Abstract
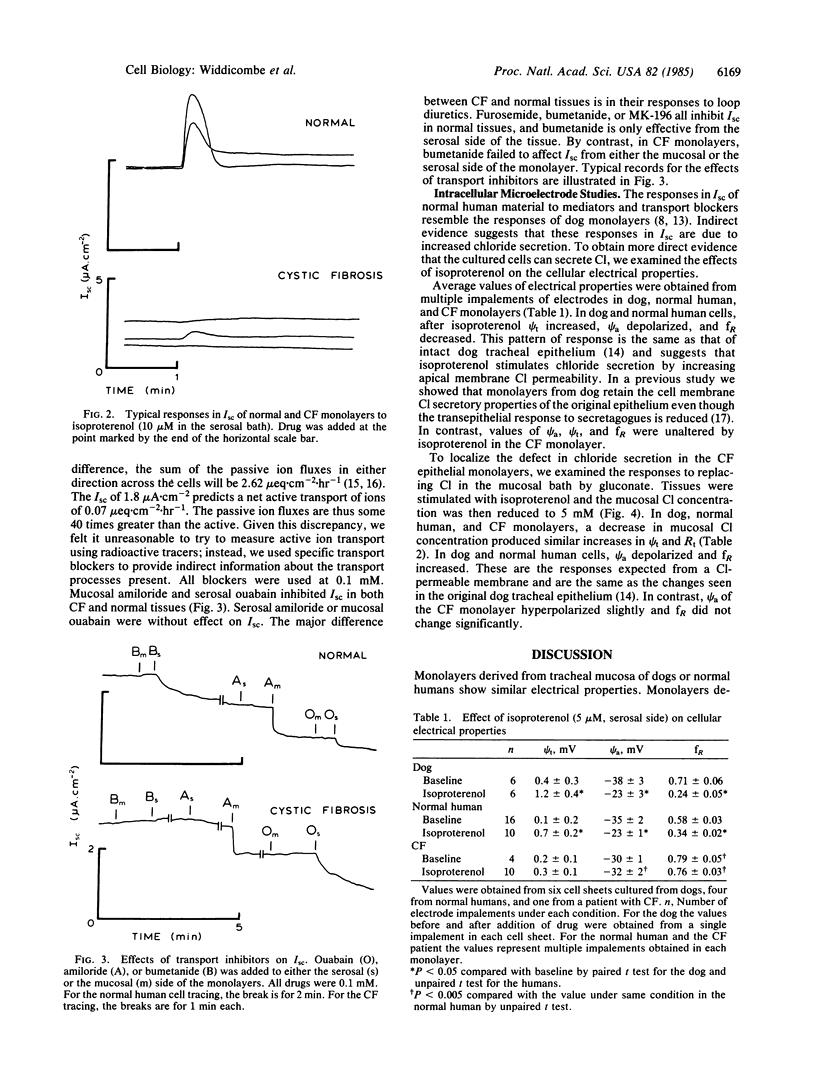
The tracheal mucosa from a 12-year-old girl was digested with collagenase 4 hr after her death from cystic fibrosis. Forty million viable cells were obtained. The cells, plated at 10(6) per cm2 onto four Nuclepore filters coated with human placental collagen, formed confluent monolayers after 1 day. Their ultrastructure was similar to that of normal human cells. They were studied in conventional Ussing chambers or with intracellular microelectrodes on days 5-7 after plating. The monolayers displayed resistance of 380 +/- 50 omega X cm2 and short-circuit current (Isc) of 1.8 +/- 0.4 microA X cm-2. This resistance is similar to that obtained for dog or normal human monolayers. The Isc is less than normal human (approximately 3 microA X cm-2) or dog (approximately 10 microA X cm-2) cells. The cystic fibrosis cells resembled normal monolayers in that serosal ouabain and mucosal amiloride inhibited Isc, while mucosal ouabain or serosal amiloride had no effect. They differed from normal human or dog cells in that Isc was not inhibited by bumetanide and the stimulation of Isc by isoproterenol or prostaglandin E2 was greatly reduced or abolished. Addition of isoproterenol depolarized apical membrane potential (psi a) and decreased fractional resistance (fR) in normal human and dog but had no effect on psi a or fR in cystic fibrosis cells. Reduction of mucosal chloride from 120 to 5 mM by replacement with gluconate increased fR of dog and normal human monolayers and depolarized psi a by 22 (dog) or 30 (human) mV. In cystic fibrosis monolayers, chloride replacement hyperpolarized psi a by 2 mV and had little effect on fR. These results suggest that the primary defect in cystic fibrosis is reduced apical membrane chloride conductance.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bisbee C. A., Machen T. E., Bern H. A. Mouse mammary epithelial cells on floating collagen gels: transepithelial ion transport and effects of prolactin. Proc Natl Acad Sci U S A. 1979 Jan;76(1):536–540. doi: 10.1073/pnas.76.1.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg M., Green N., Sohraby S., Steele R., Handler J. Differentiated function in cultured epithelia derived from thick ascending limbs. Am J Physiol. 1982 Mar;242(3):C229–C233. doi: 10.1152/ajpcell.1982.242.3.C229. [DOI] [PubMed] [Google Scholar]
- Cereijido M., Ehrenfeld J., Meza I., Martínez-Palomo A. Structural and functional membrane polarity in cultured monolayers of MDCK cells. J Membr Biol. 1980;52(2):147–159. doi: 10.1007/BF01869120. [DOI] [PubMed] [Google Scholar]
- Coleman D. L., Tuet I. K., Widdicombe J. H. Electrical properties of dog tracheal epithelial cells grown in monolayer culture. Am J Physiol. 1984 Mar;246(3 Pt 1):C355–C359. doi: 10.1152/ajpcell.1984.246.3.C355. [DOI] [PubMed] [Google Scholar]
- Handler J. S., Steele R. E., Sahib M. K., Wade J. B., Preston A. S., Lawson N. L., Johnson J. P. Toad urinary bladder epithelial cells in culture: maintenance of epithelial structure, sodium transport, and response to hormones. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4151–4155. doi: 10.1073/pnas.76.8.4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. P., Steele R. E., Perkins F. M., Wade J. B., Preston A. S., Green S. W., Handler J. S. Epithelial organization and hormone sensitivity of toad urinary bladder cells in culture. Am J Physiol. 1981 Aug;241(2):F129–F138. doi: 10.1152/ajprenal.1981.241.2.F129. [DOI] [PubMed] [Google Scholar]
- Knowles M. R., Stutts M. J., Spock A., Fischer N., Gatzy J. T., Boucher R. C. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science. 1983 Sep 9;221(4615):1067–1070. doi: 10.1126/science.6308769. [DOI] [PubMed] [Google Scholar]
- Knowles M., Gatzy J., Boucher R. Relative ion permeability of normal and cystic fibrosis nasal epithelium. J Clin Invest. 1983 May;71(5):1410–1417. doi: 10.1172/JCI110894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. J., Williams M. C., Widdicombe J. H., Sanders M. J., Misfeldt D. S., Berry L. C., Jr Transepithelial transport by pulmonary alveolar type II cells in primary culture. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6033–6037. doi: 10.1073/pnas.79.19.6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J. W., Macknight A. D., Dayer J. M., Ausiello D. A. Localization of [3H]ouabain-sensitive Na+ pump sites in cultured pig kidney cells. Am J Physiol. 1979 Mar;236(3):C157–C162. doi: 10.1152/ajpcell.1979.236.3.C157. [DOI] [PubMed] [Google Scholar]
- Quinton P. M. Chloride impermeability in cystic fibrosis. Nature. 1983 Feb 3;301(5899):421–422. doi: 10.1038/301421a0. [DOI] [PubMed] [Google Scholar]
- SCHULTZ S. G., ZALUSKY R., GASS A. E., Jr ION TRANSPORT IN ISOLATED RABBIT ILEUM. 3. CHLORIDE FLUXES. J Gen Physiol. 1964 Nov;48:375–378. doi: 10.1085/jgp.48.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Sato F. Defective beta adrenergic response of cystic fibrosis sweat glands in vivo and in vitro. J Clin Invest. 1984 Jun;73(6):1763–1771. doi: 10.1172/JCI111385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. L., Welsh M. J., Stoff J. S., Frizzell R. A. Chloride secretion by canine tracheal epithelium: I. Role of intracellular c AMP levels. J Membr Biol. 1982;70(3):217–226. doi: 10.1007/BF01870564. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Smith P. L., Frizzell R. A. Chloride secretion by canine tracheal epithelium: II. The cellular electrical potential profile. J Membr Biol. 1982;70(3):227–238. doi: 10.1007/BF01870565. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Smith P. L., Frizzell R. A. Chloride secretion by canine tracheal epithelium: III. Membrane resistances and electromotive forces. J Membr Biol. 1983;71(3):209–218. doi: 10.1007/BF01875462. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. H., Coleman D. L., Finkbeiner W. E., Tuet I. K. Electrical properties of monolayers cultured from cells of human tracheal mucosa. J Appl Physiol (1985) 1985 May;58(5):1729–1735. doi: 10.1152/jappl.1985.58.5.1729. [DOI] [PubMed] [Google Scholar]




