Abstract
Tinnitus, phantom sound perception, is a worldwide highly prevalent disorder for which no clear underlying pathology has been established and for which no approved drug is on the market. Thus, there is an urgent need for new approaches to understand this condition. We used a network pharmacology side-effect analysis to search for genes that are involved in tinnitus generation. We analyzed a network of 1,313 drug–target pairs, based on 275 compounds that elicit tinnitus as side effect and their targets reported in databases, and used a quantitative score to identify emergent significant targets that were more common than expected at random. Cyclooxigenase 1 and 2 were significant, which validates our approach, since salicylate is a known tinnitus generator. More importantly, we predict previously unknown tinnitus-related targets. The present results have important implications toward understanding tinnitus pathophysiology and might pave the way toward the design of novel pharmacotherapies.
Tinnitus, the phantom perception of sound, in the absence of a corresponding external sound source, is a highly prevalent and debilitating disorder.1 At present, between 10 and 20% of the world population experiences tinnitus,2 prevalence rates are increasing, and it augments with age.3 In addition, severe tinnitus, which afflicts 1–2% of the general population, can lead to anxiety, depression, cognitive dysfunction, insomnia, and to an important decrease in the quality of life. Thus, it has recently been suggested that it should be considered a global burden.4
A wide variety of approaches have been used in an attempt to delineate the neural correlates of tinnitus. These range from experiments in animal models to brain imaging, magnetoencephalography, and electrophysiology studies in humans. Although early studies considered it a pathology of cochlear origin, recent data suggest that tinnitus is a central nervous system (CNS) disorder and that in can be considered an emergent property of multiple, parallel, dynamically changing, and partially overlapping subnetworks.5 However, the exact mechanisms underlying this phantom perception are still unknown. In fact, when compared with other CNS pathologies for which perturbations in different neurotransmitter systems or in ion channel activity have been associated,6,7,8 no clear target systems have been associated with tinnitus. Moreover, although a wide variety of compounds are used off-label to treat tinnitus patients, there is still no US Food and Drug Administration or European Medicines Agency approved drug on the market.9 Thus, further comprehensive approaches are needed to better understand this pathology.
In the last decade, all aspects of life sciences have witnessed a fabulous explosion of available data which are deposited in databanks. This is true for drug and drug–target information. Network approaches have proven useful for organizing these high-dimensional biological data sets and extracting meaningful information,10 and the term network pharmacology or systems pharmacology has been coined.11 Interestingly, a high-throughput electronic-biology approach based on in silico data mining of existing databases and integration of this information has been proposed for drug discovery and repurposing.12,13 Networks have been applied to the analysis of the topological and global properties of drug–protein interactions, demonstrating that most CNS drugs are promiscuous, aiming at more than one target.14,15 Moreover, new molecular targets for old drugs have been predicted and in some cases, they have been validated experimentally.14,16
A variety of ways whereby network approaches can be used toward understanding tinnitus have been proposed recently.17 In particular, the high-throughput analysis of drug side-effect information to predict targets leading to this pathology might result informative. The strategy is based on the notion that treatment of patients with drugs is an in vivo chemical perturbation experiment in a complex organism,18 that similar side effects of unrelated drugs can be caused by their common off-targets,18 and that the analysis of the mutual interactions of chemicals and proteins by means of network analysis enables a better understanding of the molecular mechanisms of disease, drug action, and associated adverse effects.14 In the present work, we built and analyzed a drug–target network based on compounds that have been documented to elicit tinnitus as side effect, in order to explore the tinnitus target space. Making use of this network pharmacology side-effect approach, we describe novel emergent protein targets predicted to be associated with tinnitus pathology. The present work further aids the understanding of the underlying mechanisms leading to tinnitus and opens the path for the development of new pharmacotherapies.
Results
Tinnitus drug–target network
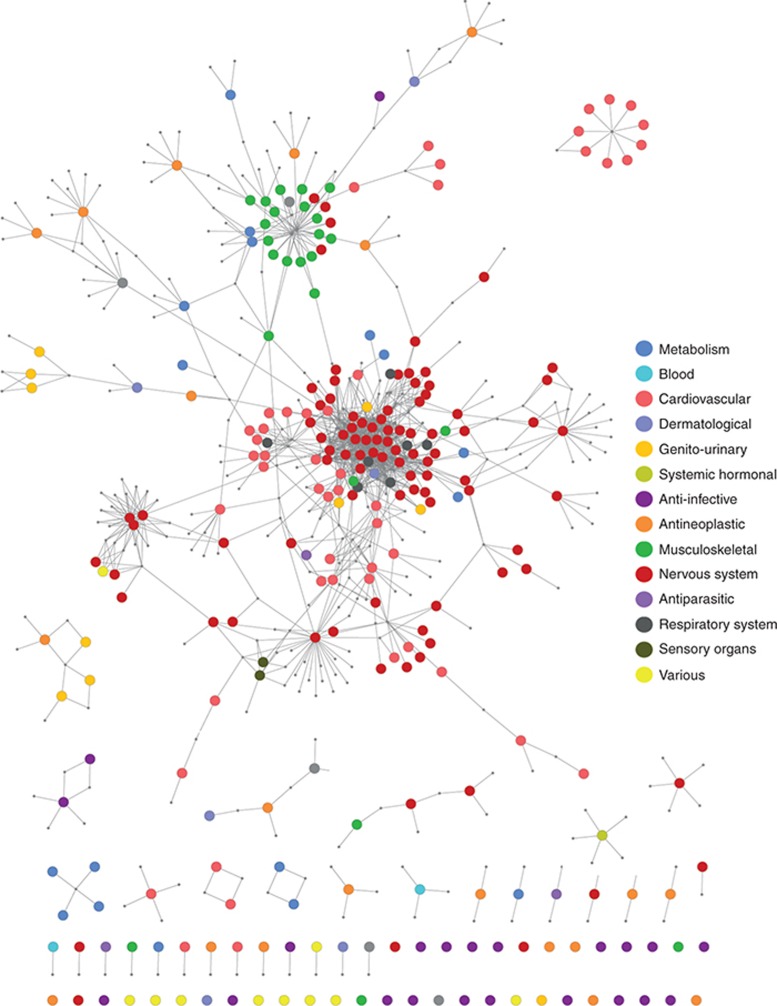
The Side Effect Resource Database (SIDER 2; http://sideeffects.embl.de) reports a total of 4,192 side effects and 996 drugs;19 of these, 275 with side effect tinnitus and synonyms occurring on labels, such as “ringing in ears,” “ringing in the ear,” and “ear noise” (Supplementary Table S1). We retrieved all targets for drugs that produce tinnitus as side effect from our reference database (created as described in Methods based on DrugBank, http://www.drugbank.ca/ and Psychoactive Drug Screening Program (PDSP) Ki, http://pdsp.med.unc.edu/pdsp.php) and generated a bipartite graph of 1,313 drug–protein interactions in which a drug and a protein are connected to each other by an edge or link if the protein is a reported target of the drug, resulting in a drug–target network. To analyze the drug space of tinnitus as side effect, target node sizes were minimized, and drug nodes were colored according to their Anatomical Therapeutic Chemical code, generating the Drug Network (Figure 1). Drug nodes were not weighted, e.g., all nodes were given equal size. If most drugs only targeted a single protein, the network would consist of isolated nodes with few or no edges between them. However, as previously reported, most drugs are promiscuous and bind to more than one target;14,15,20 therefore, the network was highly connected. There was a main giant component (the largest connected component) which comprised 180 drugs. Within this giant component, 47% of drugs were nervous system acting (red nodes in Figure 1 and Supplementary Table S2) and were mainly clustered, with an average number of neighbors of 10.6 compared with 4.3 (including the nervous system drugs) for the whole network, indicating an important number of shared targets. Moreover, 90% of nervous system compounds were found within the giant component, including antidepressants, antipsychotics, and opioids. Cardiovascular system drugs were the second (20%) most highly represented group within the giant component (Supplementary Table S2), comprising beta blockers, calcium channel blockers, angiotensin antagonists, and statins. An additional cluster of drugs was clearly identified within the giant component as a separate neighborhood with an average number of neighbors of 4.6. This mainly contained musculoskeletal first-level Anatomical Therapeutic Chemical code drugs (green nodes in Figure 1) with anti-inflammatory activity. There were several small, isolated components. One component worth noting is the one composed of nine cardiovascular acting drugs (dark orange, upper right panel, Figure 1), which were all angiotensin-converting enzyme (ACE) inhibitors.
Figure 1.
Tinnitus drug network. A drug–target network was generated with Cytoscape 3.0, by retrieving all drugs that produce tinnitus as side effect from SIDER and their targets from DrugBank and PDSP Ki. An edge was placed between a drug node and a target node if the protein is a target of that drug in the reference database. Target nodes were minimized to visualize the tinnitus drug space. Drugs were color coded according to first-level Anatomical Therapeutic Chemical Classification codes indicated in Supplementary Table S3.
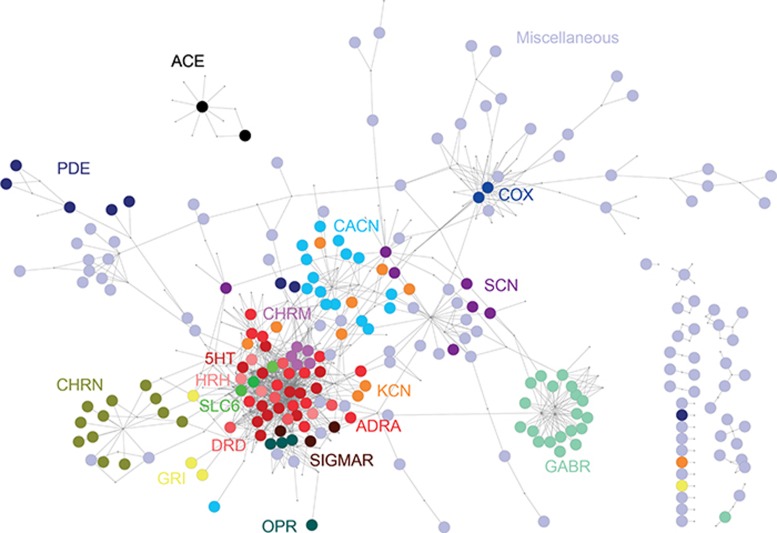
To further analyze the target space of tinnitus as side effect, drug nodes were minimized, and targets were colored according to their family group, giving rise to the Target Network (Figure 2). The network was not weighted, e.g., all target nodes were given equal size. A total of 339 different targets were found (Supplementary Tables S3 and S4). As described for the Drug Network (Figure 1), a main giant component was observed, which contained 258 targets. Within it, protein family members were mostly clustered in different neighborhoods: cholinergic nicotinic receptor subunits (CHRN), type A γ-aminobutyric acid receptor subunits (GABR), voltage-gated sodium channel subunits (SCN), voltage-gated calcium channel subunits (CACN), voltage-gated potassium channels (KCN), glutamate receptors (GRI), and cyclooxigenase (COX) 1 and 2. An important neighborhood of highly clustered nodes from different protein families, derived from the promiscuity of nervous system acting drugs,20 included monoaminergic receptors (histaminergic, HRH; adrenergic, ADRA; serotonergic, 5HT; and dopaminergic, DRD), cholinergic muscarinic receptors (CHRM), and monoaminergic transporters (SLC6). There were several small, isolated components, including the one composed of the ACEs. Although not shown in Figure 2, nodes in the Target Network had different degrees, e.g., number of links to different drugs. Supplementary Figure S1 shows the degree of the most targeted proteins and the total of hits for each one in the reference database. Some targets, like 5HT2A and HRH1, had the highest degree (33 and 25, respectively). However, they were also highly represented in the database (69 and 67, respectively). On the other hand, some targets like ACE had a low degree (9), but this was similar to the total number of hits in the reference database (10). Therefore, the degree of targets did not result in a good index for how significant any given target was in the network.
Figure 2.
Tinnitus target network. Same as in Figure 1, but drug nodes were minimized to visualize the tinnitus target space. Targets were color coded according to protein family members.
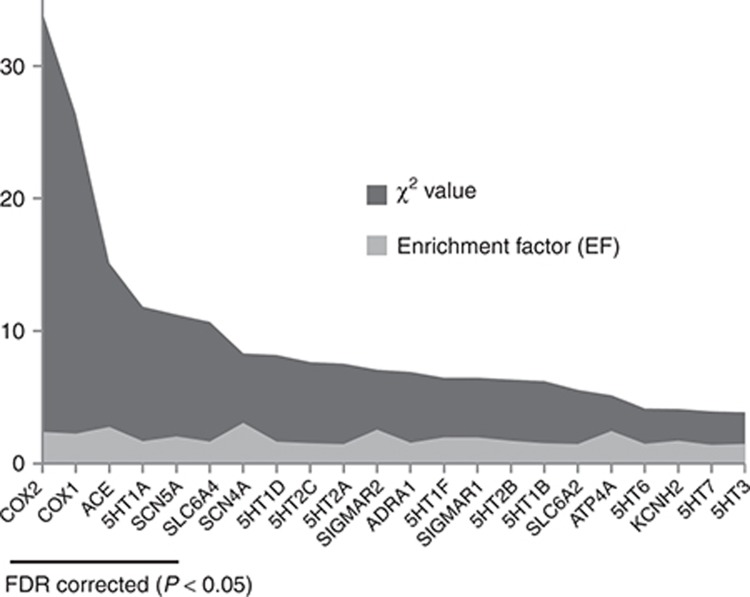
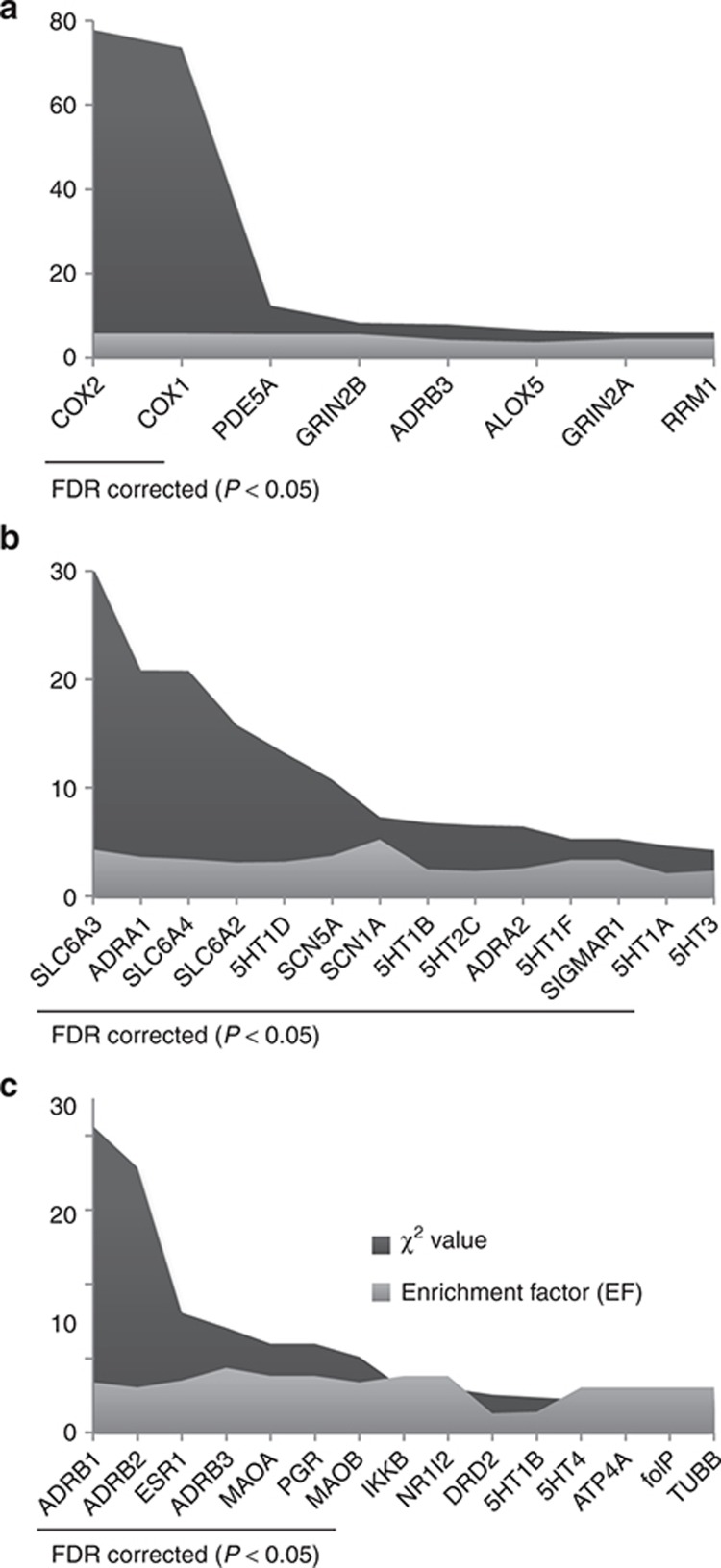
Emergent significant targets within the tinnitus network were identified as described in Methods, calculating an enrichment factor which identified targets that were more common than expected by chance and significant by χ2, corrected by false-discovery rate for multiple comparisons (Figure 3 and Supplementary Table S5). These were COX2 (enrichment factor (EF): 2.39, q = 1 × 10−20), COX1 (EF: 2.26, q = 1 × 10−20), ACE (EF: 2.77, q = 0.006), 5HT1A (EF: 1.69, q = 0.03), SCN5A (EF: 2.05, q = 0.03), and SLC6A4 (EF: 1.66, q = 0.04). Targets belonged to four different neighborhoods (Figure 4): ACE, COX1/COX2, SCN5A, and the nervous system acting drug cluster mainly composed of aminergic receptors which contained 5HT1A and SLC6A4. Thus, ACE formed a completely isolated component. COX1 and COX2 shared 25 of the 25 and 27 drugs that targeted COX1 and COX2, respectively. They shared no drugs with 5HT1A, SLC6A4, or SCN5A. 5HT1A and SLC6A4 shared 16 of the 28 and 27 that targeted 5HT1A and SLC6A4 and shared no drugs with SCN5A.
Figure 3.
Significant tinnitus targets. Enrichment factors and χ2 values are shown. Underlined are significant targets after correction for multiple comparisons by false-discovery rate (FDR). Statistical values for all targets are shown in Supplementary Table S5.
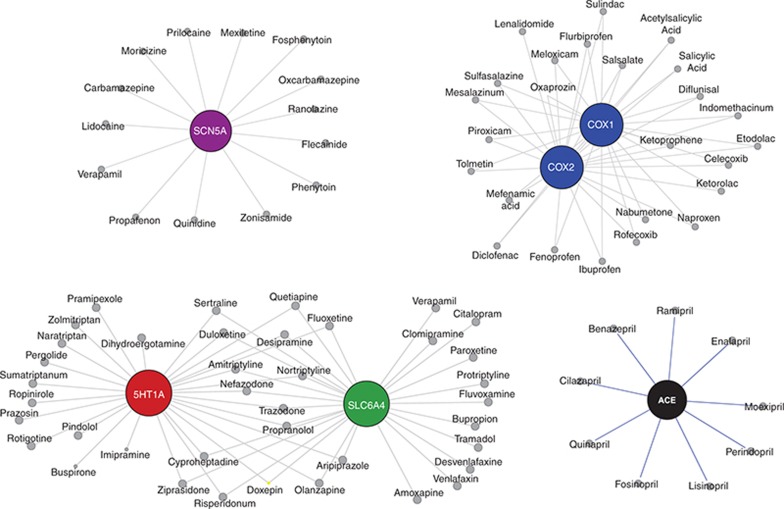
Figure 4.
Neighborhoods of significant tinnitus targets. Shown are drug neighborhoods corresponding to the significant tinnitus targets (COX1/COX2, SCN5, ACE, and 5HT1A/SLC6A4).
Hearing impaired and hyperacusis drug–target networks
Tinnitus is very often accompanied by hearing impairment and/or hyperacusis.21,22 To differentiate between tinnitus, hearing impairment, and hyperacusis, we analyzed the drug and target space of “hearing impaired” and “hyperacusis” as side effect. For this purpose, we retrieved all drugs that produce these side effects from SIDER, 102 and 36, respectively (Supplementary Table S1). We obtained all drug targets from our reference database and generated bipartite graphs of drug–protein interactions. Target networks are shown in Supplementary Figures S2 and S3. Drug node sizes were minimized, and target nodes were colored according to family members.
The hearing impaired Target Network contained 186 targets, 96 of them within the biggest component of the network. This contained SCN, voltage-gated potassium channels, cholinergic nicotinic receptor subunits, glutamate receptors, COX, and a clustered neighborhood which included monoaminergic receptors (HRH, adrenergic, 5HT, and dopaminergic), cholinergic muscarinic receptors, and SLC6. Emergent hearing impaired targets are shown in Figure 5a. Worth noting are COX2 (EF: 5.72, q = 1 × 10−20) and COX1 (EF: 5.73, 1 × 10−20), emergent shared targets with tinnitus. The hyperacusis Target Network only contained 69 targets, 54 of them in a clustered neighborhood which included monoaminergic receptors (HRH, adrenergic, 5HT, and dopaminergic), cholinergic muscarinic receptors, and SLC6. Emergent hyperacusis targets are shown in Figure 5b. Worth noting are SLC6A4 (EF: 3.46, q = 0.00008), SCN5A (EF: 3.75, q = 0.004), and 5HT1A (EF: 2.16, q = 0.05), emergent shared targets with tinnitus.
Figure 5.
Significant side effect targets. Enrichment factors and χ2 values are shown for (a) hearing impaired, (b) hyperacusis, and (c) depression. Underlined are significant targets after correction for multiple comparisons by false-discovery rate (FDR). Statistical values for all targets are shown in Supplementary Tables S6–S8.
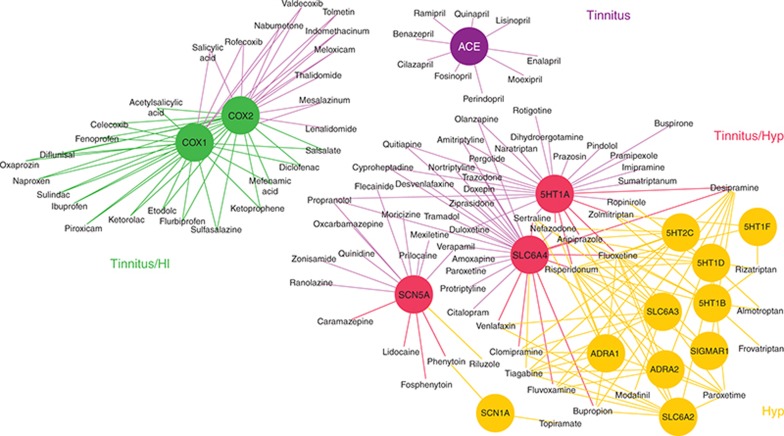
Figure 6 shows an integrated drug–target network for tinnitus, hearing impairment, and hyperacusis. Only emergent significant targets for each side effect are included and color coded. Shared targets between side effects have a different color, and drug–target links follow the same color code. For example, in purple, emergent targets that are only significant (and drugs that are only present) in the tinnitus drug–target network are shown. Thus, ACE is only significant for tinnitus, and ACE inhibitors only present in the tinnitus network. Tinnitus shares with hearing impairment COX1 and COX2 and with hyperacusis, SLC6A4, 5HT1A, and SCN5A. Finally, hearing impairment and hyperacusis shared no emergent targets and drugs.
Figure 6.
Integrated drug–target network. Emergent significant targets for tinnitus, hearing impairment, and hyperacusis and their connecting drugs are integrated in one network. Color codes: tinnitus only, targets and drugs in purple; hyperacusis only, targets and drugs in orange; tinnitus/hearing impaired, targets and drugs in green; and tinnitus/hyperacusis, targets and drugs in red. HI, hearing impaired; Hyp, hyperacusis.
Depression as side effect
An increasing body of evidence suggests that tinnitus is not restricted to the characteristic “ringing in the ears” but instead encompasses a wide range of symptoms with emotional components, such as depression.23 A priori, one could propose that depressed people refer more to “ringing in the ears,” because it is more bothersome for them and therefore they are more aware of it. To control for this potential confounder, we searched for an overlap of emergent targets between tinnitus and depression as side effect. Only 30 drugs are reported to produce side effect depression in SIDER 2 (Supplementary Table S1), which are linked to 80 different targets (Supplementary Figure S4). Figure 5c shows the significant emergent targets in the depression network. None were significant in the tinnitus network.
Discussion
Disorders of the CNS are complex disease states. They are a major challenge for clinical medicine and drug discovery. Pathology is the result of multiple factors, which include environmental, genetic, and epigenetic imbalance in excitatory or inhibitory neurotransmission and alteration in modulatory neurotransmitter pathways (e.g., dopaminergic, cholinergic, and serotonergic).6,7,8 Although etiology is often unknown, the link between pathology and specific neurotransmitter systems and/or protein targets has been reported in most cases. Thus, in its acute psychotic state, schizophrenia is associated with an increase in dopamine synthesis and release as well as in resting-state synaptic dopamine concentrations.8 Similarly, several lines of evidence show a direct link between serotonin neurotransmission and depressive disorders.7 Moreover, approved drugs that target the dopaminergic and serotonergic system are used to treat schizophrenia and depression, respectively. A different scenario is observed in the case of tinnitus, for which no clear endophenotype nor neural substrate is known and for which there is no approved drug on the market.9 By making use of a network pharmacology approach based on drugs that produce tinnitus as side effect, in this article, we predict protein targets that might be involved in the genesis of tinnitus.
The use of large-scale side-effect data analysis for making valid predictions in pharmacology has been recently demonstrated. Thus, Campillos et al.18 have successfully explored side-effect information generated from the use of 746 marketed drugs to infer their molecular activity and predict additional targets for many existing drugs often implicated in different therapeutic categories, some of which were validated experimentally. In addition, Lounkine et al.24 used a computational large-scale strategy to predict new molecular targets for known drugs, based on chemical similarity and thereafter targets that explain their side effects. The fact that COX1 and COX2, which are the main targets of salicylate, were found as the most significant emergent targets in our analysis is a further proof of principle of the validity of these network pharmacology side-effect approaches. Thus, salicylate ototoxicity has been reported since the early 1900s. High-dose salicylate induces alterations in perceived sounds, temporary hearing loss, and tinnitus in humans and is used as an animal model to study tinnitus.25 Interestingly, in their large-scale prediction and testing of drug activity on side-effect targets, Lounkine et al.24 also found that COX1 and COX2 are significantly associated with tinnitus. Furthermore, they also found SCN5A significantly linked to tinnitus. However, different from our present study, they describe the association of tinnitus with HRH1, but not with ACE, SLC6A4, and 5HTA1. Differences could derive from the different databases used: SIDER vs. World Drug Index from Thompson Reuters (not freely available) for side effects and DrugBank plus PDSP Ki vs. DrugBank plus ChEMBL, Thompson Reuters Integrity, GeneGo Metabase, and GVKBio for targets, in this article and in the study by Lounkine et al.,24 respectively. Moreover, our present analysis was only based on drug–target associations linked to tinnitus, hyperacusis, hearing impairment, and depression, whereas the latter analysis integrated drug–target information for 1,685 unique adverse drug reaction terms, which did not include hyperacusis and hearing impairment. For an extended body of literature supporting the involvement of salicylate and suggesting the participation of the serotonergic system and voltage-gated sodium channels in the genesis of tinnitus, see Supplementary Table S9.
The fact that COX1/COX2, SCN5A, SLC6A4/5HTA1, and ACE belong to different neighborhoods most likely indicates that the underlying tinnitus-generating mechanism differs between drugs that aim those targets. This is consistent with the notion that tinnitus can be triggered by different mechanisms and that different tinnitus subtypes most likely exist.4 These underlying mechanisms could be unrelated to the described therapeutically relevant pharmacological effect of the side-effect–generating drug. For example, inhibition of COX1 and COX2 might not be the cause that leads to tinnitus. Instead, both cochlear and CNS effects of salicylate have been described.25 High-dose salicylate inhibits the binding of chloride to the anion-binding site on prestin, thereby suppressing outer hair cell electromotility and cochlear amplification,26 induces a reversible increase in amplitude of cortical responses evoked by tone bursts over a wide range of frequencies and intensities,27 and increases expression of c-fos, an activity-dependent protein, in the auditory cortex, as well as in several nonclassical auditory regions (e.g., amygdala) associated with stress, anxiety, and emotion.28
The observation that the voltage-gated sodium channel SCN5A was significantly increased above a random observation supports the proposal that tinnitus might result from an imbalance in excitability anywhere along the auditory pathway.29 Moreover, the fact that SCN5 are sensitive to lidocaine30 and that the effects of lidocaine as a tinnitus suppressor or enhancer have been well documented31 further highlight the validity of the present side-effect approach. Although SCN5, which encodes a tetrodotoxin-resistant sodium channel, is mainly a heart voltage-gated sodium channel subunit, expression of a variant in the brain has been described. SCN5 transcripts have been localized in limbic structures of rat and human brain,32 and the encoded protein has been found in various mouse brain regions including the cerebral cortex, thalamus, hypothalamus, and brain stem,33 areas that are implicated in tinnitus-related brain subnetworks.5 In addition, tetrodotoxin-resistant Na+ currents have been detected in neurons from the neocortex, hippocampus, and striatum.34,35
Because 5HTA1 and SLC6A4 belong to the same neighborhood (Figures 2 and 4), it is difficult to determine if either one of the two targets or both are necessary as tinnitus triggers. SLC6A4 encodes the serotonin transporter, which reuptakes the neurotransmitter serotonin from synaptic spaces into presynaptic neurons, and it is the main target of serotonin reuptake inhibitors which increase the concentration of serotonin at the biophase.20 Because only the 5HTA1 receptor was significant, the effect of serotonin as a tinnitus trigger seems not to be widespread acting on several serotonin receptor subtypes. Therefore, the significance of SLC6A4 might result from an increase in serotonin concentration acting on 5HTA1 receptors. According to DrugBank and PDSP Ki, drugs that targeted 5HTA1 receptors are agonists (e.g., zolmitriptan, naratriptan, sumatriptan, pergolide, ropirinole, and rotigotine), partial agonists (e.g., buspirone and trazodone), and antagonists (e.g., quetiapine, doxepine, nortryptiline, nefazodone, and olanzapine). Thus, it is difficult to conclude which pharmacological effect results in the onset of tinnitus. However, one could propose that perturbation of serotonin neurotransmission is linked to tinnitus. Although serotonergic descending fibers to the cochlea have been documented,36 no clear effect of serotonin has been described at the auditory periphery. On the contrary, several CNS serotonergic-related effects have been documented. In particular, the thalamic reticular nucleus and the dorsal thalamus are innervated by serotonergic axons from the dorsal raphe nucleus, the nucleus accumbens, and other paralimbic regions.37 By exciting GABAergic neurons of the thalamic reticular nucleus, these serotonergic projections inhibit sensory thalamic relay cells.38 Recently, the perturbation of a nucleus accumbens–thalamic reticular nucleus serotonergic “noise-cancellation system” has been proposed as the origin of the perception of persistent unpleasant noises, including phantom sensations such as tinnitus.39
The finding of ACE as a significant target is surprising, and to the best of our knowledge, the first time described to be associated with tinnitus. The classic renin–angiotensin system is a peripheral hormone system designed to mediate cardiovascular and body water regulation, with angiotensin II as its major effector.40 One could propose that ACE-related alterations of cardiovascular and body water regulation might cause pulsatile or nonpulsatile tinnitus via alterations of cerebral blood flow and related flow noises. Alternatively, ACE-targeting drugs might induce tinnitus by a direct impact on brain activity via the brain renin–angiotensin system. A potential role of this system has been suggested for stress regulation and in pathologies such as depression and memory-related disorders.40 The angiotensin II receptor (AT1 subtype) is present in the auditory system including the cochlear nucleus and superior olivary nuclei,41 and the cochlear nucleus has been considered an important anatomical and physiological area in the development of tinnitus.42 In addition, a potential role of the brain renin–angiotensin system has been suggested in auditory attention.43 Thus, this system might participate in sound and auditory attention and affective brain circuits related to tinnitus.
The fact that tinnitus shared targets with hearing impairment and hyperacusis reflects the observation that it is often associated with these symptoms.21,22 Thus, COX1 and COX2 were shared targets between tinnitus and hearing impairment, which is consistent with the finding that salicylate leads not only to tinnitus but also to temporary hearing loss.25 On the other hand, 5HTA1 and SLC6A4 were shared targets between tinnitus and hyperacusis. An increased central nonlinear gain has been posited as the origin of both hyperacusis and tinnitus,44,45 which could result from a dysfunction of central serotonergic neurotransmission.46 Thus, the finding of 5HTA1 and SLC6A4 in the hyperacusis/tinnitus network but not in the hearing impaired/tinnitus network might support a serotonergic mechanism underlying a central gain origin of tinnitus and hyperacusis, in the absence of overt deafferentation due to hearing loss. This is consistent with the observation that in some tinnitus patients, auditory sensitivity is enhanced even in the presence of a normal audiogram.45,47,48 The observation that hearing impairment and hyperacusis shared no common targets is surprising, since hyperacusis is sometimes accompanied with hearing loss.22 However, it is possible that patients often report hyperacusis as a side effect due to the high discomfort it produces, whereas hearing impairment is not reported unless it is very profound.
In conclusion, the network integration of targets based on drugs that produce tinnitus as side effect indicates that several distinct mechanisms are involved in the generation of tinnitus. Whereas ACE inhibitors cause tinnitus without hearing loss or hyperacusis, many drugs that target COX1/COX2 result in tinnitus with hearing loss, whereas drugs that target SCN5A or SLC6A4/5HTA1 generate tinnitus with associated hyperacusis. These results have important pathophysiological implications and can further aid in dissecting the underlying neuronal correlates of sound phantom perception and associated disorders. In addition, the present study provides important clues concerning targets that can be investigated toward the further development of drugs to treat this enigmatic condition of high prevalence.
Methods
Reference database. A drug–target reference database was built with drugs and targets derived from DrugBank (http://www.drugbank.ca/)49 and the National Institute of Mental Health PDSP Ki Database (http://pdsp.med.unc.edu/pdsp.php). DrugBank combines comprehensive drug (e.g., chemical, pharmacological, and pharmaceutical) data with detailed drug–target (e.g., sequence, structure, and pathway) information. PDSP Ki serves as a data warehouse for published and internally derived Ki, or affinity, values for a large number of drugs and drug candidates at an expanding number of G-protein–coupled receptors, ion channels, transporters, and enzymes. In some cases, the PDSP Ki database contains entries for binding constants with complexes or groups of proteins that cannot be mapped to individual proteins (e.g., “calcium channel” and “sodium channel”) and were therefore not retrieved from this database. Both databases were downloaded as of September 2012 into excel files. Matlab (The MathWorks, http://www.mathworks.com) was used to obtain from DrugBank all drugs and their targets, and data were thereafter manually curated to only leave approved drugs that were also present in SIDER 2.19 PDSP was manually curated to retrieve approved drugs present in SIDER 2 and their targets (with binding affinities lower than 10 µmol/l). The reference database is composed of 781 drugs, 756 targets, and 3,728 drug–target interactions. Metabolizing enzymes, nonspecific protein binders, nonmammalian targets, DNA, and RNA were not included: e.g., targets with keywords multidrug resistance, cytochrome, ATP-binding cassette, glutathione S-transferases, flavin-containing monooxygenase, and albumin.
Side-effect networks. Drugs producing side effect “tinnitus,” “hearing impaired,” “hyperacusis,” and “depression” (Supplementary Table S1) were downloaded from SIDER 2.19 SIDER contains information on marketed medicines and their recorded adverse drug reactions extracted from public documents and package inserts. Frequency of adverse events was not taken into account. For each drug, all known targets were retrieved from the reference database. Supplementary Tables S1, S3, and S4 show drug and target information for tinnitus, hearing impaired, hyperacusis, and depression. Based on drug–target associations, we generated bipartite networks for each side effect using Cytoscape 3.0 (ref. 50) to visualize the network. A link or edge was placed between a drug node and a target node if the protein is a reported target of that drug in the reference database. Nodes were denoted as circles and were not weighted. In the drug networks, target nodes were drawn at a smaller size to better view drug nodes. Drug nodes were classified according to the first level (the main category) of the Anatomical Therapeutic Chemical classification provided by the World Health Organization Centre for Drug Statistics Methodology (Supplementary Table S3). Each category was assigned a different color. For drugs with more than one classification, we assigned it “nervous system” when applicable. In the target networks, drug nodes were drawn at a smaller size to better visualize the target nodes. Target proteins were colored according to their protein family. Layouts for all networks were generated by a force-directed algorithm with default Cytoscape settings, followed by a local manual rearrangement for visual clarity without modifying the overall layout of the network. Network parameters were obtained through the Cytoscape Network Analysis Plugin.
Statistical analysis. To assess the potential relevance of a specific target for a specific adverse event (i.e., tinnitus, hearing loss, hyperacusis, and depression), we applied a χ2 method previously introduced by Lounkine et al.24 (Supplementary Tables S5–S8). To verify whether a specific target is expected above chance, we calculated an enrichment factor for the occurrence of a target using the following formula:
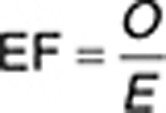 |
where EF is the enrichment factor of adverse drug reaction for predicted targets, O the occurrence of a target, and E is the expected occurrence of a specific target. We calculated the expected occurrence of a specific target with the following formula:
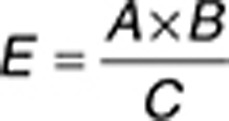 |
where A is the amount of drugs that have a specific target in the reference database, B is the total amount of drug–target pairs that are linked to a side effect, and C is the total amount of drug–target pairs in the database.
For example, COX2 is targeted by 28 drugs that create tinnitus as an adverse event and by 36 drugs in the reference database. In total, there are 3,728 drug–target pairs in the reference database. A total of 1,211 drug–target pairs are linked to tinnitus. Accordingly, the pair tinnitus–COX2 was enriched 2.39-fold above random, with a χ2 P value of 5.5 × 10−9, which was still significant after correction for multiple comparisons by false-discovery rate (q = 1.0 × 10−20) (Supplementary Table S5). For calculations, drug–target pairs that were only represented once in the database were not considered. Moreover, after statistical analysis, only targets that were represented more than three times in the general database were taken into consideration.
Author contributions
A.B.E. wrote the manuscript. A.B.E. and S.V. designed the research. A.B.E., S.V., W.N., and M.S. performed the research. A.B.E., B.L., D.D.R., and S.V. analyzed the data.
Conflict of interest
A.B.E. has received research funding from Merz; B.L. received honoraria and speakers' fee from Advanced Neuro Modulation, AstraZeneca, Autifony, Lundbeck, Merz, Magventure, Novartis, Pfizer, and Servier; research funding from AstraZeneca and Cerbomed; funding for equipment from Magventure; and travel and accommodation payments from Lilly, Servier, and Pfizer. D.D.R. received speaker's fees and travel and accommodation payments and research funding from Saint Jude Medical. A.B.E., B.L., and D.D.R. have a patent application for the use of cyclobenzaprine in tinnitus treatment. The other authors declared no conflict of interest.
Study Highlights

Acknowledgments
This work was funded by a research grant from the Tinnitus Research Initiative. The authors thank Mariana Obertello for her help with the use of Cytoscape.
Supplementary Material
References
- Jastreboff P.J. Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci. Res. 1990;8:221–254. doi: 10.1016/0168-0102(90)90031-9. [DOI] [PubMed] [Google Scholar]
- Shargorodsky J., Curhan G.C., Farwell W.R. Prevalence and characteristics of tinnitus among US adults. Am. J. Med. 2010;123:711–718. doi: 10.1016/j.amjmed.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Nondahl D.M., et al. Generational differences in the reporting of tinnitus. Ear Hear. 2012;33:640–644. doi: 10.1097/AUD.0b013e31825069e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langguth B., Kreuzer P.M., Kleinjung T., De Ridder D. Tinnitus: causes and clinical management. Lancet Neurol. 2013;12:920–930. doi: 10.1016/S1474-4422(13)70160-1. [DOI] [PubMed] [Google Scholar]
- De Ridder D., Elgoyhen A.B., Romo R., Langguth B. Phantom percepts: tinnitus and pain as persisting aversive memory networks. Proc. Natl. Acad. Sci. USA. 2011;108:8075–8080. doi: 10.1073/pnas.1018466108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer D.J., Frank E., Phillips M.L. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet. 2012;379:1045–1055. doi: 10.1016/S0140-6736(11)60602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G. Gene, brains, and environment-genetic neuroimaging of depression. Curr. Opin. Neurobiol. 2013;23:133–142. doi: 10.1016/j.conb.2012.08.004. [DOI] [PubMed] [Google Scholar]
- van Os J., Kapur S. Schizophrenia. Lancet. 2009;374:635–645. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- Elgoyhen A.B., Langguth B. Pharmacological approaches to the treatment of tinnitus. Drug Discov. Today. 2010;15:300–305. doi: 10.1016/j.drudis.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Barabási A.L., Oltvai Z.N. Network biology: understanding the cell's functional organization. Nat. Rev. Genet. 2004;5:101–113. doi: 10.1038/nrg1272. [DOI] [PubMed] [Google Scholar]
- Hopkins A.L. Network pharmacology: the next paradigm in drug discovery. Nat. Chem. Biol. 2008;4:682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- Loging W., Harland L., Williams-Jones B. High-throughput electronic biology: mining information for drug discovery. Nat. Rev. Drug Discov. 2007;6:220–230. doi: 10.1038/nrd2265. [DOI] [PubMed] [Google Scholar]
- Kuhn M., Campillos M., González P., Jensen L.J., Bork P. Large-scale prediction of drug-target relationships. FEBS Lett. 2008;582:1283–1290. doi: 10.1016/j.febslet.2008.02.024. [DOI] [PubMed] [Google Scholar]
- Paolini G.V., Shapland R.H., van Hoorn W.P., Mason J.S., Hopkins A.L. Global mapping of pharmacological space. Nat. Biotechnol. 2006;24:805–815. doi: 10.1038/nbt1228. [DOI] [PubMed] [Google Scholar]
- Yildirim M.A., Goh K.I., Cusick M.E., Barabási A.L., Vidal M. Drug-target network. Nat. Biotechnol. 2007;25:1119–1126. doi: 10.1038/nbt1338. [DOI] [PubMed] [Google Scholar]
- Keiser M.J., et al. Predicting new molecular targets for known drugs. Nature. 2009;462:175–181. doi: 10.1038/nature08506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgoyhen A.B., Langguth B., Vanneste S., De Ridder D. Tinnitus: network pathophysiology-network pharmacology. Front. Syst. Neurosci. 2012;6:1. doi: 10.3389/fnsys.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campillos M., Kuhn M., Gavin A.C., Jensen L.J., Bork P. Drug target identification using side-effect similarity. Science. 2008;321:263–266. doi: 10.1126/science.1158140. [DOI] [PubMed] [Google Scholar]
- Kuhn M., Campillos M., Letunic I., Jensen L.J., Bork P. A side effect resource to capture phenotypic effects of drugs. Mol. Syst. Biol. 2010;6:343. doi: 10.1038/msb.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth B.L., Sheffler D.J., Kroeze W.K. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat. Rev. Drug Discov. 2004;3:353–359. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- Baguley D.M. Hyperacusis. J. R. Soc. Med. 2003;96:582–585. doi: 10.1258/jrsm.96.12.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauman R., Bouscau-Faure F. Assessment and amelioration of hyperacusis in tinnitus patients. Acta Otolaryngol. 2005;125:503–509. doi: 10.1080/00016480510027565. [DOI] [PubMed] [Google Scholar]
- Langguth B., Landgrebe M., Kleinjung T., Sand G.P., Hajak G. Tinnitus and depression. World J. Biol. Psychiatry. 2011;12:489–500. doi: 10.3109/15622975.2011.575178. [DOI] [PubMed] [Google Scholar]
- Lounkine E., et al. Large-scale prediction and testing of drug activity on side-effect targets. Nature. 2012;486:361–367. doi: 10.1038/nature11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.D., Stolzberg D., Lobarinas E., Sun W., Ding D., Salvi R. Salicylate-induced cochlear impairments, cortical hyperactivity and re-tuning, and tinnitus. Hear. Res. 2013;295:100–113. doi: 10.1016/j.heares.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J., Song L., Zheng J., Nuttall A.L. Control of mammalian cochlear amplification by chloride anions. J. Neurosci. 2006;26:3992–3998. doi: 10.1523/JNEUROSCI.4548-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noreña A.J., Moffat G., Blanc J.L., Pezard L., Cazals Y. Neural changes in the auditory cortex of awake guinea pigs after two tinnitus inducers: salicylate and acoustic trauma. Neuroscience. 2010;166:1194–1209. doi: 10.1016/j.neuroscience.2009.12.063. [DOI] [PubMed] [Google Scholar]
- Wallhäusser-Franke E., Mahlke C., Oliva R., Braun S., Wenz G., Langner G. Expression of c-fos in auditory and non-auditory brain regions of the gerbil after manipulations that induce tinnitus. Exp. Brain Res. 2003;153:649–654. doi: 10.1007/s00221-003-1614-2. [DOI] [PubMed] [Google Scholar]
- Eggermont J.J., Roberts L.E. The neuroscience of tinnitus. Trends Neurosci. 2004;27:676–682. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Saint D.A. The cardiac persistent sodium current: an appealing therapeutic target. Br. J. Pharmacol. 2008;153:1133–1142. doi: 10.1038/sj.bjp.0707492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes S.A., et al. Brain imaging of the effects of lidocaine on tinnitus. Hear. Res. 2002;171:43–50. doi: 10.1016/s0378-5955(02)00346-5. [DOI] [PubMed] [Google Scholar]
- Hartmann H.A., Colom L.V., Sutherland M.L., Noebels J.L. Selective localization of cardiac SCN5A sodium channels in limbic regions of rat brain. Nat. Neurosci. 1999;2:593–595. doi: 10.1038/10147. [DOI] [PubMed] [Google Scholar]
- Wu L., Nishiyama K., Hollyfield J.G., Wang Q. Localization of Nav1.5 sodium channel protein in the mouse brain. Neuroreport. 2002;13:2547–2551. doi: 10.1097/01.wnr.0000052322.62862.a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J.A., Alonso A., Kay A.R. A heart-like Na+ current in the medial entorhinal cortex. Neuron. 1993;11:1037–1047. doi: 10.1016/0896-6273(93)90217-f. [DOI] [PubMed] [Google Scholar]
- Hoehn K., Watson T.W., MacVicar B.A. A novel tetrodotoxin-insensitive, slow sodium current in striatal and hippocampal neurons. Neuron. 1993;10:543–552. doi: 10.1016/0896-6273(93)90341-n. [DOI] [PubMed] [Google Scholar]
- Bartolomé M.V., Gil-Loyzaga P. Serotonergic innervation of the inner ear: is it involved in the general physiological control of the auditory receptor. Int. Tinnitus J. 2005;11:119–125. [PubMed] [Google Scholar]
- Brown P., Molliver M.E. Dual serotonin (5-HT) projections to the nucleus accumbens core and shell: relation of the 5-HT transporter to amphetamine-induced neurotoxicity. J. Neurosci. 2000;20:1952–1963. doi: 10.1523/JNEUROSCI.20-05-01952.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery R.W., Sherman S.M. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- Rauschecker J.P., Leaver A.M., Mühlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron. 2010;66:819–826. doi: 10.1016/j.neuron.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J.W., Harding J.W. The brain renin-angiotensin system: a diversity of functions and implications for CNS diseases. Pflugers Arch. 2013;465:133–151. doi: 10.1007/s00424-012-1102-2. [DOI] [PubMed] [Google Scholar]
- Phillips M.I., Shen L., Richards E.M., Raizada M.K. Immunohistochemical mapping of angiotensin AT1 receptors in the brain. Regul. Pept. 1993;44:95–107. doi: 10.1016/0167-0115(93)90233-x. [DOI] [PubMed] [Google Scholar]
- Kaltenbach J.A. The dorsal cochlear nucleus as a contributor to tinnitus: mechanisms underlying the induction of hyperactivity. Prog. Brain Res. 2007;166:89–106. doi: 10.1016/S0079-6123(07)66009-9. [DOI] [PubMed] [Google Scholar]
- Derad I., Pietrowsky R., Dodt C., Fehm H.L., Born J. Enhanced psychophysiological signs of attention after angiotensin-converting enzyme inhibition by captopril. Psychophysiology. 1996;33:295–301. doi: 10.1111/j.1469-8986.1996.tb00426.x. [DOI] [PubMed] [Google Scholar]
- Noreña A.J. An integrative model of tinnitus based on a central gain controlling neural sensitivity. Neurosci. Biobehav. Rev. 2011;35:1089–1109. doi: 10.1016/j.neubiorev.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Hébert S., Fournier P., Noreña A. The auditory sensitivity is increased in tinnitus ears. J. Neurosci. 2013;33:2356–2364. doi: 10.1523/JNEUROSCI.3461-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriage J., Barnes N.M. Is central hyperacusis a symptom of 5-hydroxytryptamine (5-HT) dysfunction. J. Laryngol. Otol. 1995;109:915–921. doi: 10.1017/s0022215100131676. [DOI] [PubMed] [Google Scholar]
- Noreña A.J., Eggermont J.J. Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hear. Res. 2003;183:137–153. doi: 10.1016/s0378-5955(03)00225-9. [DOI] [PubMed] [Google Scholar]
- Gu J.W., Halpin C.F., Nam E.C., Levine R.A., Melcher J.R. Tinnitus, diminished sound-level tolerance, and elevated auditory activity in humans with clinically normal hearing sensitivity. J. Neurophysiol. 2010;104:3361–3370. doi: 10.1152/jn.00226.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox C., et al. DrugBank 3.0: a comprehensive resource for ‘omics' research on drugs. Nucleic Acids Res. 2011;39:D1035–D1041. doi: 10.1093/nar/gkq1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoot M.E., Ono K., Ruscheinski J., Wang P.L., Ideker T. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics. 2011;27:431–432. doi: 10.1093/bioinformatics/btq675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.