared to the NP, RUPP and NP+Ex groups.
Figure 3. Circulating vascular endothelial growth factor (VEGF) and angiogenic balance in late gestation.
Circulating VEGF (panel A) was decreased in the reduced uterine perfusion pressure (RUPP) compared to normal pregnant (NP) rats and exercise training increased free VEGF in the RUPP+Ex compared to RUPP and in the NP+Ex compared to NP. Panel B illustrates that the sFlt-1:VEGF ratio was increased in the RUPP compared to NP rats was decreased by exercise training in pregnancy. Data are expressed as mean ± SEM. Statistical significance for comparisons that are different by post-hoc testing are indicated by lines above the bars and * P<0.05.
Figure 4. Skeletal muscle (gastrocnemius) VEGF expression.
VEGF expression expressed relative to β-actin was increased (P<0.05) in the skeletal muscle of the RUPP +Ex rats compared to the NP and RUPP groups. Representative blots are shown below graph. Data are expressed as mean ± SEM. Statistical significance for comparisons that are different by post-hoc testing are indicated by lines above the bars and * P<0.05.
Figure 5. Placental prolyl hydroxylase 1 (PHD1) expression.
PHD1 expression was increased (P<0.05) in placentas of RUPP+Ex compared to the NP+Ex, NP and RUPP groups. Representative blot images for PHD1 and β-actin are below the graph. Data are expressed as mean ± SEM. Statistical significance for comparisons that are different by post-hoc testing are indicated by lines above the bars and * P<0.05.
Endothelial cell function
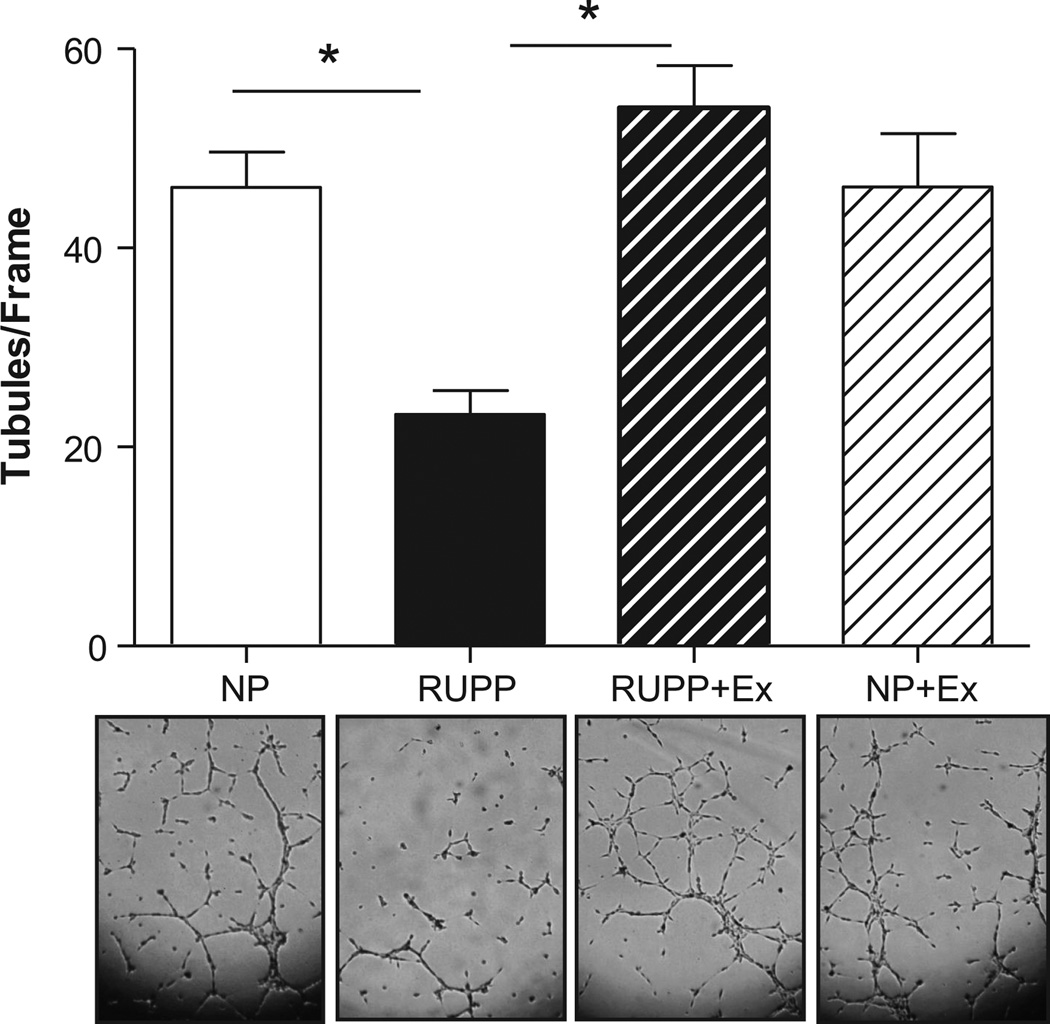
Figure 6 shows that endothelial cell tubule formation is decreased (P<0.05) with the addition of RUPP serum to the cells. The serum from RUPP+Ex rats showed more tubule formation when compared to the RUPP.
Figure 6. Endothelial cell tube formation assay.
Endothelial cell tubule formation was decreased with the addition of RUPP serum to the cells when compared to cells treated with serum from NP rats. This effect was not observed when cells were treated with serum from RUPP rats that exercised. No effect was observed due to exercise in the cells treated with serum from NP rats that exercised. Data are expressed as mean ± SEM. Statistical significance for comparisons that are different by post-hoc testing are indicated by lines above the bars and * P<0.05.
Oxidative stress data
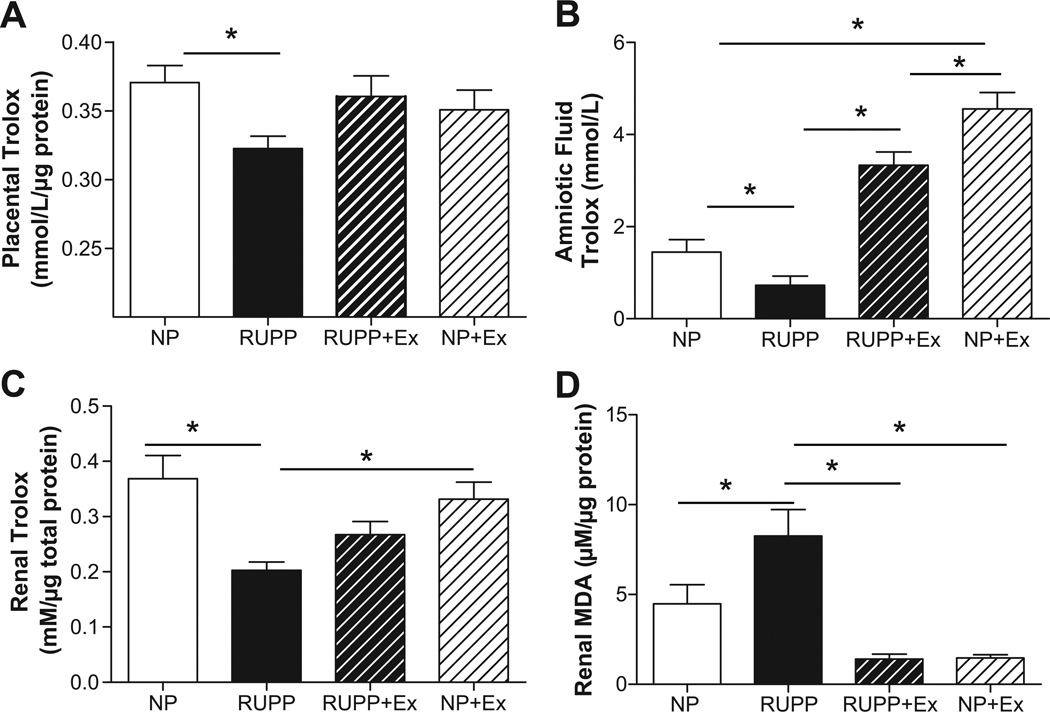
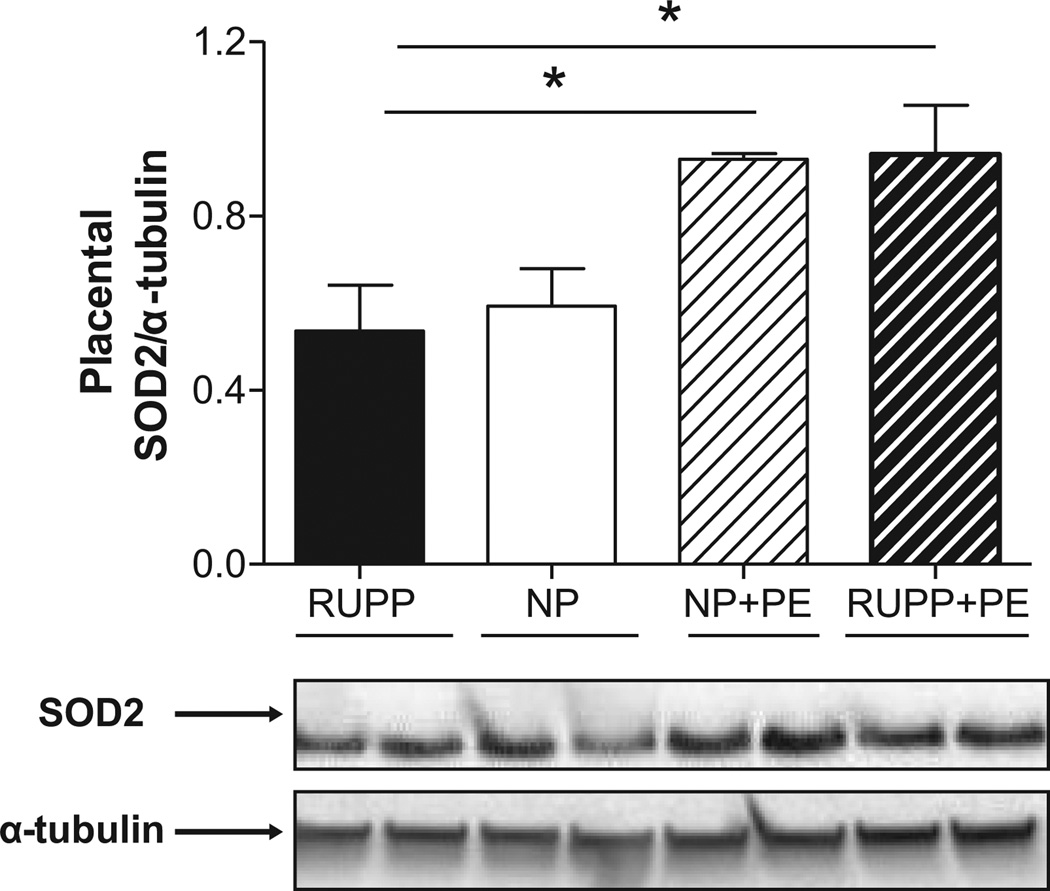
Figure 7A shows that placental antioxidant capacity was decreased (P<0.05) in the RUPP group and this was attenuated by exercise in the RUPP group. Exercise did not augment total antioxidant status of placental tissue in the NP rats. Figure 7B shows that amniotic fluid antioxidant status was decreased (P<0.05) in RUPP and this was reversed by exercise training. Moreover, Figure 7C shows that exercise training increased (P<0.05) antioxidant capacity in the kidneys of the NP rats. Figure 7D shows that renal MDA was increased (P<0.05) in the RUPP kidneys and that this was attenuated by exercise in the RUPP group. Figure 8 illustrates that SOD2 was increased (P<0.05) in both exercise groups compared to the respective non-exercise groups. Western blot analysis indicated renal SOD2 was not altered in any of the groups (not shown).
Figure 7. Total antioxidant capacity in placenta, amniotic fluid and kidney, and TBARS (malondialdehyde) in kidney.
Placental antioxidant capacity was decreased (P<0.05) in the RUPP group and this was attenuated by exercise in the RUPP rats. Exercise did not augment total antioxidant status of placental tissue in the NP rats. Panel B shows that amniotic fluid antioxidant status was decreased (P<0.05) in RUPP compared to NP and this was reversed by exercise training. Panel C shows renal antioxidant capacity was decreased (P<0.05) in the kidneys from reduced uterine perfusion pressure (RUPP) compared to normal pregnant (NP) and that this was attenuated by exercise in the RUPP group. Exercise alone did not augment total antioxidant status of renal tissue. Panel D shows that renal MDA was increased in the RUPP compared to NP rats and this was abrogated by exercise training. Data are expressed as mean ± SEM. Statistical significance for comparisons that are different by post-hoc testing are indicated by lines above the bars and * P<0.05.
Figure 8. Placental superoxide dismutase 2 (SOD2) expression.
SOD2 was increased (P<0.05) in the RUPP+Ex and NP+Ex groups compared to the RUPP rats. Representative blot images for PHD1 and β-actin are below the graph. Data are expressed as mean ± SEM. Statistical significance for comparisons that are different by post-hoc testing are indicated by lines above the bars and * P<0.05.
DISCUSSION
The present study reveals several interesting and novel findings regarding the effects of exercise before and during pregnancy on several aspects of maternal and fetal physiology in a robust model of hypertension during preeclampsia. Foremost, we observed that the placental ischemia-induced hypertension in this model was attenuated by exercise and this was accompanied by restoration of angiogenic balance (i.e. increased free VEGF, decreased sFlt-1 and increased endothelial cell tube formation in vitro). We also report that exercise training significantly increased VEGF expression in skeletal muscle of RUPP+Ex rats and increased PHD1 expression in the placentas from RUPP+Ex rats. Further, exercise resulted in reduced oxidative stress in the RUPP hypertensive rat by reversing decreased anti-oxidant capacity in the placental, kidney and amniotic fluid and mitigating the RUPP-induced increase in renal TBARS. Thus, these findings demonstrate that exercise has several beneficial effects in rats with placental ischemia-induced hypertension.
We chose to employ a voluntary wheel running paradigm that has been previously shown to elicit metabolic adaptations in the skeletal muscle (i.e. increased gastrocnemius ATP synthase and PGC1-α expression) of the animals given free access to an activity wheel for the same amount of time as the rats in the current study (12) and to avoid the stress that has previously been associated with treadmill running as an activity intervention (19). In the current study, we also found that the spontaneous wheel activity was not different in rats with or without a RUPP clip. Thus, despite reductions in blood flow to lower extremities due to the clips there was sufficient autoregulation of blood flow to meet the metabolic demands of voluntary running.
Blood pressure is one of the chief concerns in preeclampsia and our present data shows that exercise before and during pregnancy is effective at reducing the extent of hypertension in the RUPP rat. This is in agreement with a recent study that used a transgenic approach in mice to study hypertension during pregnancy (11). Despite the significant reduction in blood pressure from the RUPP to the RUPP+Ex groups in this study, blood pressure was still elevated compared to the NP group. While in some hypertensive settings this may not be desirable, in the case of hypertension that is likely due to a decrease in perfusion pressure this may be an optimal compromise that gains benefits of lowered blood pressure with maintenance of tissue perfusion. Taken together, the data from studies suggest that exercise may hold therapeutic potential in hypertensive pregnancies despite the traditional approach that has contraindicated exercise in these pregnancies (18).
We also found that fetal weight was not compromised by exercise in NP or RUPP rats. In addition, there were no obvious signs of fetal stress in the exercised RUPP rats, thus we are unsure of the significance of this observation at the present time. Taken together, there did not appear to be any significant fetal distress in the RUPP rats that exercised before and during pregnancy. In contrast, placental weight was decreased in the RUPP rats that exercised. In contrast to previous reports we did not observe any decrease in placental efficiency between the RUPP and NP groups in the present cohort (21) but we did observe an increase in placental efficiency due to exercise in the RUPP rats. This observation is consistent with the restoration of angiogenic balance in the animals and the pro-angiogenic effects we observed when HUVECs were cultured with sera from the groups in this study and may suggest the presence of increased angiogenesis in the placentas of these animals. It should be noted that the placental weight change is difficult to evaluate in isolation as this could be interpreted as either increased placental efficiency or as a sign of placental stress (26). Thus, further studies are needed to evaluate placental function and vascularity as well as possible effects on the fetus and offspring health; especially considering recent reports suggesting maternal exercise during pregnancy may attenuate the fetal programming effects of low protein diet in rats (9).
The importance of angiogenic factors in the pathophysiology of hypertension during preeclampsia has become increasingly clear in recent years (1;27) and this has been confirmed in animal models (23;28;29). Thus, a primary focus of this study was to determine if exercise training before and during pregnancy had any effect on angiogenic balance in RUPP hypertension. To this end, we found that free plasma VEGF was increased and plasma sFlt-1 was decreased. Moreover, we found that the angiogenic potential of the serum from exercised RUPP rats was also restored to normal pregnant levels. These studies are in agreement with previous work in women, rats and mice that suggest exercise during pregnancy has beneficial effects on angiogenic balance (6;11;12). Taken together, these data provide evidence that the mechanism by which exercise lowers blood pressure in placental ischemia-induced hypertension involves the restoration of angiogenic balance.
While our observations that exercise training before and during pregnancy lowers sFlt-1 and increases VEGF levels are exciting, the mechanism by which exercise influences these angiogenic factors remains unclear. We recently showed that exercise training in rats increases circulating VEGF in pregnant but not in non-pregnant rats which suggested that the placenta is a likely source of the increased VEGF(12). In this study we show that VEGF was increased in the gastrocnemius muscle of the rats in the RUPP+Ex compared to the RUPP group suggesting that there may be an non-placenta source of VEGF in these studies.
We have previously shown that RUPP hypertension and angiogenic imbalance is associated with increased placental HIF-1α expression (30) and the HIF pathway along with associated regulatory proteins such as PHDs have been suggested as putative targets to control factors governing angiogenic functions (31). We found that PHD1 was increased in the placentas of the RUPP+Ex rats compared to the NP, RUPP and NP+Ex groups. Although we only found that PHD1 was increased in the RUPP rats, the dependence on ischemia for pathway activation in the placenta has been reported recently by George et al. with respect to adenosine regulation of angiogenic factors as well as heme oxygenase-1 (32;33). Moreover, recent work has shown that stimulation of PGC1-α in skeletal muscle represents a non-HIF mediated pathway to stimulate VEGF (34). This observation remains correlative at this point but reveals a potential regulatory pathway by which regular physical activity may influence angiogenic balance in the setting of placental ischemia.
We also observed that exercise before and during pregnancy improved the antioxidant status of RUPP rats and attenuated the oxidative stress that we presently observed and others (24) have previously reported in RUPP rats. In the present study, renal oxidative stress in the RUPP rat was improved by chronic exercise training before and during pregnancy. We report that antioxidant capacity was increased by exercise and renal TBARs, which are increased in the RUPP kidneys, was attenuated by exercise. Previous work has shown that antioxidant treatment lowers blood pressure in the RUPP model (24), thus the decrease in renal oxidative stress may contribute to an improvement in renal excretory function and the reduction in blood pressure observed in the present study. We also show that placental and amniotic fluid antioxidant capacity was decreased in the RUPP group and this was attenuated by exercise in the RUPP group. Excess reactive oxygen species (ROS) in the intrauterine environment has been implicated in developmental programming but whether or not a decrease of ROS alters fetal development remains unclear (35). Nevertheless, this is an intriguing observation that deserves further attention in subsequent experiments.
Perspectives and Significance
Currently, preeclampsia is a disorder with very limited treatment options. The present data suggest that regular exercise before and during pregnancy reduces blood pressure in hypertensive pregnant rats and attenuates several sequelae of placental ischemia such as angiogenic imbalance and oxidative stress. While the design of the present study precluded us from extending these findings to the well-being of the offspring, it is apparent from the present work that there was no decrease in fetal survival or fetal size due to the voluntary exercise by the hypertensive rats.
Our findings also support the hypothesis that exercise training promotes angiogenic balance during pregnancy with placental ischemia. Further, the observation that increased PHD1 was associated with decreased sFlt-1 suggests that disruption of HIF signaling may play a role. While these observations are encouraging, it remains unclear whether regular exercise training prior to pregnancy is important to these observations. Considering that both pregnancy and exercise can be considerable physiological stressors, it seems likely that pre-pregnancy exercise status may play a role in whether or not exercise during pregnancy is effective and possibly safe as a preventive measure for increased blood pressure in pregnancy. Further studies are planned in our laboratory to evaluate these possibilities.
Viewed in concert with previous studies indicating that physical activity lowers risk for preeclampsia and other hypertensive disorders of pregnancy (10;36;37), the data from our study raise the possibility that exercise regimens may be an important way for women to mitigate risk of preeclampsia. There are certainly questions that remain such as when and how much exercise is required and whether exercise can safely be employed as a therapeutic modality to mitigate effects of placental-ischemia induced hypertension; nevertheless, we feel these positive results are encouraging and further studies are warranted.
NOVELTY AND SIGNIFICANCE.
1)What is new?
This study shows that exercise training before and during pregnancy stimulates a pro-angiogenic state, reduces oxidative stress and lowers blood pressure in a rat model of hypertension during preeclampsia that results from placental ischemia and mimics many of the features of the human condition. We also provide evidence that exercise training before and during a hypertensive pregnancy is associated with increased PHD1, a factor that may decrease HIF mediated sFlt-1 signaling, and did not result in any adverse fetal consequences in late gestation.
2)What is relevant?
There are currently no treatments for preeclampsia other than close management of the expectant mother and delivery of the baby when needed. Despite the long recognized benefits of exercise on cardiovascular health, exercise has been traditionally contraindicated during hypertensive pregnancies. This study provides important evidence indicating that exercise training before and during pregnancy has beneficial effects on maternal cardiovascular function.
3)Summary
Exercise before and during pregnancy may have beneficial effects on blood pressure and angiogenic balance in preeclampsia.
Acknowledgements
Sources of Funding
This work was supported in part by grants from the American Heart Association 10SDG2600040 and National Institutes of Health HL114096.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Jeffrey Gilbert- funding from AHA and NIH
Christopher Banek- NONE
Ashley Bauer- NONE
Anne Gingery-NONE
Karen Needham- NONE
REFERENCE
- 1.Rana S, Powe CE, Salahuddin S, Verlohren S, Perschel FH, Levine RJ, Lim KH, Wenger JB, Thadhani R, Karumanchi SA. Angiogenic Factors and the Risk of Adverse Outcomes in Women With Suspected Preeclampsia / Clinical Perspective. Circulation. 2012;125:911–919. doi: 10.1161/CIRCULATIONAHA.111.054361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raijmakers MTM, Dechend R, Poston L. Oxidative Stress and Preeclampsia. Rationale for Antioxidant Clinical Trials. Hypertension. 2004;44:374–380. doi: 10.1161/01.HYP.0000141085.98320.01. [DOI] [PubMed] [Google Scholar]
- 3.LaMarca BD, Gilbert J, Granger JP. Recent progress toward the understanding of the pathophysiology of hypertension during preeclampsia. Hypertension. 2008;51:982–988. doi: 10.1161/HYPERTENSIONAHA.107.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of hypertension during preeclampsia linking placental ischemia with endothelial dysfunction. Hypertension. 2001;38:718–722. doi: 10.1161/01.hyp.38.3.718. [DOI] [PubMed] [Google Scholar]
- 5.Clapp JF, III, Kim H, Burciu B, Schmidt S, Petry K, Lopez B. Continuing regular exercise during pregnancy: effect of exercise volume on fetoplacental growth. Am J Obstet Gynecol. 2002;186:142–147. doi: 10.1067/mob.2002.119109. [DOI] [PubMed] [Google Scholar]
- 6.Weissgerber TL, Davies GAL, Roberts JM. Modification of angiogenic factors by regular and acute exercise during pregnancy. Journal of Applied Physiology. 2010;108:1217–1223. doi: 10.1152/japplphysiol.00008.2010. [DOI] [PubMed] [Google Scholar]
- 7.Clapp JF. Long-term outcome after exercising throughout pregnancy: fitness and cardiovascular risk. American Journal of Obstetrics and Gynecology. 2008;199:489. doi: 10.1016/j.ajog.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clapp JF., III Morphometric and neurodevelopmental outcome at age five years of the offspring of women who continued to exercise regularly throughout pregnancy. J Pediatr. 1996;129:856–863. doi: 10.1016/s0022-3476(96)70029-x. [DOI] [PubMed] [Google Scholar]
- 9.Fidalgo M, Falcão-Tebas F, Bento-Santos A, de Oliveira E, Nogueira-Neto JF, de Moura EG, Lisboa PcC, de Castro RM, Leandro CGi. Programmed changes in the adult rat offspring caused by maternal protein restriction during gestation and lactation are attenuated by maternal moderate-low physical training. British Journal of Nutrition. 2012:1–8. doi: 10.1017/S0007114512001316. FirstView. [DOI] [PubMed] [Google Scholar]
- 10.Yeo S, Steele NM, Chang MC, Leclaire SM, Ronis DL, Hayashi R. Effect of exercise on blood pressure in pregnant women with a high risk of gestational hypertensive disorders. J Reprod Med. 2000;45:293–298. [PubMed] [Google Scholar]
- 11.Falcao S, Bisotto S, Michel C, Lacasse AA, Vaillancourt C, Gutkowska J, Lavoie JL. Exercise training can attenuate preeclampsia-like features in an animal model. J Hypertens. 2010;28:2446–2453. doi: 10.1097/HJH.0b013e32833e97d0. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert JS, Banek CT, Bauer AJ, Gingery A, Dreyer HC. Placental and vascular adaptations to exercise training before and during pregnancy in the rat. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2012 doi: 10.1152/ajpregu.00253.2012. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamashita N, Hoshida S, Otsu K, Asahi M, Kuzuya T, Hori M. Exercise Provides Direct Biphasic Cardioprotection via Manganese Superoxide Dismutase Activation. The Journal of Experimental Medicine. 1999;189:1699–1706. doi: 10.1084/jem.189.11.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atalay M, Oksala NKJ, Laaksonen DE, Khanna S, Nakao C, Lappalainen J, Roy S, Hanninen O, Sen CK. Exercise training modulates heat shock protein response in diabetic rats. Journal of Applied Physiology. 2004;97:605–611. doi: 10.1152/japplphysiol.01183.2003. [DOI] [PubMed] [Google Scholar]
- 15.Siu PM, Bryner RW, Martyn JK, Alway SE. Apoptotic adaptations from exercise training in skeletal and cardiac muscles. The FASEB Journal. 2004 doi: 10.1096/fj.03-1291fje. 03-1291fje. [DOI] [PubMed] [Google Scholar]
- 16.Chicco AJ, McCune SA, Emter CA, Sparagna GC, Rees ML, Bolden DA, Marshall KD, Murphy RC, Moore RL. Low-Intensity Exercise Training Delays Heart Failure and Improves Survival in Female Hypertensive Heart Failure Rats. Hypertension. 2008;51:1096–1102. doi: 10.1161/HYPERTENSIONAHA.107.107078. [DOI] [PubMed] [Google Scholar]
- 17.Freyssenet D. Energy sensing and regulation of gene expression in skeletal muscle. Journal of Applied Physiology. 2007;102:529–540. doi: 10.1152/japplphysiol.01126.2005. [DOI] [PubMed] [Google Scholar]
- 18.ACOG Committee on Obstetric Practice. Committee opinion #267: exercise during pregnancy and the postpartum period. Obstetrics & Gynecology. 2002;99:171–173. doi: 10.1016/s0029-7844(01)01749-5. [DOI] [PubMed] [Google Scholar]
- 19.Moraska A, Deak T, Spencer RL, Roth D, Fleshner M. Treadmill running produces both positive and negative physiological adaptations in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1321–R1329. doi: 10.1152/ajpregu.2000.279.4.R1321. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP. Recombinant Vascular Endothelial Growth Factor 121 Infusion Lowers Blood Pressure and Improves Renal Function in Rats With Placental Ischemia-Induced Hypertension. Hypertension. 2009;55:380–385. doi: 10.1161/HYPERTENSIONAHA.109.141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilbert JS, Bauer AJ, Gingery A, Banek CT, Chasson S. Circulating and utero-placental adaptations to chronic placental ischemia in the rat. Placenta. 2012;33:100–105. doi: 10.1016/j.placenta.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 22.Heltemes A, Gingery A, Soldner ELB, Bozadjieva N, Jahr K, Johnson B, Gilbert JS. Chronic placental ischemia alters amniotic fluid milieu and results in impaired glucose tolerance, insulin resistance and hyperleptinemia in young rats. Experimental Biology and Medicine. 2010;235:892–899. doi: 10.1258/ebm.2010.009357. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert JS, Babcock SA, Granger JP. Hypertension Produced by Reduced Uterine Perfusion in Pregnant Rats Is Associated With Increased Soluble Fms-Like Tyrosine Kinase-1 Expression. Hypertension. 2007;50:1142–1147. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 24.Sedeek M, Gilbert JS, LaMarca BB, Sholook M, Chandler DL, Wang Y, Granger JP. Role of Reactive Oxygen Species in Hypertension Produced by Reduced Uterine Perfusion in Pregnant Rats. Am J Hypertens. 2008;21:1152–1156. doi: 10.1038/ajh.2008.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banek CT, Bauer AJ, Gingery A, Gilbert JS. Timing of ischemic insult alters fetal growth trajectory, maternal angiogenic balance and markers of renal oxidative stress in the pregnant rat. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2012 doi: 10.1152/ajpregu.00250.2012. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myatt L. Placental adaptive responses and fetal programming. The Journal of Physiology. 2006;572:25–30. doi: 10.1113/jphysiol.2006.104968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension. 2005;46:1077–1085. doi: 10.1161/01.HYP.0000187899.34379.b0. [DOI] [PubMed] [Google Scholar]
- 28.Maynard SE, Venkatesha S, Thadhani R, Karumanchi SA. Soluble Fms-like tyrosine kinase 1 and endothelial dysfunction in the pathogenesis of preeclampsia. Pediatr Res. 2005;57:1R–7R. doi: 10.1203/01.PDR.0000159567.85157.B7. [DOI] [PubMed] [Google Scholar]
- 29.Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 30.Gilbert JS, Gilbert SAB, Arany M, Granger JP. Hyp{Banek, 2012 3317 /id;Falcao, 2010 2985 /id}ertension Produced by Placental Ischemia in Pregnant Rats Is Associated With Increased Soluble Endoglin Expression. Hypertension. 2008;53:399–403. doi: 10.1161/HYPERTENSIONAHA.108.123513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, Gleadle JM. Differential Function of the Prolyl Hydroxylases PHD1, PHD2, and PHD3 in the Regulation of Hypoxia-inducible Factor. Journal of Biological Chemistry. 2004;279:38458–38465. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- 32.George EM, Cockrell K, Aranay M, Csongradi E, Stec DE, Granger JP. Induction of heme oxygenase 1 attenuates placental ischemia-induced hypertension. Hypertension. 2011;57:941–948. doi: 10.1161/HYPERTENSIONAHA.111.169755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.George EM, Cockrell K, Adair TH, Granger JP. Regulation of sFlt-1 and VEGF secretion by adenosine under hypoxic conditions in rat placental villous explants. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2010;299:R1629–R1633. doi: 10.1152/ajpregu.00330.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chinsomboon J, Ruas J, Gupta RK, Thom R, Shoag J, Rowe GC, Sawada N, Raghuram S, Arany Z. The transcriptional coactivator PGC-1alpha mediates exercise-induced angiogenesis in skeletal muscle. Proceedings of the National Academy of Sciences. 2009;106:21401–21406. doi: 10.1073/pnas.0909131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Myatt L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta. 2010;31:S66–S69. doi: 10.1016/j.placenta.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weissgerber TL, Wolfe LA, Davies GA. The role of regular physical activity in preeclampsia prevention. Med Sci Sports Exerc. 2004;36:2024–2031. doi: 10.1249/01.mss.0000147627.35139.dc. [DOI] [PubMed] [Google Scholar]
- 37.Kasawara K, Nascimento S, Costa M, Surita F, SILVA E. Exercise and physical activity in the prevention of preeclampsia: systematic review. Acta Obstetricia et Gynecologica Scandinavica. 2012 doi: 10.1111/j.1600-0412.2012.01483.x. In Press. [DOI] [PubMed] [Google Scholar]