Abstract
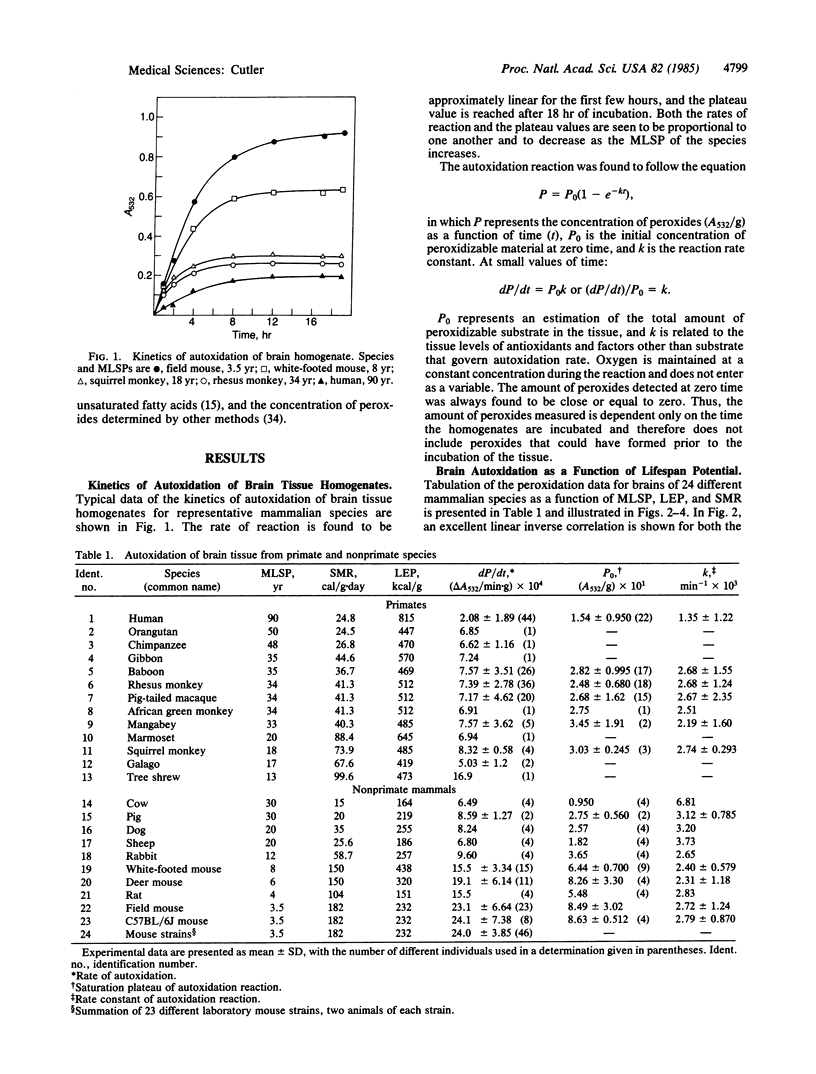
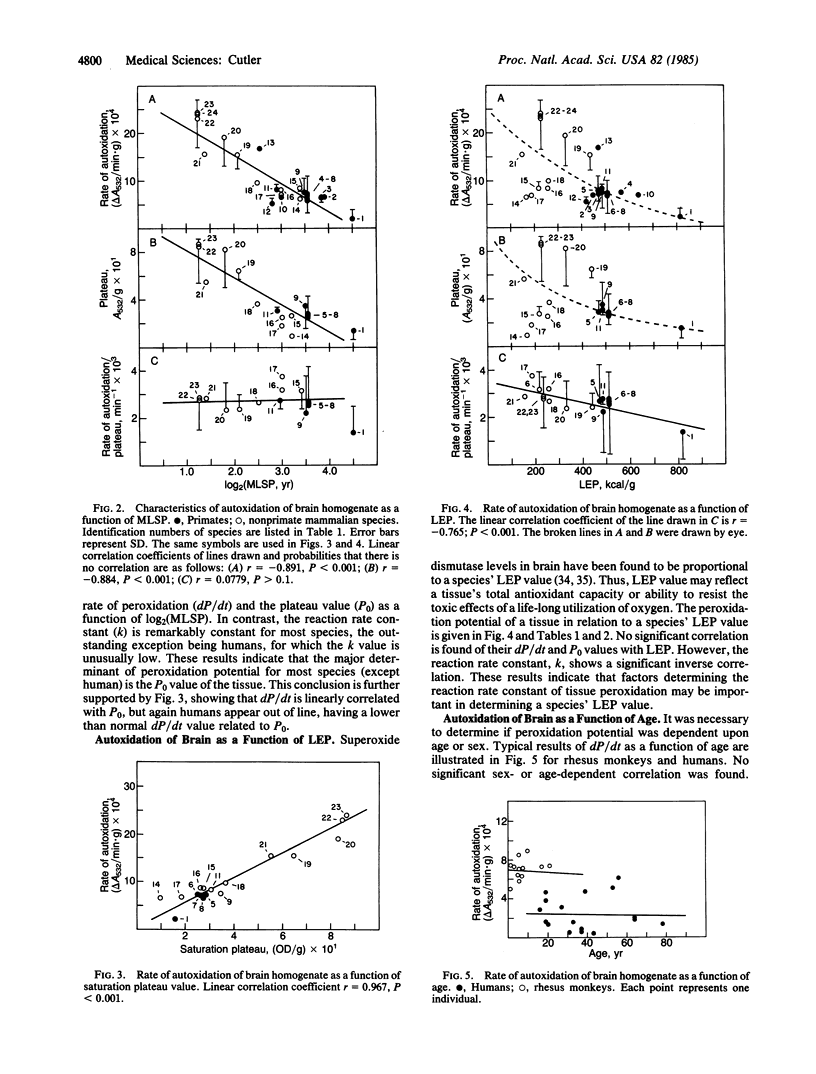
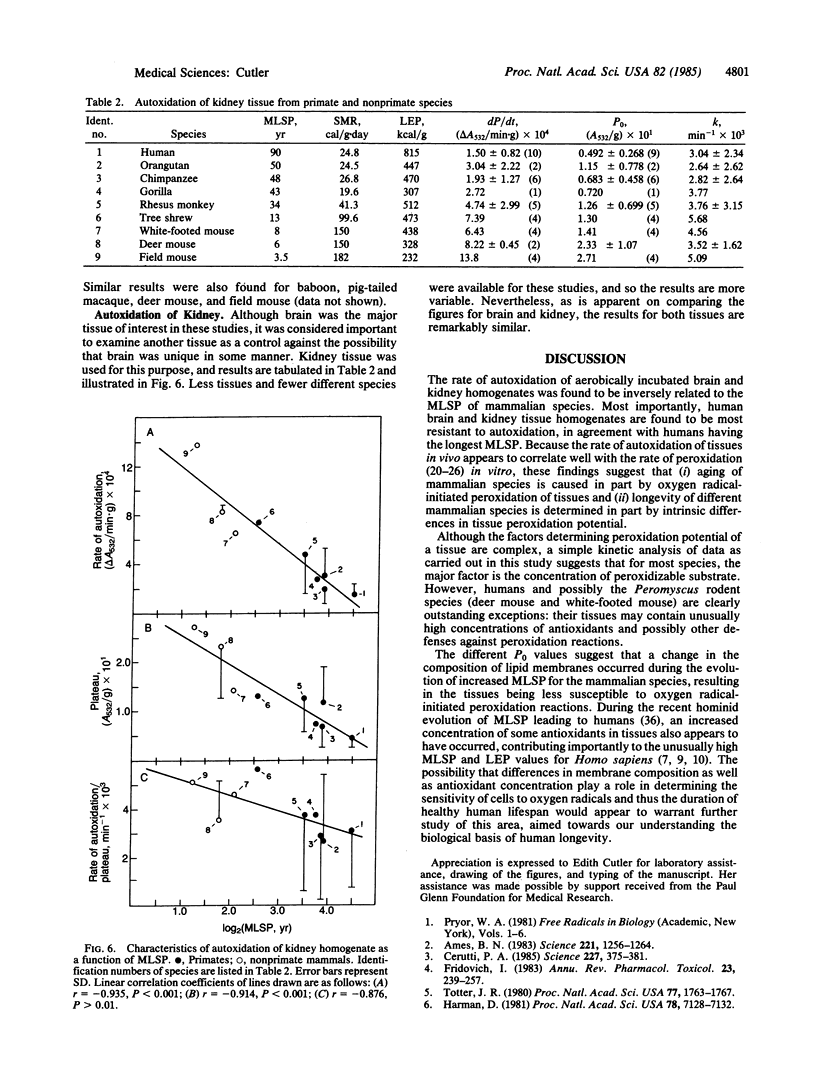
Peroxidation reactions may cause many of the dysfunctions associated with aging. Accordingly, the 30-fold differences in aging rate among the mammalian species could be determined in part by peroxidation defense processes. This possibility was tested by measuring the spontaneous autoxidation of aerobically incubated brain and kidney tissue homogenates of 24 different mammalian species as a function of their maximum lifespan potential. Results show a statistically significant inverse correlation between both the rate of autoxidation and the amount of peroxidizable substrate with maximum lifespan potential. Kinetic analysis of the data indicates that the amount of peroxidizable substrate was the major factor determining the rate of autoxidation. For human tissues, antioxidants also appear to contribute to their unusually low sensitivity to peroxidation. These results support the hypothesis that aging may be caused in part by oxygen radicals initiating peroxidation reactions and that peroxidation defense processes are involved in governing the longevity of mammalian species.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- BARBER A. A. Addendum: mechansims of lipid peroxide formation in rat tissue homogenates. Radiat Res. 1963;Suppl 3:33–43. [PubMed] [Google Scholar]
- BERNHEIM F. Biochemical implications of pro-oxidants and antioxidants. Radiat Res. 1963;Suppl 3:17–32. [PubMed] [Google Scholar]
- BRAUER R. W. Liver circulation and function. Physiol Rev. 1963 Jan;43:115–213. doi: 10.1152/physrev.1963.43.1.115. [DOI] [PubMed] [Google Scholar]
- Boehme D. H., Kosecki R., Carson S., Stern F., Marks N. Lipoperoxidation in human and rat brain tissue: developmental and regional studies. Brain Res. 1977 Nov 4;136(1):11–21. doi: 10.1016/0006-8993(77)90127-5. [DOI] [PubMed] [Google Scholar]
- Booth J., Boyland E., Cooling C. The respiration of human liver tissue. Biochem Pharmacol. 1967 Apr;16(4):721–724. doi: 10.1016/0006-2952(67)90085-8. [DOI] [PubMed] [Google Scholar]
- Cadenas E., Varsavsky A. I., Boveris A., Chance B. Oxygen- or organic hydroperoxide-induced chemiluminescence of brain and liver homogenates. Biochem J. 1981 Sep 15;198(3):645–654. doi: 10.1042/bj1980645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Cutler R. G. Carotenoids and retinol: their possible importance in determining longevity of primate species. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7627–7631. doi: 10.1073/pnas.81.23.7627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler R. G. Evolution of human longevity and the genetic complexity governing aging rate. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4664–4668. doi: 10.1073/pnas.72.11.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler R. G. Superoxide dismutase, longevity and specific metabolic rate. A reply. Gerontology. 1983;29(2):113–120. doi: 10.1159/000213102. [DOI] [PubMed] [Google Scholar]
- DAVIES M. On body size and tissue respiration. J Cell Comp Physiol. 1961 Jun;57:135–147. doi: 10.1002/jcp.1030570302. [DOI] [PubMed] [Google Scholar]
- Dillard C. J., Tappel A. L. Fluorescent products from reaction of peroxidizing polyunsaturated fatty acids with phosphatidyl ethanolamine and phenylalanine. Lipids. 1973 Apr;8(4):183–189. doi: 10.1007/BF02544632. [DOI] [PubMed] [Google Scholar]
- Dormandy T. L. Biological rancidification. Lancet. 1969 Sep 27;2(7622):684–688. doi: 10.1016/s0140-6736(69)90390-0. [DOI] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982 Nov;47(5):412–426. [PubMed] [Google Scholar]
- Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol. 1983;23:239–257. doi: 10.1146/annurev.pa.23.040183.001323. [DOI] [PubMed] [Google Scholar]
- Harman D. The aging process. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7124–7128. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday M. A., Potter D., Jarrah A., Bearg S. The relation of metabolic rate to body weight and organ size. Pediatr Res. 1967 May;1(3):185–195. doi: 10.1203/00006450-196705000-00005. [DOI] [PubMed] [Google Scholar]
- Kartha V. N., Krishnamurthy S. Effect of vitamins, antioxidants and sulfhydryl compounds on in vitro rat brain lipid peroxidation. Int J Vitam Nutr Res. 1978;48(1):38–43. [PubMed] [Google Scholar]
- Kornbrust D. J., Mavis R. D. Relative susceptibility of microsomes from lung, heart, liver, kidney, brain and testes to lipid peroxidation: correlation with vitamin E content. Lipids. 1980 May;15(5):315–322. doi: 10.1007/BF02533546. [DOI] [PubMed] [Google Scholar]
- Sohal R. S., Farmer K. J., Allen R. G., Ragland S. S. Effects of diethyldithiocarbamate on life span, metabolic rate, superoxide dismutase, catalase, inorganic peroxides and glutathione in the adult male housefly, Musca domestica. Mech Ageing Dev. 1984 Feb;24(2):175–183. doi: 10.1016/0047-6374(84)90069-1. [DOI] [PubMed] [Google Scholar]
- Stocks J., Gutteridge J. M., Sharp R. J., Dormandy T. L. Assay using brain homogenate for measuring the antioxidant activity of biological fluids. Clin Sci Mol Med. 1974 Sep;47(3):215–222. doi: 10.1042/cs0470215. [DOI] [PubMed] [Google Scholar]
- Tappel A. L. Vitamin E and selenium protection from in vivo lipid peroxidation. Ann N Y Acad Sci. 1980;355:18–31. doi: 10.1111/j.1749-6632.1980.tb21324.x. [DOI] [PubMed] [Google Scholar]
- Tolmasoff J. M., Ono T., Cutler R. G. Superoxide dismutase: correlation with life-span and specific metabolic rate in primate species. Proc Natl Acad Sci U S A. 1980 May;77(5):2777–2781. doi: 10.1073/pnas.77.5.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totter J. R. Spontaneous cancer and its possible relationship to oxygen metabolism. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1763–1767. doi: 10.1073/pnas.77.4.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladimirov Y. A., Olenev V. I., Suslova T. B., Cheremisina Z. P. Lipid peroxidation in mitochondrial membrane. Adv Lipid Res. 1980;17:173–249. doi: 10.1016/b978-0-12-024917-6.50011-2. [DOI] [PubMed] [Google Scholar]
- ZALKIN H., TAPPEL A. L. Studies of the mechanism of vitamin E action. IV. Lipide peroxidation in the vitamin E-deficient rabbit. Arch Biochem Biophys. 1960 May;88:113–117. doi: 10.1016/0003-9861(60)90205-8. [DOI] [PubMed] [Google Scholar]



