Abstract
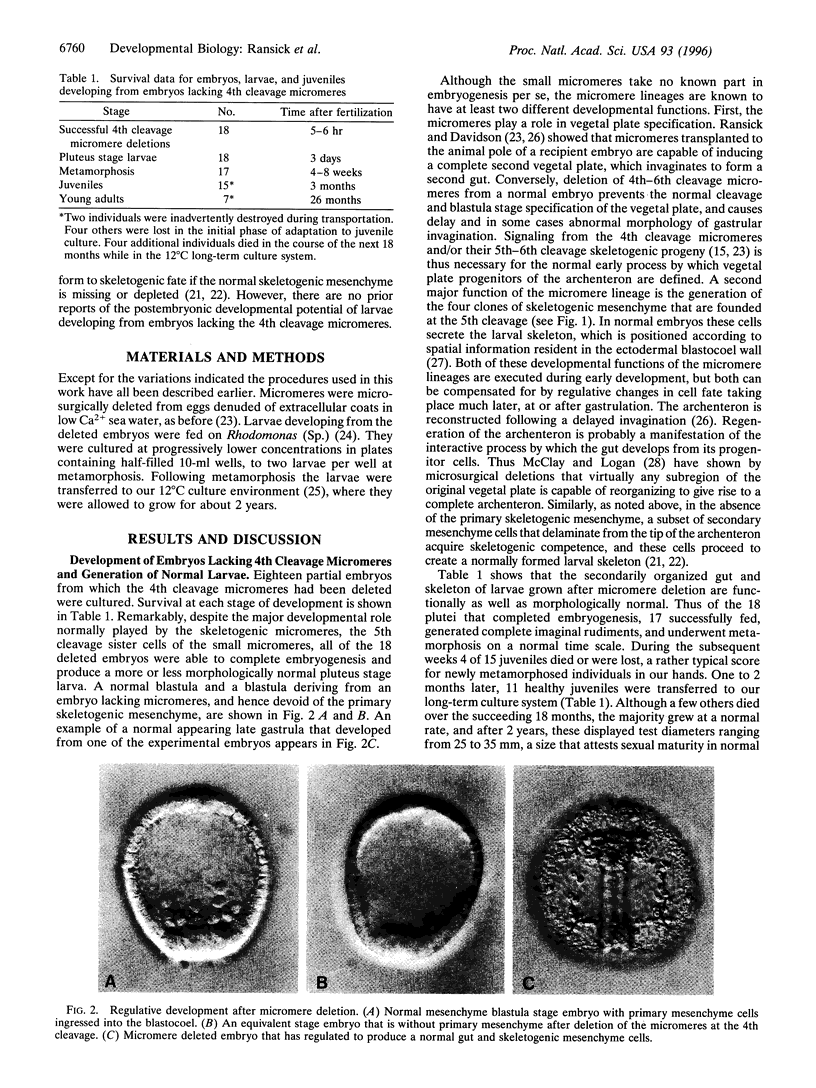
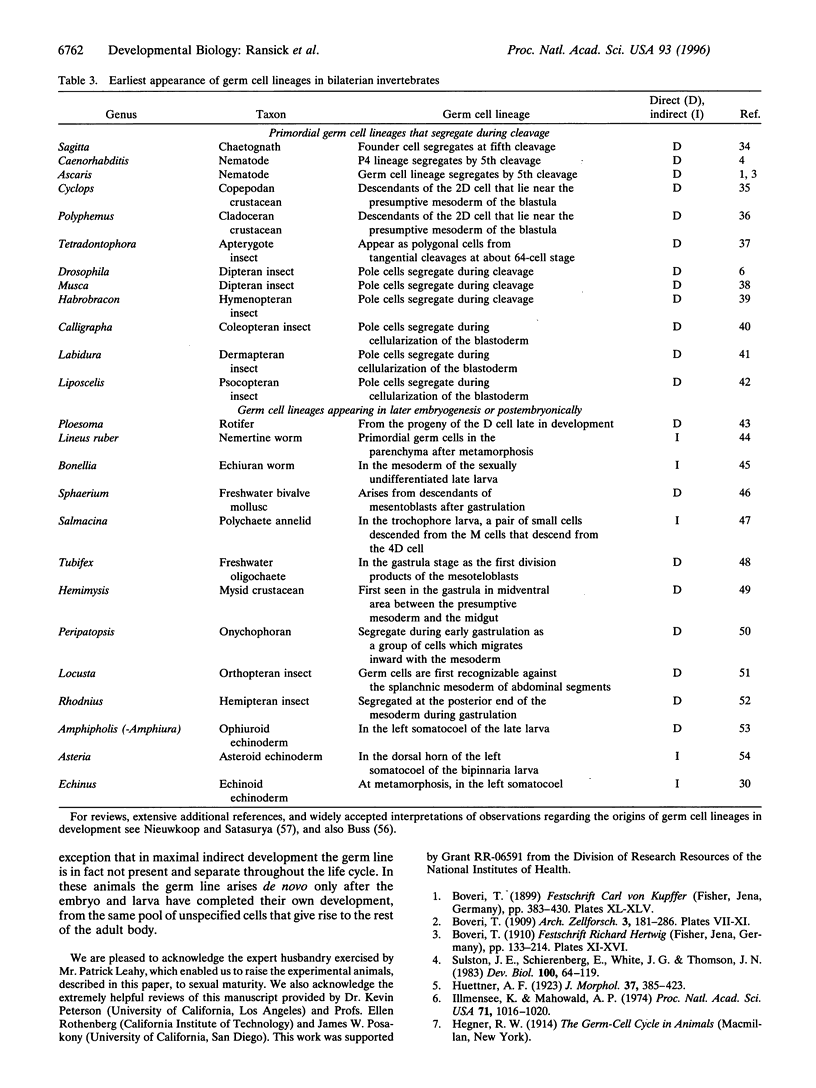
The four small micromeres of the sea urchin embryo contribute only to the coelomic sacs, which produce major components of the adult body plan during postembryonic development. To test the proposition that the small micromeres are the definitive primordial germ cell lineage of the sea urchin, we deleted their 4th cleavage parents, and raised the deleted embryos through larval life and metamorphosis to sexual maturity. Almost all of the experimental animals produced functional gametes, excluding the possibility that the germ cell lineage arises exclusively and obligatorily from descendants of the small micromeres; rather, the germ cell lineage arises during the postembryonic development of the rudiment. A survey of the literature indicates that there is no known case of an embryonic primordial germ cell lineage in a bilaterian species that displays maximal indirect development.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong N., Hardin J., McClay D. R. Cell-cell interactions regulate skeleton formation in the sea urchin embryo. Development. 1993 Nov;119(3):833–840. doi: 10.1242/dev.119.3.833. [DOI] [PubMed] [Google Scholar]
- Buss L. W. Evolution, development, and the units of selection. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1387–1391. doi: 10.1073/pnas.80.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron R. A., Fraser S. E., Britten R. J., Davidson E. H. Macromere cell fates during sea urchin development. Development. 1991 Dec;113(4):1085–1091. doi: 10.1242/dev.113.4.1085. [DOI] [PubMed] [Google Scholar]
- Cameron R. A., Hough-Evans B. R., Britten R. J., Davidson E. H. Lineage and fate of each blastomere of the eight-cell sea urchin embryo. Genes Dev. 1987 Mar;1(1):75–85. doi: 10.1101/gad.1.1.75. [DOI] [PubMed] [Google Scholar]
- Davidson E. H. Lineage-specific gene expression and the regulative capacities of the sea urchin embryo: a proposed mechanism. Development. 1989 Mar;105(3):421–445. doi: 10.1242/dev.105.3.421. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Peterson K. J., Cameron R. A. Origin of bilaterian body plans: evolution of developmental regulatory mechanisms. Science. 1995 Nov 24;270(5240):1319–1325. doi: 10.1126/science.270.5240.1319. [DOI] [PubMed] [Google Scholar]
- Ettensohn C. A., McClay D. R. Cell lineage conversion in the sea urchin embryo. Dev Biol. 1988 Feb;125(2):396–409. doi: 10.1016/0012-1606(88)90220-5. [DOI] [PubMed] [Google Scholar]
- Gardner R. L., Lyon M. F., Evans E. P., Burtenshaw M. D. Clonal analysis of X-chromosome inactivation and the origin of the germ line in the mouse embryo. J Embryol Exp Morphol. 1985 Aug;88:349–363. [PubMed] [Google Scholar]
- Ginsburg M., Snow M. H., McLaren A. Primordial germ cells in the mouse embryo during gastrulation. Development. 1990 Oct;110(2):521–528. doi: 10.1242/dev.110.2.521. [DOI] [PubMed] [Google Scholar]
- Illmensee K., Mahowald A. P. Transplantation of posterior polar plasm in Drosophila. Induction of germ cells at the anterior pole of the egg. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1016–1020. doi: 10.1073/pnas.71.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaner O., Wilt F. Interactions of different vegetal cells with mesomeres during early stages of sea urchin development. Development. 1991 Jul;112(3):881–890. doi: 10.1242/dev.112.3.881. [DOI] [PubMed] [Google Scholar]
- Lawson K. A., Hage W. J. Clonal analysis of the origin of primordial germ cells in the mouse. Ciba Found Symp. 1994;182:68–91. doi: 10.1002/9780470514573.ch5. [DOI] [PubMed] [Google Scholar]
- Leahy P. S. Laboratory culture of Strongylocentrotus purpuratus adults, embryos, and larvae. Methods Cell Biol. 1986;27:1–13. doi: 10.1016/s0091-679x(08)60339-8. [DOI] [PubMed] [Google Scholar]
- Leahy P. S., Tutschulte T. C., Britten R. J., Davidson E. H. A large-scale laboratory maintenance system for gravid purple sea urchins (Strongylocentrotus purpuratus). J Exp Zool. 1978 Jun;204(3):369–380. doi: 10.1002/jez.1402040308. [DOI] [PubMed] [Google Scholar]
- McClay D. R., Logan C. Y. Regulative capacity of the archenteron during gastrulation in the sea urchin. Development. 1996 Feb;122(2):607–616. doi: 10.1242/dev.122.2.607. [DOI] [PubMed] [Google Scholar]
- Pehrson J. R., Cohen L. H. The fate of the small micromeres in sea urchin development. Dev Biol. 1986 Feb;113(2):522–526. doi: 10.1016/0012-1606(86)90188-0. [DOI] [PubMed] [Google Scholar]
- Ransick A., Davidson E. H. A complete second gut induced by transplanted micromeres in the sea urchin embryo. Science. 1993 Feb 19;259(5098):1134–1138. doi: 10.1126/science.8438164. [DOI] [PubMed] [Google Scholar]
- Ransick A., Davidson E. H. Micromeres are required for normal vegetal plate specification in sea urchin embryos. Development. 1995 Oct;121(10):3215–3222. doi: 10.1242/dev.121.10.3215. [DOI] [PubMed] [Google Scholar]
- Seydoux G., Fire A. Soma-germline asymmetry in the distributions of embryonic RNAs in Caenorhabditis elegans. Development. 1994 Oct;120(10):2823–2834. doi: 10.1242/dev.120.10.2823. [DOI] [PubMed] [Google Scholar]
- Sulston J. E., Schierenberg E., White J. G., Thomson J. N. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983 Nov;100(1):64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- West J. A., Cantwell G. E., Shorino T. J. Embryology of the house fly, Musca domestica (Diptera: Muscidae), to the blastoderm stage. Ann Entomol Soc Am. 1968 Jan;61(1):13–17. doi: 10.1093/aesa/61.1.13. [DOI] [PubMed] [Google Scholar]



