Abstract
We studied the molecular composition of the complement C5b-9 complex required for optimal killing of Escherichia coli strain J5. J5 cells were incubated in 3.3%, 6.6%, or 10.0% C8-deficient serum previously absorbed to remove specific antibody and lysozyme. This resulted in the stable deposition after washing of 310, 560, and 890 C5b67 molecules per colony-forming unit, respectively, as determined by binding of 125I-labeled C7. Organisms were then incubated with excess C8 and various amounts of 131I-labeled C9. Plots of the logarithm (base 10) of E. coli J5 cells killed (log kill) vs. C9 input were sigmoidal, confirming the multihit nature of the lethal process. When C9 was supplied in excess, 3300, 5700, and 9600 molecules of C9 were bound per organism for cells bearing 310, 560, and 890 C5b-8 complexes, respectively, leading to C9-to-C7 ratios of 11.0:1, 10.8:1, and 11.4:1 and to log kill values of 1.3, 2.1, and 3.9. However, at low inputs of C9 that lead to C9-to-C7 ratios of less than 3.3:1, no killing occurred, and this was independent of the number of C5b-9 complexes bound. Formation of multimeric C9 at C9-to-C7 ratios permissive for killing was confirmed by electron microscopy and by binding of 125I-labeled antibody with specificity for multimeric but not monomeric C9. These experiments are the first to demonstrate a biological function for C9 polymerization and suggest that multimeric C9 is necessary for optimal killing of E. coli J5 cells by C5b-9.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N., Doane F. W. Agar diffusion method for negative staining of microbial suspensions in salt solutions. Appl Microbiol. 1972 Sep;24(3):495–496. doi: 10.1128/am.24.3.495-496.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz S. J., Isliker H. Antibody-independent interactions between Escherichia coli J5 and human complement components. J Immunol. 1981 Nov;127(5):1748–1754. [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. On the cause and nature of C9-related heterogeneity of terminal complement complexes generated on target erythrocytes through the action of whole serum. J Immunol. 1984 Sep;133(3):1453–1463. [PubMed] [Google Scholar]
- Boyle M. D., Borsos T. Studies on the terminal stages of immune hemolysis. V. Evidence that not all complement-produced transmembrane channels are equal. J Immunol. 1979 Jul;123(1):71–76. [PubMed] [Google Scholar]
- Boyle M. D., Gee A. P., Borsos T. Studies on the terminal stages of immune hemolysis. VI. Osmotic blockers of differing Stokes' radii detect complement-induced transmembrane channels of differing size. J Immunol. 1979 Jul;123(1):77–82. [PubMed] [Google Scholar]
- Clas F., Loos M. Requirement for an additional serum factor essential for the antibody-independent activation of the classical complement sequence by Gram-negative bacteria. Infect Immun. 1982 Sep;37(3):935–939. doi: 10.1128/iai.37.3.935-939.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELBEIN A. D., HEATH E. C. THE BIOSYNTHESIS OF CELL WALL LIPOPOLYSACCHARIDE IN ESCHERICHIA COLI. I. THE BIOCHEMICAL PROPERTIES OF A URIDINE DIPHOSPHATE GALACTOSE 4-EPIMERASELESS MUTANT. J Biol Chem. 1965 May;240:1919–1925. [PubMed] [Google Scholar]
- Feingold D. S., Goldman J. N., Kuritz H. M. Locus of the action of serum and the role of lysozyme in the serum bactericidal reaction. J Bacteriol. 1968 Dec;96(6):2118–2126. doi: 10.1128/jb.96.6.2118-2126.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold D. S., Goldman J. N., Kuritz H. M. Locus of the lethal event in the serum bactericidal reaction. J Bacteriol. 1968 Dec;96(6):2127–2131. doi: 10.1128/jb.96.6.2127-2131.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giavedoni E. B., Chow Y. M., Dalmasso A. P. The functional size of the primary complement lesion in resealed erythrocyte membrane ghosts. J Immunol. 1979 Jan;122(1):240–245. [PubMed] [Google Scholar]
- Goldman J. N., Austen K. F. Reaction mechanisms of nascent C567 (reactive lysis). II. Killing of a rough form of Escherichia coli by C567, C8, and C9. J Infect Dis. 1974 Apr;129(4):444–450. doi: 10.1093/infdis/129.4.444. [DOI] [PubMed] [Google Scholar]
- Goldman J. N., Ruddy S., Austen K. F., Feingold D. S. The serum bactericidal reaction. 3. Antibody and complement requirements for killing a rough Escherichia coli. J Immunol. 1969 Jun;102(6):1379–1387. [PubMed] [Google Scholar]
- Hammer C. H., Wirtz G. H., Renfer L., Gresham H. D., Tack B. F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981 Apr 25;256(8):3995–4006. [PubMed] [Google Scholar]
- Harriman G. R., Esser A. F., Podack E. R., Wunderlich A. C., Braude A. I., Lint T. F., Curd J. G. The role of C9 in complement-mediated killing of Neisseria. J Immunol. 1981 Dec;127(6):2386–2390. [PubMed] [Google Scholar]
- Inoue K., Kinoshita T., Okada M., Akiyama Y. Release of phospholipids from complement-mediated lesions on the surface structure of Escherichia coli. J Immunol. 1977 Jul;119(1):65–72. [PubMed] [Google Scholar]
- Inoue K., Yonemasu K., Takamizawa A., Amano T. [Studies on the immune bacteriolysis. XIV. Requirement of all nine components of complement for immune bacteriolysis]. Biken J. 1968 Sep;11(3):203–206. [PubMed] [Google Scholar]
- Joiner K. A., Hammer C. H., Brown E. J., Cole R. J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. I. Terminal complement components are deposited and released from Salmonella minnesota S218 without causing bacterial death. J Exp Med. 1982 Mar 1;155(3):797–808. doi: 10.1084/jem.155.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Schmetz M. A., Goldman R. C., Leive L., Frank M. M. Mechanism of bacterial resistance to complement-mediated killing: inserted C5b-9 correlates with killing for Escherichia coli O111B4 varying in O-antigen capsule and O-polysaccharide coverage of lipid A core oligosaccharide. Infect Immun. 1984 Jul;45(1):113–117. doi: 10.1128/iai.45.1.113-117.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami M., Ihara I., Suzuki A., Harada Y. Properties of a new complement-dependent bactericidal factor specific for Ra chemotype salmonella in sera of conventional and germ-free mice. J Immunol. 1982 Nov;129(5):2198–2201. [PubMed] [Google Scholar]
- Kroll H. P., Bhakdi S., Taylor P. W. Membrane changes induced by exposure of Escherichia coli to human serum. Infect Immun. 1983 Dec;42(3):1055–1066. doi: 10.1128/iai.42.3.1055-1066.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lint T. F., Zeitz H. J., Gewurz H. Inherited deficiency of the ninth component of complement in man. J Immunol. 1980 Nov;125(5):2252–2257. [PubMed] [Google Scholar]
- Podack E. R., Tschoop J., Müller-Eberhard H. J. Molecular organization of C9 within the membrane attack complex of complement. Induction of circular C9 polymerization by the C5b-8 assembly. J Exp Med. 1982 Jul 1;156(1):268–282. doi: 10.1084/jem.156.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Tschopp J. Circular polymerization of the ninth component of complement. Ring closure of the tubular complex confers resistance to detergent dissociation and to proteolytic degradation. J Biol Chem. 1982 Dec 25;257(24):15204–15212. [PubMed] [Google Scholar]
- Podack E. R., Tschopp J. Membrane attack by complement. Mol Immunol. 1984 Jul;21(7):589–603. doi: 10.1016/0161-5890(84)90044-0. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Tschopp J. Polymerization of the ninth component of complement (C9): formation of poly(C9) with a tubular ultrastructure resembling the membrane attack complex of complement. Proc Natl Acad Sci U S A. 1982 Jan;79(2):574–578. doi: 10.1073/pnas.79.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramm L. E., Whitlow M. B., Mayer M. M. Size distribution and stability of the trans-membrane channels formed by complement complex C5b-9. Mol Immunol. 1983 Feb;20(2):155–160. doi: 10.1016/0161-5890(83)90126-8. [DOI] [PubMed] [Google Scholar]
- SHUGAR D. The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme. Biochim Biophys Acta. 1952 Mar;8(3):302–309. doi: 10.1016/0006-3002(52)90045-0. [DOI] [PubMed] [Google Scholar]
- Schreiber R. D., Morrison D. C., Podack E. R., Müller-Eberhard H. J. Bactericidal activity of the alternative complement pathway generated from 11 isolated plasma proteins. J Exp Med. 1979 Apr 1;149(4):870–882. doi: 10.1084/jem.149.4.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims P. J., Lauf P. K. Analysis of solute diffusion across the C5b-9 membrane lesion of complement: evidence that individual C5b-9 complexes do not function as discrete, uniform pores. J Immunol. 1980 Dec;125(6):2617–2625. [PubMed] [Google Scholar]
- Spitznagel J. K. Normal serum cytotoxicity for P32-labeled smooth Enterobacteriaceae. II. Fate of macromolecular and lipid phosphorus of damaged cells. J Bacteriol. 1966 Jan;91(1):148–152. doi: 10.1128/jb.91.1.148-152.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. L., Monahan J. B., Brickner A., Sodetz J. M. Measurement of the ratio of the eighth and ninth components of human complement on complement-lysed membranes. Biochemistry. 1984 Aug 28;23(18):4016–4022. doi: 10.1021/bi00313a002. [DOI] [PubMed] [Google Scholar]
- Stolfi R. L. Immune lytic transformation: a state of irreversible damage generated as a result of the reaction of the eighth component in the guinea pig complement system. J Immunol. 1968 Jan;100(1):46–54. [PubMed] [Google Scholar]
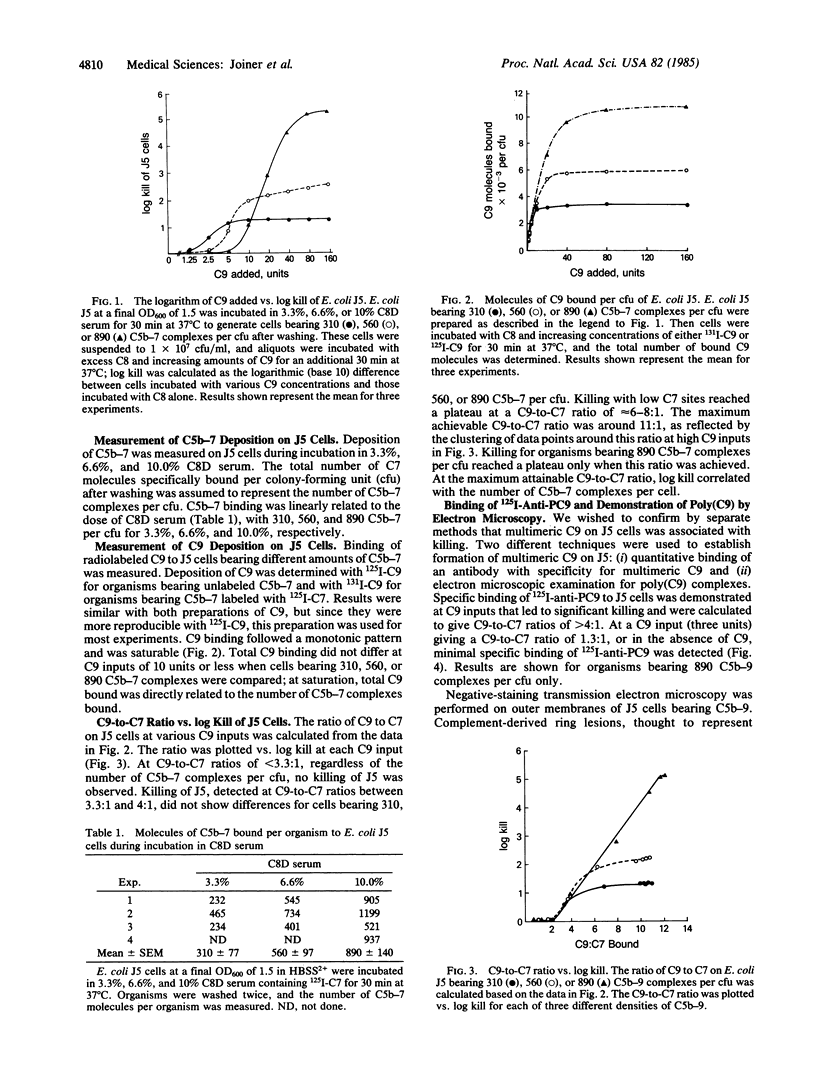
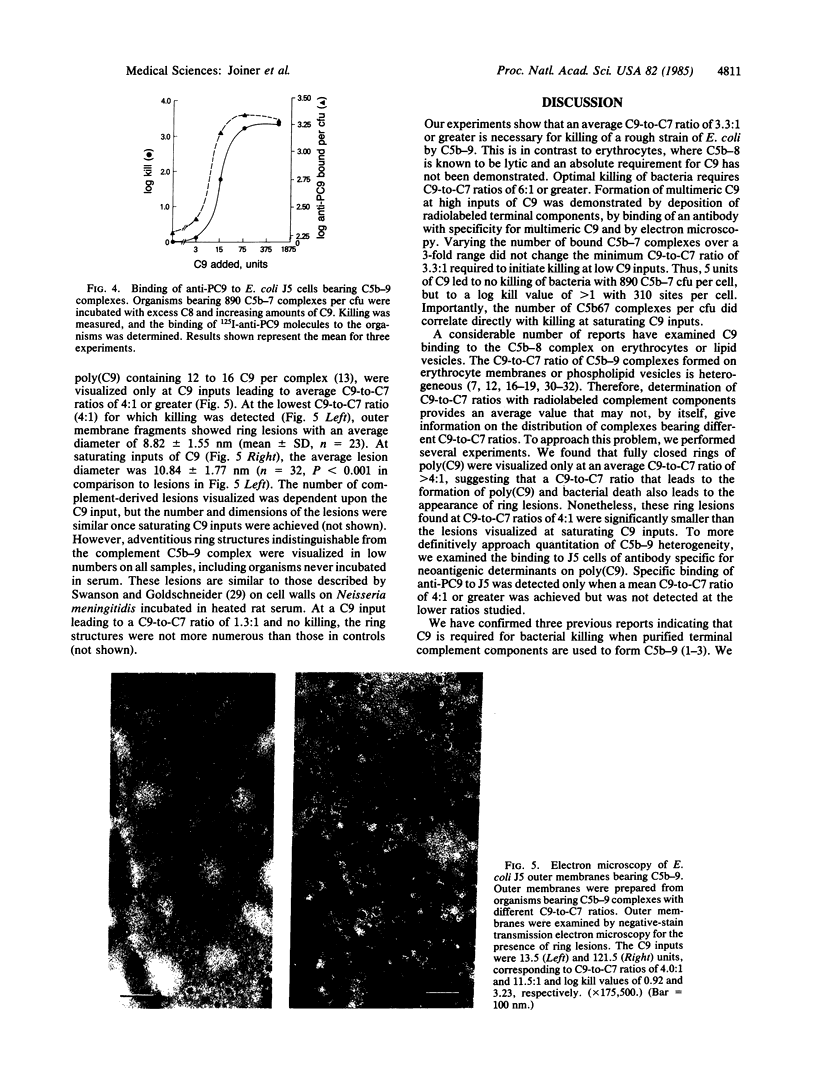
- Swanson J., Goldschneider I. The serum bactericidal system: ultrastructural changes in Neisseria meningitidis exposed to normal rat serum. J Exp Med. 1969 Jan 1;129(1):51–79. doi: 10.1084/jem.129.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W., Kroll H. P. Interaction of human complement proteins with serum-sensitive and serum-resistant strains of Escherichia coli. Mol Immunol. 1984 Jul;21(7):609–620. doi: 10.1016/0161-5890(84)90046-4. [DOI] [PubMed] [Google Scholar]
- Tschopp J., Engel A., Podack E. R. Molecular weight of poly(C9). 12 to 18 C9 molecules form the transmembrane channel of complement. J Biol Chem. 1984 Feb 10;259(3):1922–1928. [PubMed] [Google Scholar]
- Tschopp J., Müller-Eberhard H. J., Podack E. R. Formation of transmembrane tubules by spontaneous polymerization of the hydrophilic complement protein C9. Nature. 1982 Aug 5;298(5874):534–538. doi: 10.1038/298534a0. [DOI] [PubMed] [Google Scholar]
- Tschopp J. Ultrastructure of the membrane attack complex of complement. Heterogeneity of the complex caused by different degree of C9 polymerization. J Biol Chem. 1984 Jun 25;259(12):7857–7863. [PubMed] [Google Scholar]
- Wright S. D., Levine R. P. How complement kills E. coli. II. The apparent two-hit nature of the lethal event. J Immunol. 1981 Sep;127(3):1152–1156. [PubMed] [Google Scholar]
- Ziegler E. J., Douglas H., Sherman J. E., Davis C. E., Braude A. I. Treatment of E. coli and klebsiella bacteremia in agranulocytic animals with antiserum to a UDP-gal epimerase-deficient mutant. J Immunol. 1973 Aug;111(2):433–438. [PubMed] [Google Scholar]