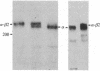
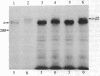
Abstract
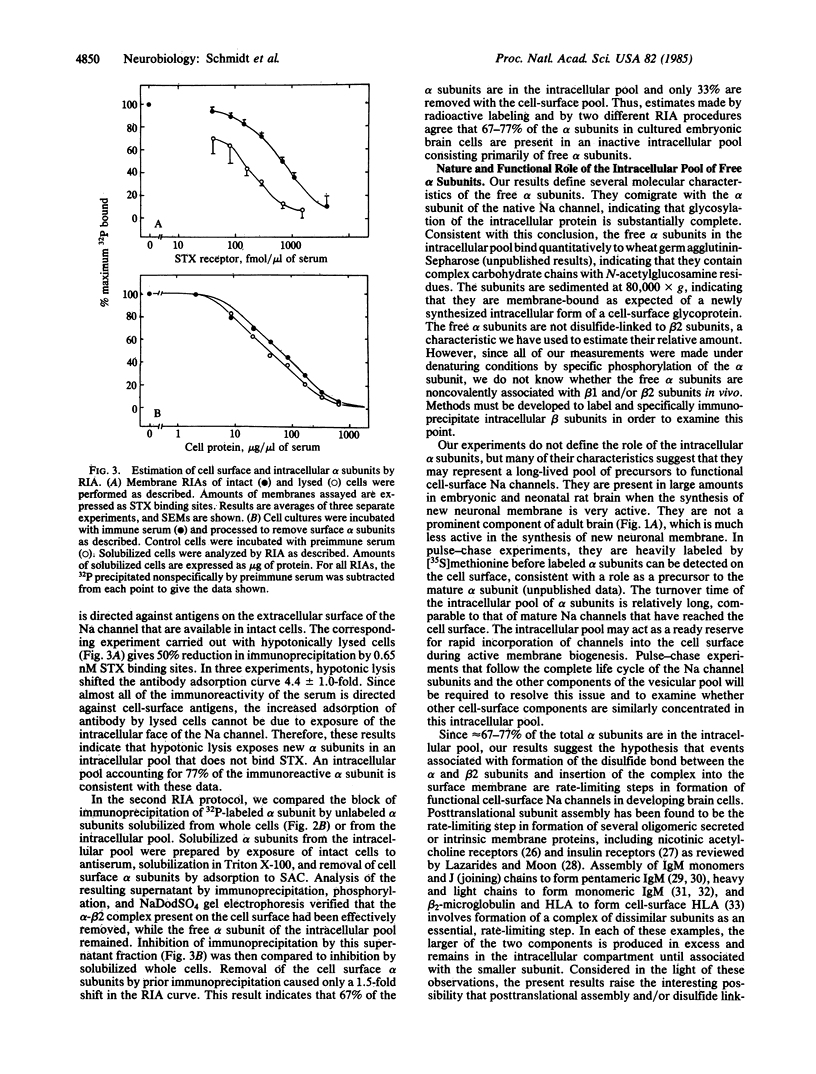
An intracellular pool of Na channel alpha subunits has been detected in developing brain cells in vivo and in vitro by phosphorylation with cAMP-dependent protein kinase, immunoprecipitation with specific antiserum, and NaDodSO4 gel electrophoresis or by radioimmunoassay. These alpha subunits are membrane-bound, contain complex carbohydrate chains, and have an apparent molecular weight of 260,000 like mature alpha subunits. In contrast to mature alpha subunits, the intracellular subunits are not covalently attached to a beta 2 subunit, and they do not bind saxitoxin with high affinity. They comprise 67-77% of the total immunoreactive alpha subunit in developing rat brain cells but are not a prominent component in the adult brain. It is proposed that this intracellular pool of alpha subunits forms a ready reserve of preformed subunits for incorporation into the surface membrane during periods of active membrane biogenesis. The results suggest that disulfide linkage of the alpha and beta 2 subunits, insertion into the cell surface membrane, and attainment of a functional conformation are closely related late events in the biogenesis of the Na channel. These processes may regulate the number of functional Na channels in the developing brain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnew W. S., Moore A. C., Levinson S. R., Raftery M. A. Identification of a large molecular weight peptide associated with a tetrodotoxin binding protein from the electroplax of Electrophorus electricus. Biochem Biophys Res Commun. 1980 Feb 12;92(3):860–866. doi: 10.1016/0006-291x(80)90782-2. [DOI] [PubMed] [Google Scholar]
- Barchi R. L. Protein components of the purified sodium channel from rat skeletal muscle sarcolemma. J Neurochem. 1983 May;40(5):1377–1385. doi: 10.1111/j.1471-4159.1983.tb13580.x. [DOI] [PubMed] [Google Scholar]
- Barhanin J., Pauron D., Lombet A., Norman R. I., Vijverberg H. P., Giglio J. R., Lazdunski M. Electrophysiological characterization, solubilization and purification of the Tityus gamma toxin receptor associated with the gating component of the Na+ channel from rat brain. EMBO J. 1983;2(6):915–920. doi: 10.1002/j.1460-2075.1983.tb01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgold J., Zimmerman I., Bambrick L. Appearance of [3H]saxitoxin binding sites in developing rat brain. Brain Res. 1983 Sep;285(3):405–407. doi: 10.1016/0165-3806(83)90040-8. [DOI] [PubMed] [Google Scholar]
- Beneski D. A., Catterall W. A. Covalent labeling of protein components of the sodium channel with a photoactivable derivative of scorpion toxin. Proc Natl Acad Sci U S A. 1980 Jan;77(1):639–643. doi: 10.1073/pnas.77.1.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwald-Netter Y., Martin-Moutot N., Koulakoff A., Couraud F. Na+-channel-associated scorpion toxin receptor sites as probes for neuronal evolution in vivo and in vitro. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1245–1249. doi: 10.1073/pnas.78.2.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer W. F., Jones E. A., Barnstable C. J., Bodmer J. G. Genetics HLA: the major human histocompatibility system. Proc R Soc Lond B Biol Sci. 1978 Jun 5;202(1146):93–116. doi: 10.1098/rspb.1978.0059. [DOI] [PubMed] [Google Scholar]
- Casadei J. M., Gordon R. D., Lampson L. A., Schotland D. L., Barchi R. L. Monoclonal antibodies against the voltage-sensitive Na+ channel from mammalian skeletal muscle. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6227–6231. doi: 10.1073/pnas.81.19.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A., Morrow C. S., Hartshorne R. P. Neurotoxin binding to receptor sites associated with voltage-sensitive sodium channels in intact, lysed, and detergent-solubilized brain membranes. J Biol Chem. 1979 Nov 25;254(22):11379–11387. [PubMed] [Google Scholar]
- Costa M. R., Catterall W. A. Cyclic AMP-dependent phosphorylation of the alpha subunit of the sodium channel in synaptic nerve ending particles. J Biol Chem. 1984 Jul 10;259(13):8210–8218. [PubMed] [Google Scholar]
- Dulis B. H. Regulation of protein expression in differentiation by subunit assembly. Human membrane and secreted IgM. J Biol Chem. 1983 Feb 25;258(4):2181–2187. [PubMed] [Google Scholar]
- Fambrough D. M., Devreotes P. N. Newly synthesized acetylcholine receptors are located in the Golgi apparatus. J Cell Biol. 1978 Jan;76(1):237–244. doi: 10.1083/jcb.76.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne R. P., Catterall W. A. Purification of the saxitoxin receptor of the sodium channel from rat brain. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4620–4624. doi: 10.1073/pnas.78.7.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne R. P., Catterall W. A. The sodium channel from rat brain. Purification and subunit composition. J Biol Chem. 1984 Feb 10;259(3):1667–1675. [PubMed] [Google Scholar]
- Hartshorne R. P., Messner D. J., Coppersmith J. C., Catterall W. A. The saxitoxin receptor of the sodium channel from rat brain. Evidence for two nonidentical beta subunits. J Biol Chem. 1982 Dec 10;257(23):13888–13891. [PubMed] [Google Scholar]
- Lazarides E., Moon R. T. Assembly and topogenesis of the spectrin-based membrane skeleton in erythroid development. Cell. 1984 Jun;37(2):354–356. doi: 10.1016/0092-8674(84)90364-7. [DOI] [PubMed] [Google Scholar]
- Lombet A., Kazazoglou T., Delpont E., Renaud J. F., Lazdunski M. Ontogenic appearance of Na+ channels characterized as high affinity binding sites for tetrodotoxin during development of the rat nervous and skeletal muscle systems. Biochem Biophys Res Commun. 1983 Feb 10;110(3):894–901. doi: 10.1016/0006-291x(83)91046-x. [DOI] [PubMed] [Google Scholar]
- Lombet A., Lazdunski M. Characterization, solubilization, affinity labeling and purification of the cardiac Na+ channel using Tityus toxin gamma. Eur J Biochem. 1984 Jun 15;141(3):651–660. doi: 10.1111/j.1432-1033.1984.tb08241.x. [DOI] [PubMed] [Google Scholar]
- Mains P. E., Sibley C. H. The requirement of light chain for the surface deposition of the heavy chain of immunoglobulin M. J Biol Chem. 1983 Apr 25;258(8):5027–5033. [PubMed] [Google Scholar]
- Mather E. L., Alt F. W., Bothwell A. L., Baltimore D., Koshland M. E. Expression of J chain RNA in cell lines representing different stages of B lymphocyte differentiation. Cell. 1981 Feb;23(2):369–378. doi: 10.1016/0092-8674(81)90132-x. [DOI] [PubMed] [Google Scholar]
- Miller J. A., Agnew W. S., Levinson S. R. Principal glycopeptide of the tetrodotoxin/saxitoxin binding protein from Electrophorus electricus: isolation and partial chemical and physical characterization. Biochemistry. 1983 Jan 18;22(2):462–470. doi: 10.1021/bi00271a032. [DOI] [PubMed] [Google Scholar]
- Nakayama H., Withy R. M., Raftery M. A. Use of a monoclonal antibody to purify the tetrodotoxin binding component from the electroplax of Electrophorus electricus. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7575–7579. doi: 10.1073/pnas.79.23.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T., Haas H. G., Therrien E. F. Saxitoxin and tetrodotoxin: comparison of nerve blocking mechanism. Science. 1967 Sep 22;157(3795):1441–1442. doi: 10.1126/science.157.3795.1441. [DOI] [PubMed] [Google Scholar]
- Norman R. I., Schmid A., Lombet A., Barhanin J., Lazdunski M. Purification of binding protein for Tityus gamma toxin identified with the gating component of the voltage-sensitive Na+ channel. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4164–4168. doi: 10.1073/pnas.80.13.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. N., Glaser L., Merlie J. P., Lindstrom J. Expression of acetylcholine receptor alpha-subunit mRNA during differentiation of the BC3H1 muscle cell line. J Biol Chem. 1984 Mar 10;259(5):3330–3336. [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Ronnett G. V., Knutson V. P., Kohanski R. A., Simpson T. L., Lane M. D. Role of glycosylation in the processing of newly translated insulin proreceptor in 3T3-L1 adipocytes. J Biol Chem. 1984 Apr 10;259(7):4566–4575. [PubMed] [Google Scholar]
- Roth R. A., Koshland M. E. Identification of a lymphocyte enzyme that catalyzes pentamer immunoglobulin M assembly. J Biol Chem. 1981 May 10;256(9):4633–4639. [PubMed] [Google Scholar]
- Unsworth B. R., Hafemann D. R. Tetrodotoxin binding as a marker for functional differentiation of various brain regions during chick and mouse development. J Neurochem. 1975 Feb;24(2):261–270. doi: 10.1111/j.1471-4159.1975.tb11874.x. [DOI] [PubMed] [Google Scholar]
- Waechter C. J., Schmidt J. W., Catterall W. A. Glycosylation is required for maintenance of functional sodium channels in neuroblastoma cells. J Biol Chem. 1983 Apr 25;258(8):5117–5123. [PubMed] [Google Scholar]
- Wollner D. A., Catterall W. A. Antigenic differences among the voltage-sensitive sodium channels in the peripheral and central nervous systems and skeletal muscle. Brain Res. 1985 Apr 1;331(1):145–149. doi: 10.1016/0006-8993(85)90724-3. [DOI] [PubMed] [Google Scholar]