Abstract
Human embryonic stem cells have emerged as the prototypical source from which cardiomyocytes can be derived for use in drug discovery and cell therapy. However, such applications require that these cardiomyocytes (hESC-CMs) faithfully recapitulate the physiology of adult cells, especially in relation to their electrophysiological and contractile function. We review what is known about the electrophysiology of hESC-CMs in terms of beating rate, action potential characteristics, ionic currents, and cellular coupling as well as their contractility in terms of calcium cycling and contraction. We also discuss the heterogeneity in cellular phenotypes that arises from variability in cardiac differentiation, maturation, and culture conditions, and summarize present strategies that have been implemented to reduce this heterogeneity. Finally, we present original electrophysiological data from optical maps of hESC-CM clusters.
Keywords: Human embryonic stem cell, Cardiac cell, Embryoid body, Electrophysiology, Contraction, Optical mapping
1. Introduction
Over the past several decades, revolutionary advances in the cardiac field have occurred with the advent of human pluripotent stem cells and their differentiation into cardiomyocytes (CMs). Much attention has been directed toward the potential clinical application of these cells in the context of regenerative medicine and cell-based therapy [for reviews, see (Codina et al., 2010; Habib et al., 2008; Laflamme et al., 2007b; Li et al., 2009)]. Despite early successes with basic science studies and experimentation using animal hearts, clinical studies in general have not yet met with similar success (Lovell and Mathur, 2010). On the other hand, these cells hold immediate promise as a new generation of experimental models never before possible. Previously, the availability of human tissue for experimentation was limited to tissue biopsies and to hearts that are unsuitable for or made available by organ transplantation. For the most part, fundamental knowledge regarding the functioning of cardiac tissue under normal or diseased conditions has relied on animal models [for reviews, see (Hamlin, 2007; McCauley and Wehrens, 2009; Milan and MacRae, 2005)]. These models approximate the human condition and have been used for mechanistic studies at a molecular level.
Today, many types of human pluripotent stem cells are being investigated for their potential to produce functional CMs, including adult bone marrow-derived cells, bone marrow-derived mesenchymal stem cells, adipose-derived cells, embryonic stem cells, endothelial progenitor cells, skeletal myoblasts, resident cardiac stem cells, and, most recently, induced pluripotent stem cells (Li et al., 2009). In this article, we will focus on CMs derived from human embryonic stem cells (hESC-CMs), first reported in 2001 (Kehat et al., 2001). At present, this cell type best produces functional CMs and is particularly relevant because it is widely used as the gold standard against which other pluripotent stem cells are compared.
The potential usages of hESC-CMs are several-fold. First, they are expected to be more clinically relevant than animal models for the purposes of toxicity testing and drug discovery and development (Davis et al., 2011; He et al., 2007; Mandenius et al., 2011; Zeevi-Levin et al., 2012). The cells can be subjected to detailed analysis of their molecular, pharmacological, electrophysiological and contractile properties (Goh et al., 2005; Kong et al., 2010; Poon et al., 2011). Second, they have been used to study broad aspects of disease, including myocyte vulnerability to bile acids (Abdul Kadir et al., 2009), oxidative stress and ischemic preconditioning (Sepac et al., 2010), and hypertrophy (Foldes et al., 2011). Third, hESC-CMs may serve as a scalable cell source that can be used for cardiac myoplasty and regeneration (Capi and Gepstein, 2006; Dai and Kloner, 2006; Habib et al., 2008; Zeevi-Levin et al., 2010) and electrophysiological therapy (Gepstein, 2006), provided that the additional hurdles of survival, immunogenicity, heterogeneity, maturation, and electrophysiological safety can be worked out.
Essential for all of these applications is the proviso that the cells recapitulate native physiological function. Thus, the goal of this article is to review the known functional characteristics of hESC-CMs. Although previous studies have examined these characteristics largely from a genomic perspective, we focus here on contractile and electrophysiological function. At the elemental level, this means the contractile structure of the cell (sarcomere and myofilament organization) and expression of ion channel and calcium cycling proteins. At the integrative level is cellular function, which includes contractility, intracellular calcium release and uptake, action potentials, drug responses, and intercellular coupling. The final level is physiological function, which has been well characterized in adult cells and tissue in terms of contractility [force-frequency relation (Endoh, 2004), force-length relation (Shiels and White, 2008) and intracellular calcium kinetics (Sobie et al., 2006)], electrophysiology [restitution (rate-dependence) of action potential duration (Franz, 2003), conduction velocity restitution (Weiss et al., 2002)] and excitation-contraction coupling (Bers, 2008)].
In the sections that follow, we summarize the reported electrophysiological and contractile properties of hESC-CMs, recognizing that the properties may be rather diverse. Heterogeneity can arise from the variability in cardiac differentiation that occurs across cell lines, differentiation protocols, time of differentiation and maturation of the cells, and culture conditions in different laboratories. The absence of a well-defined phenotype of the cells is an unavoidable limitation at this time and may present a significant hurdle to the usage of hESC-CMs. The issues of heterogeneity and maturation and avenues for improvement will be addressed in detail in the last section. Finally, we include original electrophysiological data that we have obtained from optical maps of clusters of hESC-CMs.
2. Beating rate
Cardiac differentiation in hESCs has been induced by a variety of techniques (Burridge et al., 2012), but commonly involves culturing an aggregate of hESCs termed an embryoid body1 (hEB). The first visual clue of CM differentiation and the presence of hESC-CMs within hEBs is the onset of spontaneous synchronized contraction. This automaticity implies pacemaker activity within hEBs while the synchronization suggests the presence of a functional syncytium. Kehat et al. was the first to demonstrate these properties in hEBs (Kehat et al., 2002), finding stable pacemaker activities within the hEBs along with gap junctions that electrically and functionally connect the cells. In this section, we summarize the hormonal and pharmacological responses of hESC-CMs in terms of beating rate, as this is often the first metric used to characterize hESC-CM function. However, it should be noted that beating rate is not invariant and may vary at different stages of cell maturation. For example, hEBs derived from the H1 hESC line have been shown to have a stable, high beating rate in the first weeks after the onset of spontaneous beating, with a significant drop in rate after 9 weeks in culture (Reppel et al., 2004).
2.1. Hormonal responses
The autonomic nervous system regulates pacemaker activities in vivo through adrenergic and cholinergic pathways. Evaluating whether hESC-CMs express similar pathways is important to understanding their physiology, and has been extensively performed.
The adrenergic agonists adrenaline and noradrenaline (Norstrom et al., 2006; Yokoo et al., 2009) and β-adrenergic agonist isoproterenol (Anderson et al., 2007; Gupta et al., 2010; Kapucu et al., 2008; Mandel et al., 2012; Pekkanen-Mattila et al., 2009; Reppel et al., 2004; Xu et al., 2006a; Xu et al., 2002; Xu et al., 2009; Yokoo et al., 2009) all increase beating rate in hEBs in a dose-dependent manner. Furthermore, the β2-adrenergic agonist clenbuterol increases beating rates of hESC-CMs only at a late stage of differentiation (61-72 days) (Xu et al., 2002), whereas the α1-adrenergic agonist phenylephrine, increases the beating rate of hESC-CMs at an early stage of differentiation (15-20 days) (Xu et al., 2006a; Xu et al., 2002). The stimulatory effect of adrenaline and noradrenaline can be reversed by α- or β-adrenergic receptor blockers (Norstrom et al., 2006), while the positive chronotropic effect of isoproterenol can be negated by the β-blockers propranolol (Anderson et al., 2007) and metoprolol (Mandel et al., 2012). These findings suggest that the adrenergic responses of hEBs may be regulated by different receptors at various differentiation stages. Conversely, carbachol, a cholinergic agonist, decreases beating rate in hEBs (Gupta et al., 2010; Mandel et al., 2012; Reppel et al., 2004). Acetylcholine also decreases beating rates of hESC-CMs in a dose-dependent manner, and at high concentrations ceases beating (Norstrom et al., 2006). The presence of both positive and negative chronotropic hormonal regulation suggest that hESC-CMs develop intact hormonal receptors similar to those in native myocardium, indicating the potential use of hESC-CMs in studies of cardiac adrenergic and cholinergic regulation.
2.2. Pharmacological responses
Ionic currents underlie pacemaker activity in hESC-CMs (see Section 3.2), and pharmacological manipulation of these currents has been shown to affect beating rates. The L-type calcium channel blocker diltiazem reversibly decreases beating rates of hESC-CMs in a dose-dependent manner, and can cease spontaneous activity altogether (Xu et al., 2006a; Xu et al., 2002). Application of another L-type calcium channel blocker, verapamil, yielded similar effects in hESC-CMs (Liang et al., 2010; Yokoo et al., 2009), with decreased beating rate occurring in a dose-dependent manner and cessation of spontaneous beating at high concentration. The rapid delayed rectifier channel blocker E-4031 (Guo et al., 2011a; Liang et al., 2010) and the sodium channel blocker TTX decrease beating rate in hESC-CMs (Satin et al., 2004) .
Some cardioactive drugs also affect beating rates in hESC-CMs. The antiarrhythmic agents propafenone (Caspi et al., 2009) and quinidine (Liang et al., 2010) decrease beating rates of hESC-CMs, while procainamide, mexiletine, and flecainide have no effect (Yokoo et al., 2009). The QT-prolonging drug sotalol, however, was shown to decrease beating rates in one study (Liang et al., 2010) and have no effect in another study (Reppel et al., 2005). Additionally, beating rate increases in a dose-dependent manner with isobutyl methylxanthine (IBMX), a phosphodiesterase inhibitor, and with forskolin, a stimulator of adenylate cyclase, suggesting the existence of a functioning cAMP system in hESC-CMs (Norstrom et al., 2006; Xu et al., 2002).
3. Electrophysiology
3.1. Action potentials
3.1.1. Microelectrode and patch clamp recordings
Following differentiation, hESC-CMs obtained from the beating clump of cells in the EB consist of a mixture of electrophysiological phenotypes. Classification of hESC-CMs as nodal-, atrial-, and ventricular-like is based on the resemblance of their action potential (AP) to each of the three principal phenotypes found in the adult heart (Schram et al., 2002) and has been referred to as a “functional signature” (He et al., 2003). The relative fractions of nodal:atrial:ventricular-like cells, estimated from AP recordings, vary for different cell lines and differentiation conditions and are summarized in Table 1, although it should be noted that these fractions depend on the criteria used and can change with time of differentiation. Furthermore, within a given phenotype (e.g., ventricular) there can be substantial variation in action potential shape (Pekkanen-Mattila et al., 2010).
Table 1. Proportion of principal cellular electrophysiological phenotypes in hESC-CM cultures.
| N | Cell Line(s) | % Nodal-like | % Atrial-like | % Ventlike | Quantitative criteriaa | Reference |
|---|---|---|---|---|---|---|
| 33 | HES-2 | 3 | 6 | 85 | No | (Mummery et al., 2003) |
| 21 | H1,H7,H9 and H14 | 24 | 24 | 52 | No | (He et al., 2003) |
| 33 | HES-2 | 3 | 33 | 64 | No | (Moore et al., 2008) |
| 20 | H1 | 5 | 60 | 35 | No | (Moore et al., 2008) |
| 15 | H1 | 20 | 20 | 60 | No | (Zhang et al., 2009) |
| 27 | H9 | 11 | 30 | 59 | No | (Zhang et al., 2009) |
| 114 | SA002 | 16 | 47 | 37 | No | (Jonsson et al., 2010) |
| 125 | H7 | < 1 | 18 | 82 | Yes | (Peng et al., 2010) |
| not given | HES-2 | 5 | 2 | 93 | No | (Braam et al., 2010) |
| 93 | HES-2 | 4b | 48c | 48d | Yes | (Fu et al., 2011) |
| 35 | H7 and HES-3 | 43 | 23 | 34 | No | (Lee et al., 2011) |
Notes:
Quantitative criteria are those using numerical values of AP parameters to distinguish different phenotypes.
Abolished with miR-499
Decreased with miR-1 or miR-499
Increased with miR-1 or miR-499
Many groups have recorded APs in developing hESC-CMs and hEBs, and it is well known that the APs are immature analogs of those of adult heart cells (Braam et al., 2010; Cao et al., 2008; Fu et al., 2011; He et al., 2003; Jonsson et al., 2010; Lee et al., 2011; Moore et al., 2008; Mummery et al., 2003; Peng et al., 2010; Wang et al., 2011; Zhang et al., 2009). The criteria used to classify electrophysiological phenotype varies among studies, but in general is based on some qualitative or quantitative combination of the maximum diastolic potential (MDP), upstroke velocity (dV/dtmax), action potential duration (APD), action potential amplitude (APA), beating rate (BR), and phase 4 (diastolic) depolarization rate (DDR). For example, cells with more negative MDP, longer APD, lower BR, higher dV/dtmax, larger APA, and lower DDR may be considered to be ventricular-like.
The most information on AP parameters is for the ventricular-like phenotype (Bettiol et al., 2007; Fu et al., 2011; He et al., 2003; Jonsson et al., 2010; Kim et al., 2010; Mummery et al., 2003; Pekkanen-Mattila et al., 2010; Peng et al., 2010; Wang et al., 2011; Xu et al., 2006a; Xu et al., 2011; Zhang et al., 2009; Zhang et al., 2011; Zhu et al., 2010). These data are summarized in Table 2 and illustrate the large variability in AP properties of hESC-CMs. For example, a general trend is that isolated cells have much higher upstroke velocities (118 V/s) compared with those in hEBs (approximately 12 V/s) (Satin et al., 2004). A recent study showed that upstroke velocity in small cell aggregates also varied between spontaneous beating (51 V/s) and pacing (26 V/s) (Jonsson et al., 2012). Different cell lines preferentially adopt different AP phenotypes (Moore et al., 2008). Another factor contributing to AP variability is that APD is rate-dependent (He et al., 2003; Zhang et al., 2009) (also see Section 3.1.3) and in most studies is measured at the spontaneous beating rate, which is highly variable.
Table 2. Ventricular-type APs in hESC-CMs.
| Cell Line(s) | N | dV/dtmax (V/s) | APA | MDP | APD90a | Rate | Level | Reference |
|---|---|---|---|---|---|---|---|---|
| H7b | 31 | 44.2±6.7 | 96.8±2.7 | - 57.5 ±1.6 | 211.1 ±17.2 | 85.8±6.9 | Single | (Zhu et al., 2010) |
| H7 | 42 | 11.4±2.8 | 86.8 ±12.4 | - 62.3 ±8.6 | 285.8 ±52.6 | Not specified | Single | (Zhang et al., 2011) |
| H9 | 45 | 33.5±3.7 | 83.5±2.4 | - 64.3 ±3.2 | 312.6 ±15.9 | 44.6±2.8 | EB | (Zhang et al., 2009) |
| H1 | 27 | 17.6±2.6 | 82.7±3.0 | - 60.8 ±2.8 | 298.7 ±13.4 | 33.3±2.3 | EB | (Zhang et al., 2009) |
| H7b | 28 | 48.0±6.7 | 99.6±2.7 | - 58.7 ±1.6 | 212.9 ±17.2 | 86.9±6.9 | Single | (Xu et al., 2011) |
| HES-2 | 23 | Not specified | 102.5 ±1.7 | - 62.4 ±1.2 | 513.6 ±28.4 | Stimulation at 1 Hz | Single | (Wang et al., 2011) |
| H7 | 102 | 12.1±0.9 | 102.3 ±1.6 | 73.1 ±1.2 | 355.9 ±12.5 | Stimulation at 1 Hz | Single | (Peng et al., 2010) |
| Otherc,d | 28 | 129.4 ±105.0 | 113.3 ±10.7 | 67.3 ±4.5 | 264.5 ±55.7 | 72±6 | Single | (Pekkanen-Mattila et al., 2010) |
| Otherc,e | 34 | 154.6 ±55.7 | 120.3 ±10.0 | - 74.8 ±3.9 | 232.4 ±89.5 | 72±6 | Single | (Pekkanen-Mattila et al., 2010) |
| HES-2d | 28 | 7.0±0.8 | 80.0±3.5 | - 48.0 ±1.7 | 436.4 ±55.3 | 36±6 | Small Aggregates | (Mummery et al., 2003) |
| SA002 | 42 | 14±9 | 61±17 | 220 ±51 | 81±31 | EB | (Jonsson et al., 2010) | |
| H9 and H14 | 11 | 13.2±6.2 | 85.3±9.3 | - 53.9 ±8.6 | 247.2 ±66.7 | 47.1 ±23.3 | EB | (He et al., 2003) |
Notes:
APD at 90% repolarization
No distinction between atrial- and ventricular-type cells
Regea 06/015, Regea 06/040, HS346, or HS360
END-2 coculture
Spontaneous EB
AP responses to various ionic channel blockers have been examined. The sodium channel blocker TTX significantly decreased dV/dtmax, and veratridine, an inhibitor of sodium channel inactivation, prolonged APD (Jonsson et al., 2012; Pekkanen-Mattila et al., 2010; Peng et al., 2010; Satin et al., 2004). Nifedipine, an L-type calcium channel blocker, shortens APD in single cells (Peng et al., 2010). E-4031 blocks the rapid delayed rectifier channel, and prolongs potential duration in both single (Otsuji et al., 2010; Pekkanen-Mattila et al., 2010; Peng et al., 2010; Sartiani et al., 2007) and multicellular preparations (hEBs or small cell aggregates) (Braam et al., 2010; Caspi et al., 2009; He et al., 2003; Jonsson et al., 2010; Nalos et al., 2012). E-4031 has a greater effect on action potential duration at later stages of differentiation in multicellular preparations (Caspi et al., 2009; Otsuji et al., 2010). Prolonged application of E-4031 evokes early afterdepolarizations in both single (He et al., 2003; Nalos et al., 2012; Peng et al., 2010) and multicellular (Braam et al., 2010; He et al., 2003; Nalos et al., 2012) preparations. Delayed afterdepolarizations were observed in untreated hEBs (He et al., 2003) and in single cells treated with norepinephrine (Peng et al., 2010). APD prolongation also occurs with administration of the slow delayed rectifier channel blocker, chromanol 293B (Jonsson et al., 2012; Peng et al., 2010). DDR decreases following application of zatebradine, a blocker of the pacemaker channel (Kim et al., 2010; Sartiani et al., 2007).
3.1.2. Field potentials
Extracellular field potentials measured by microelectrode arrays (MEAs) can serve as a surrogate for AP measurements in multicellular preparations (Halbach et al., 2003). Field potential duration (FPD) relates to APD in CMs and the QT interval of an electrocardiogram. Measurements of FPD enable the use of EBs and cell monolayers to evaluate the risk of drug-induced arrhythmia (Weinberg et al., 2010).
Application of the rapid delayed rectifier channel blocker E-4031 results in prolongation of FPD. E-4031 had a larger dose-dependent effect in late stage hESC-CMs (>30 day) than in early stage cells (15-30 day), suggesting more rapid delayed rectifier channel expression in late stage differentiation (Braam and Mummery, 2010; Caspi et al., 2009; Mehta et al., 2011). Conversely, verapamil (Braam et al., 2010; Liang et al., 2010; Mehta et al., 2011) and nifedipine (Braam et al., 2010; Guo et al., 2011b) shortened FPD in hEBs and ESC-CM monolayers.
Several cardioactive drugs have been tested for their effect on FPD of hEBs. The QT-prolonging drug, sotalol, prolonged FPD in hEBs (Reppel et al., 2005), consistent with observations in several later studies that showed a dose-dependent effect (Braam et al., 2010; Caspi et al., 2009; Liang et al., 2010) and one that showed a more significant effect on early stage hEBs than late stage hEBs (Liang et al., 2010). The class I antiarrhythmia drugs quinidine and procainamide prolonged FPD in a dose-dependent manner, while lidocaine had no effect (Braam et al., 2010). Several non-cardiac drugs were also screened; astemizole (Xu et al., 2009), and cisapride, sparfloxacin, terfenadine, domperidone and ketoconazole (Braam and Mummery, 2010) prolonged FPD while sertindole (Braam and Mummery, 2010) had no effect. These results indicate the promising potential for using hESC-CMs to screen drugs for FPD prolongation, which is considered to be a proarrhythmic risk (Braam et al., 2010; He et al., 2007).
3.1.3. Optical mapping
Optical mapping is a well-established technique for studying transmembrane potential and intracellular calcium transients in various preparations, and can be applied to study hESC-CMs (Weinberg et al., 2010). It provides multicellular recordings at high spatial resolution and, unlike field potentials recorded by MEAs, can directly record the time course of transmembrane potential, thereby preserving action potential morphology. Recently, we (Burridge et al., 2011) and another group (Ren et al., 2011) obtained optical recordings of transmembrane action potentials and intracellular calcium transients from hEBs. Isoproterenol (Burridge et al., 2011) and verapamil (Ren et al., 2011) decreased APD, while procainamide (a dual blocker of sodium and potassium channels) increased APD (Ren et al., 2011). Here we provide additional optical voltage mapping results from hEBs and focus on the extent of heterogeneity of these cells.
Contracting hEBs were obtained from the H9 cell line using previously described methods (Burridge et al., 2011). Beating areas of hEBs were mechanically dissected out, plated on gelatin coated coverslips (3-6 beating aggregates per coverslip) and given at least 5 days to attach. These beating clusters were then stained with 10μM dμi-4-ANEPPS voltage-sensitive dye (Invitrogen) for 10 min. The coverslips with beating clusters were subsequently transferred to a custom made mapping chamber with continuously flowing Tyrode's solution (135 mM NaCI, 5.4 mM KCI, 1.8 mM CaCI2, 1 mM MgCI2, 0.33 mM NaH2P04, 5 mM HEPES, and 5 mM glucose (Sigma)). 50 μM blebbistatin was added to inhibit excitation-contraction coupling and prevent signal distortion due to motion. All experiments were conducted at 37°0. Imaging of transmembrane potential (Vm) was conducted using a MiCAM Ultima-L CMOS camera (100×100 pixels) at 500 frames/sec sampling rate. To improve signal quality, 4×4 binning was used, resulting in 25×25 recording sites. The outline of the beating clusters was manually determined, and only pixels within the outline were used for analysis.
In one batch of differentiated hESC-CMs, we recorded APs in beating clusters from 28 hEBs (20 to 35 days after hEB formation). While all of the beating clusters could be electrically stimulated, 22 of them had spontaneous activity. Electrical pacing was applied through a pair of parallel Pt field electrodes. Pacing rate started at 0.5 Hz, and increased with 0.5 Hz increments. As pacing rate increased, the beating clusters eventually failed to maintain 1:1 capture.
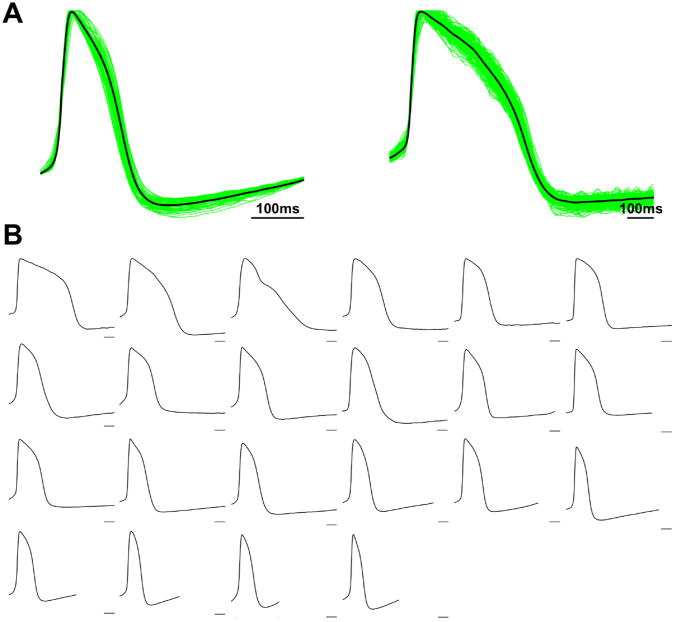
Fig. 1A shows recordings from two beating clusters with distinct AP morphologies. The green traces are APs of the 75 (left) and 209 (right) recording sites within each beating cluster, while the black traces are the representative APs obtained by aligning and spatially averaging all recordings from the beating clusters. These data show that there is relatively small heterogeneity in AP morphology across different locations within a single hEB, as has been reported previously (He et al., 2003; Jonsson et al., 2010; Zhang et al., 2009), and that the spatially averaged trace is representative of the predominant behavior of the hEB. Similar results were observed in all beating clusters mapped, under both spontaneous and paced conditions. Fig. 1B shows the representative APs from all 22 spontaneously active beating clusters, with each panel representing one beating cluster. Within this batch of hEBs, there were multiple AP morphologies, with large variability in both APD and the degree of phase 4 depolarization, contrary to the presence of a dominant electrophysiological phenotype within individual hEBs. These findings are similar to those previously reported (He et al., 2003).
Fig. 1.
Optical action potentials and activation properties of spontaneously beating cell clusters from H9 EBs. (A) Action potentials recorded from two clusters. The green traces are single recording sites within each cluster (75 and 209 sites in left and right panels, respectively). The black traces are spatial averages of all recording sites within each cluster. Scale bar is 100 ms. (Left) Beating cluster with short APD and prominent phase 4 depolarization. (Right) Beating cluster with long APD and quiescent phase 4. (B) Action potentials from clusters derived from 22 different EBs. Each panel is a spatially averaged action potential from a single cluster. All panels are normalized to 1 second time window for comparison among different spontaneous beating rates. Scale bars are 100 ms. Note that different AP morphologies clearly exist among the EB population.
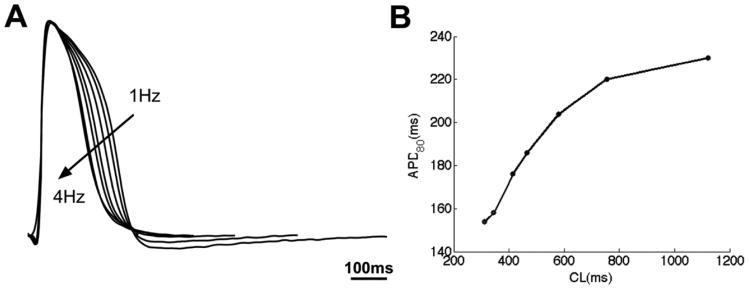
We also investigated the responses of beating clusters from hEBs stimulated at various pacing rates (example shown in Fig. 2). Electrical pacing started at ∼1 Hz and increased with 0.5 Hz increments up to 4 Hz, the maximum capture rate for this particular beating cluster. APD shortened with increasing pacing rate (Fig. 2A), as shown in the APD80 restitution curve (plotted as a function of cycle length, the inverse of pacing rate) (Fig. 2B). Similar results were observed in all beating clusters, with the maximum capture rate varying from 1.5 to 4 Hz. These findings demonstrate that hESC-CMs within the hEBs, in addition to firing spontaneous and stimulus-induced APs, have physiological rate-adaptation.
Fig. 2.
APD restitution in a beating cell cluster from an H9 EB. (A) Spatially averaged action potentials of the cluster when paced at 1 Hz to 4 Hz with 0.5 Hz intervals; scale bar is 100 ms. (B) Corresponding APD80 as function of cycle length (CL).
We have shown that optical mapping can be used to obtain hundreds of recordings of APs within a single hEB. Unlike FPs measured by MEAs, which contain only information on AP activation and repolarization time, the optical recordings preserve AP morphology, which can be important to know during drug testing. Alternatively, multiple AP measurements can be obtained by repeated impalements of the hEB with a sharp microelectrode (He et al., 2003; Jonsson et al., 2010; Zhang et al., 2009). However, the possibility of cell damage, difficulty in obtaining large sample size, and inability to measure conduction velocity are limitations of that approach. On the other hand, microelectrode recordings provide absolute voltage information such as resting potential and action potential amplitude, which are not available with optical recordings.
3.2. Ionic currents
We review the expression and function of the ionic currents INa, ICaL, IK1, Ito, IKr, IKs, If, and INCX, which underlie the shape and properties of the action potential. The expression levels of genes and gene transcripts associated with these ionic currents at various stages in hESC-CM differentiation are summarized in Table 3. Ion channel expression can be specific to the electrophysiological phenotype. HCN1, HCN2, KCNA5, and GJA5 are higher in nodal-like than in ventricular-like hESC-CMs (Jonsson et al., 2010). KCNJ2 and SCN1B are also preferentially expressed in ventricular-type cells (Jonsson et al., 2010). However, in general the specific phenotype of the cells being studied was not specified in the studies cited in this section. Further, independent studies often used different voltage protocols or other experimental conditions, so direct comparisons should be made with care. Current magnitudes are normalized to cell capacitance. Total cell capacitance for hESC-CMs is on the order of 5-30 pF compared with adult human ventricular cells, which normally have a capacitance of approximately 150 pF (Drouin et al., 1995).
Table 3. Maturation of hESC-CM ion current expression.
| Current | Gene | Gene Product | Result | Reference |
|---|---|---|---|---|
| INa | SCN5A | Nav1.5 | Much higher at 8 mo than 1 mo | (Otsuji et al., 2010)b |
| SCN1B | Navβ1 | Much higher at 8 mo than 1 mo | (Otsuji et al., 2010)b | |
| ICaL | CACNA1C | Cav1.2 | Not in undifferentiated cells, but present at 8-15 days | (Mummery et al., 2003)b |
| Constant throughout differentiation | (Sartiani et al., 2007)a | |||
| CACNA1S | Cav1.1 | Upregulated by miR-1 transduction | (Fu et al., 2011)a,c | |
| CNCNB2 | Cavβ2 | Higher at 8 mo than 1 mo | (Otsuji et al., 2010)b | |
| ICaT | CACNA1H | Cav3.2 | Lower at 8 mo than 1 mo | (Otsuji et al., 2010)b |
| IKs | KCNQ1 | Kv7.1 | Expressed in undifferentiated cells, decreases at mid-stage, then reappears at late-stages; | (Mummery et al., 2003)b |
| Higher at 8 mo than 1 mo | (Otsuji et al., 2010)b | |||
| IKr | KCNH2(HERG) | Kv11.1 | Higher at 55d than earlier (8, 20, 30 d); | (Caspi et al., 2009)a |
| Upregulated by miR-1 transduction | (Fu et al., 2011)a,c | |||
| Always present | (Sartiani et al., 2007)a | |||
| HERG1B | Kv11.1 | Higher at 8 mo than 1 mo | (Otsuji et al., 2010)b | |
| Present after day 16 | (Sartiani et al., 2007)a | |||
| KCNE2 | MiRP1 | Higher at 55d than earlier (8, 20, 30 d) | (Caspi et al., 2009)a | |
| IKATP | KCNJ11 | Kir6.2 | Higher at 8 mo than 1 mo | (Otsuji et al., 2010)b |
| If | HCN1 | HCN1 | Not present at days 5 and 10, present at days 15 and 20 | (Kim et al., 2011)c |
| Significantly higher at 8 mo than 1 mo | (Otsuji et al., 2010)b | |||
| Increased until day 25 then decreased at days 57 and 110 | (Sartiani et al., 2007)a | |||
| HCN2 | HCN2 | Not present at day 5, present at days 15 and 20 | (Kim et al., 2011)c | |
| Relatively constant | (Sartiani et al., 2007)a | |||
| HCN4 | HCN4 | Downregulated by miR-1 transduction | (Fu et al., 2011)a,c | |
| Decreased with differentiation time | (Kim et al., 2011)c (Sartiani et al., 2007)a | |||
| Ito1 | KCND3 | Kv4.3 | Not in undifferentiated cells, but present at 8-15 days | (Mummery et al., 2003)b |
| Always present even in undifferentiated cells | (Sartiani et al., 2007)a | |||
| KCNA4 | Kv1.4 | Upregulated by miR-1 transduction | (Fu et al., 2011)a,c | |
| Higher at 8 mo than 1 mo | (Otsuji et al., 2010)b | |||
| Present after 25 days | (Sartiani et al., 2007)a | |||
| IK1 | KCNJ2 | Kir2.1 | Upregulated by miR-1 transduction; | (Fu et al., 2011)a,c |
| Higher at 8 mo than 1 mo | (Otsuji et al., 2010)b | |||
| Always present, increased until day 57 | (Sartiani et al., 2007)a | |||
| INCX | NCX1 | NCX | Increased during differentiation | (Fu et al., 2010)a,c |
Notes:
Suspended embryonic bodies
END-2 coculture
Activin A and BMP growth factor treatment
Several single-cell studies using voltage clamp separation revealed the presence of the fast sodium current, INa (Jonsson et al., 2012; Otsuji et al., 2010; Pekkanen-Mattila et al., 2010; Peng et al., 2010; Satin et al., 2004). One study showed that from 1 to 8 months of differentiation INa magnitude increases in magnitude approximately five-fold to -100 pA/pF at Vm of -10 mV (Otsuji et al., 2010). This is larger than the maximal current measured of -32 pA/pF at -20 mV that has been reported in another lab at approximately 25 days of differentiation (Satin et al., 2004). For comparison, INa of adult human ventricular cardiomyocytes can be estimated from the O'Hara computational model (O'Hara et al., 2011) to have a peak amplitude of approximately -196 pA/pF at -17 mV from a holding potential of -80 mV at 37°C.
Peak L-type calcium current, ICaL, densities have been measured via voltage clamp separation (Fu et al., 2010; Fu et al., 2011; Jonsson et al., 2012; Otsuji et al., 2010; Sartiani et al., 2007; Zhu et al., 2009), and in ventricular cells range from -2.2±0.5 pA/pF at Vm of +10mV at 47 days of differentiation (Fu et al., 2010) to -10 pA/pF at -10 mV at 8 months of differentiation (Otsuji et al., 2010). These results indicate that ICaL increases in magnitude over time, in agreement with two studies that directly evaluated ICaL current densities at different times of differentiation (Otsuji et al., 2010; Sartiani et al., 2007). A recent study showed that, in comparison with native guinea pig ventricular myocytes, hESC-CMs maintained similar kinetic responses to antagonists, but different responses to activators (Kang et al., 2012). In human adult ventricular cells peak ICaL is approximately -10.2 pA/pF at +5 mV (Magyar et al., 2000).
Several single-cell studies suggest that the inward rectifier current, IK1, is absent in hESC-CMs (Fu et al., 2010; Fu et al., 2011; He et al., 2003; Satin et al., 2004). A recent study showed that it was expressed in only 3 out of 5 cells examined (Jonsson et al., 2012). Additionally, one study showed that DDR increases following application of the IK1 blocker BaCl2, and that the magnitude of IK1, measured as a BaCl2-sensitive current at Vm of -90 mV, increases from -0.6±0.3 pA/pF at 14-40 days to -3.4±1.3 pA/pF at 50-110 days of differentiation (Sartiani et al., 2007). Similarly, another study showed a significant increase in IK1 from 2 to 8 months (Otsuji et al., 2010). In adult human ventricular cells IK1 has a measured density of about -10 pA/pF at -90 mV (Wang et al., 1998). Increased IK1 expression results in more negative MDP and a lower spontaneous rate, and is thought to be an indicator of a more mature ventricular phenotype since it is preferentially expressed in the adult ventricle (Schram et al., 2002).
The transient outward current, Ito, has been measured in single cells via voltage clamp separation at both early (14-40 days) and late (50-110 days) stages of differentiation. At Vm of +50 mV, maturation led to an increase in peak Ito from 4.2 pA/pF in early stage hESC-CMs to 7.7 pA/pF at later stages (Sartiani et al., 2007). The results are in close agreement with measurements at +50 mV in control (4 pA/pF) and miR-1-transduced (6 pA/pF) hESC-CMs at approximately 25 days of differentiation (Fu et al., 2011). In adult ventricular cells, peak Ito ranges from 2.3±0.3 to 7.9±0.7 pA/pF at +60 mV for subendocardial and subepicardial cells, respectively (Wettwer et al., 1994).
The rapid delayed rectifier current, IKr, is responsible for late repolarization, has been measured as an E-4031-sensitive current, and has been the focus of interest with regard to long QT syndrome studies. IKr has a maximal current density of 0.4-0.7 pA/pF at Vm of +10 mV (Fu et al., 2011; Wang et al., 2011). Its magnitude is much higher at 8 months than at 1 month (Otsuji et al., 2010). In adult ventricular cardiomoycytes, IKr can be determined from the O'Hara model to have a magnitude of 0.82 pA/pF at +7 mV under physiological conditions.
The slow delayed rectifier current, IKs, sometimes eludes detection in hESC-CMs (Fu et al., 2010); however, more recent single-cell studies have reported its presence as a chromanol 293B-sensitive current (Fu et al., 2011; Jonsson et al., 2012; Otsuji et al., 2010; Peng et al., 2010; Wang et al., 2011). This current has a reported density of 0.6 pA/pF at Vm of +40 mV (Wang et al., 2011), and its amplitude remains relatively constant with increased differentiation time (Otsuji et al., 2010). In adult ventricular cardiomyocytes, IKs can be determined from the O'Hara model to have a magnitude of 0.78 pA/pF at +40 mV under physiological conditions.
Expression of the pacemaker current, If, is well established in hESC-CMs (Fu et al., 2010; Fu et al., 2011; Jonsson et al., 2012; Kim et al., 2010; Otsuji et al., 2010; Sartiani et al., 2007; Satin et al., 2004), and voltage clamp separation studies have shown peak If magnitudes ranging from -4 pA/pF to about -10 pA/pF at Vm of -120 mV (Fu et al., 2011; Otsuji et al., 2010; Satin et al., 2004). By comparison, adult human sinoatrial node cells have an If magnitude of approximately -7 pA/pF at -120 mV (Verkerk et al., 2007). Both If magnitude and HCN4 transcript decrease following transduction with miR-1 (Table 3) (Fu et al., 2011). If is downregulated during differentiation (Sartiani et al., 2007), in agreement with expression data that show downregulation of its fast isoform (HCN1) (Table 3) (Kim et al., 2011; Otsuji et al., 2010; Sartiani et al., 2007). However, a study that used a 3D/replating culture technique found a significant increase in the magnitude of If from 2 to 8 months (Otsuji et al., 2010). The same study showed that distinct regions exhibited nodal- or ventricular-like properties.
A functional sodium-calcium exchanger (NCX) is present in hESC-CMs (Fu et al., 2010; Kim et al., 2010), in agreement with gene expression data. INCX was characterized as a Ni-sensitive current in single cells that were differentiated for either 47 or 97 days (Fu et al., 2010). In the forward (Ca2+ outward) mode, measured at Vm of -120mV, the magnitude of INCX increased over time, from -1.2±0.6 pA/pF at 40 days to -6.9±1.3 pA/pF at 97 days. In the reverse mode (measured at +60 mV) it increased from 3.6±1.0 pA/pF to 7.9±1.3 pA/pF (Fu et al., 2010) over the same time period.
3.3. Cell-cell coupling
Cell-cell coupling has been studied in beating areas of hEBs and in hESC-CMs obtained from enzymatically dissociated hEBs (Jonsson et al., 2010).
3.3.1. Gap junctions
Gap junctional proteins have been identified between hESC-CMs (Kehat et al., 2002). Two isoforms of connexin, Cx43 and Cx45, were found to be co-localized and homogeneously distributed along the cell borders, with no preferential polar orientation. Using immunocytochemistry, several labs have confirmed the presence of Cx43 (Jonsson et al., 2010; Pekkanen-Mattila et al., 2010; Pekkanen-Mattila et al., 2009; Xu et al., 2006a; Xu et al., 2011; Yoon et al., 2006) although one has not (Norstrom et al., 2006). Another lab found that immunoreactivity for Cx43 was weak in early-stage (9 days) hEBs but discernible in late-stage (56 days) hEBs (Cui et al., 2007). Electron microscopy failed to show gap junctions in hEBs at 31 days of differentiation (Gherghiceanu et al., 2011). Further investigation is needed to resolve these different findings.
3.3.2. Conduction
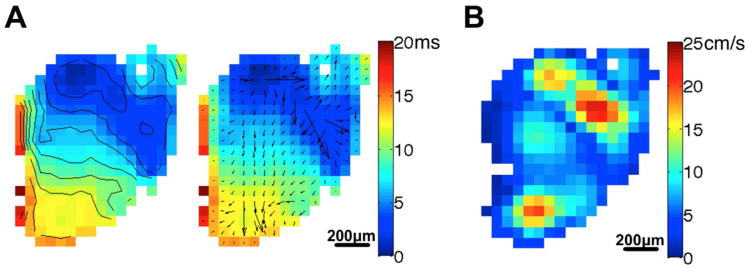
Conduction velocity measurements of hEBs using MEAs span a wide range from approximately 2 cm/s to 14 cm/s at 37 °C (Caspi et al., 2009; Kehat et al., 2002; Satin et al., 2004), and may be the result of variations in cell lines, differentiation protocols, and experimental conditions. In our optical mapping experiments on H9 hEBs described earlier, conduction in a representative hEB at 1Hz pacing is shown in Fig. 3. Activation time at each recording site within the hEB was determined as the instant of maximum slope of the AP upstroke. The left two panels show the activation map with isochrones and local conduction velocity vectors superimposed, respectively, and the right panel shows the map of local conduction velocity magnitude. The origin of the action potentials is clearly seen (deep blue areas of left two panels) as well as heterogeneous local conduction velocities across the hEB. The average local CV was 8.1 cm/s. All of these CV measurements (including our data) are consistently slower than CV measured in the intact human ventricle, which ranges from 41 to 87 cm/s (Nanthakumar et al., 2007). This may be the result of an immature phenotype, including smaller cell dimension, isotropic cell orientation, differences in ion channel expression and greater coupling with non-myocytes (Kehat et al., 2002).
Fig. 3.
Conduction properties of a beating cell cluster from an H9 EB. (A) Isochrones of 2 ms intervals and local conduction velocity vectors superimposed on activation map of the cluster subjected to 1 Hz electrical pacing. (B) Local conduction velocity magnitude map. Scale bars are 200 μm.
We also used optical mapping to investigate wavefront propagation in 20-mm diameter monolayers of CMs derived from H9 hEBs (Thompson et al., 2012). An average CV of 10.6±4.2 cm/s (n=3) was measured at 37 °C, slightly higher t han the value we measured from the intact H9 hEB.
Despite having slower conduction velocities, hEBs respond similarly to a range of cardioactive compounds as adult CMs do, as indicated by MEA recordings. TTX slows conduction in hEBs (Satin et al., 2004) in a dose-dependent manner, eventually leading to conduction block, suggesting sodium current-driven AP propagation. On the other hand, calcium channel blockers resulted in a variety of effects: verapamil slowed conduction in hEBs from the H1 line (Liang et al., 2010) while diltiazem and nifedipine had no effect on hEBs from the H9.2 line (Satin et al., 2004). Application of heptanol, a gap junctional uncoupler, decreased conduction velocities in hEBs (Caspi et al., 2009) and ceased action potential propagation at higher concentrations. The class I antiarrhythmic agents quinidine (Caspi et al., 2009; Liang et al., 2010) and propafenone (Caspi et al., 2009) slowed conduction in hEBs, and at high concentration quinidine ceased propagation. The QT-prolonging drug sotalol had no effect on conduction in hEBs (Liang et al., 2010).
4. Contractility
4.1. Contraction and force generation
Many studies of hESC-CMs have demonstrated varying degrees of structural and contractile organization -- in particular the presence of cross-striations, as well as the contractile proteins troponin, myosin heavy chain, tropomyosin, and α-actinin (Binah et al., 2007; Boudou et al., 2011; Caspi et al., 2007a; Dolnikov et al., 2005; Fu et al., 2010; Habeler et al., 2009; Kehat et al., 2001; Kim et al., 2010; Liu et al., 2007; Mummery et al., 2003; Otsuji et al., 2010; Pekkanen-Mattila et al., 2009; Schaaf et al., 2011; Sedan et al., 2010; Sedan et al., 2008). Additionally, mechanical contraction has been measured in both hEBs (Binah et al., 2007; Brito-Martins et al., 2008; Pillekamp et al., 2009; Pillekamp et al., 2007; Schaaf et al., 2011; Sedan et al., 2010; Sedan et al., 2008) and isolated hESC-CMs (Abdul Kadir et al., 2009; Germanguz et al., 2011). Contraction of hEBs increased with an increase in extracellular calcium concentration (Pillekamp et al., 2009; Schaaf et al., 2011), addition of isoproterenol (Brito-Martins et al., 2008; Schaaf et al., 2011), addition of adrenaline (Apati et al., 2012) and IP3R activation (Sedan et al., 2010; Sedan et al., 2008). Consistent with the findings of immature or absent calcium cycling described in the next section, agents that block RyR release or SERCA uptake had no effect on contraction amplitude in hEBs (Binah et al., 2007; Dolnikov et al., 2005; Dolnikov et al., 2006).
Rate- and time-dependent changes in contraction also revealed the missing contribution of SR calcium stores to contraction. The adult myocardium typically exhibits a positive force-frequency relation, primarily as a consequence of increasing SR calcium load and subsequent release (Layland and Kentish, 1999). However, a negative force-frequency relationship was measured in several studies of hEBs (Binah et al., 2007; Dolnikov et al., 2005; Dolnikov et al., 2006) and isolated hESC-CMs (Germanguz et al., 2011). Post-rest potentiation of contraction, due to an increase in SR stores during the rest period, is a normal property of the adult myocardium but is absent in hEBs (Binah et al., 2007; Dolnikov et al., 2006; Germanguz et al., 2011), consistent with the immature calcium cycling discussed above. On the other hand, hESC-CM tissue constructs exhibit physiological contractile force-length relations having positive slope, analogous to the Frank-Starling relation in the intact adult heart (Tulloch et al., 2011). Furthermore, contractile forces generated by individual hESC-CMs purified using dual drug selection have been measured using dynamic force microscopy. These contractile forces were similar to those measured in neonatal ventricular CMs and did not change with time in culture (Kita-Matsuo et al., 2009).
4.2. Calcium cycling
Given the importance of proper excitation-contraction in CMs, several studies have investigated calcium-induced calcium release (CICR) and calcium cycling processes in hESC-CMs. Compared with mature ventricular cells, isolated hESC-CMs exhibited a comparable mRNA level of Cav1.2 (encoding the α-1C subunit of the L-type calcium channel) but about a 1000-fold lower level of RyR2 (Satin et al., 2008). Expression of NCX and SERCA2a similar to that in fetal ventricular myocytes was reported for isolated hESC-CMs, and while RyR2 was present, its pattern of expression was not well organized. Also, the regulatory proteins calsequestrin (CSQ) and triadin were absent (Liu et al., 2007). A different study found expression of CSQ and also of phospholambam along with SERCA2 and NCX in hEBs (Binah et al., 2007). Furthermore, protein expression of NCX1, RyR2, and IP3R2 has been ascertained (Kim et al., 2008). A summary of SERCA2A, RyR2 and CSQ expression is given in Table 4, although the cell phenotype (atrial, ventricular, or nodal) was not specified in any of the studies.
Table 4. SERCA2A, RyR2, and CSQ expression in hESC-CMs.
| Protein or Gene Product | Cell Line(s) | Levela | Result | Reference |
|---|---|---|---|---|
| SERCA2A | H9.2, I3 | EB | Expressed at levels comparable to adult | (Binah et al., 2007; Dolnikov et al., 2006) |
| H1, HES-2 | Single | Expressed at levels comparable to fetal | (Liu et al., 2007) | |
| H7, HES-3 | Single | Expressed | (Lee et al., 2011) | |
| RyR2 | H1, HES-2 | Single | Expressed at levels comparable to fetal | (Liu et al., 2007) |
| H9.2 | Single | Expressed at levels 1000} fold-less compared with adult | (Satin et al., 2008) | |
| H7 | Single | Expressed, co-localized with L-type calcium channels | (Zhu et al., 2009) | |
| H9 | Single | Expressed in 20d and 60d CMs | (Kim et al., 2010) | |
| H7, HES-3 | Single | Expressed | (Lee et al., 2011) | |
| H9.2 | EB | Expressed | (Germanguz et al., 2011) | |
| CSQ2 | H9.2, I3 | EB | Not expressed | (Binah et al., 2007; Dolnikov et al., 2006) |
| H1, HES-2 | Single | Not expressed | (Liu et al., 2007) | |
| H7, HES-3 | Single | Expressed | (Lee et al., 2011) | |
| H9.2 | EB | Expressed in both hiPSC-and hESC-CMs | (Germanguz et al., 2011) |
Notes:
Level indicates whether contractility studies were performed in isolated cells (indicated by ‘Single’) or hEBs.
In addition to gene and protein expression, many studies have shown the presence of intracellular calcium transients in hEBs (Binah et al., 2007; Dolnikov et al., 2005; Grey et al., 2005; Kehat et al., 2001; Sedan et al., 2010; Sedan et al., 2008), engrafted hESC-CMs (Caspi et al., 2007b; Habeler et al., 2009), small cell aggregates (Kim et al., 2010; Liu et al., 2007), and isolated hESC-CMs (Fu et al., 2010; Lieu et al., 2009; Liu et al., 2009; Mummery et al., 2003; Zhu et al., 2009). However, there exists a wide range of findings in the literature regarding the maturity of calcium cycling in hESC-CMs. In adult ventricular myocytes, SR calcium release is highly sensitive to millimolar doses of caffeine (Eisner et al., 2009). With hESC-CMs, one study found that only ∼38% of isolated CMs had caffeine-sensitive calcium stores. Of these cells, calcium transients depended on RyR release and SERCA uptake (Liu et al., 2007). These findings, along with the limited expression of Ca2+ cycling proteins discussed above, (Liu et al., 2007), are suggestive of functional but immature calcium cycling in hESC-CMs. Another study came to a similar conclusion of functional but immature calcium cycling (Satin et al., 2008), demonstrating the expression of RyR2 and IP3R, the presence of RyR-dependent calcium transients and the presence of caffeine-sensitive SR calcium stores that increased with differentiation time. A summary of SR calcium release and uptake is given in Table 5, which was determined for cells with a ventricular phenotype by Fu et al. but was not specified in the other studies. In addition to studies of functional SR calcium stores, two studies measured calcium waves along the transverse axis of the CM and found a U-shaped wave, indicating there was a delay between calcium release near the cell membrane and at the center of the cell, suggestive of either immature or absent T-tubules (Lieu et al., 2009; Satin et al., 2008). Along these lines, one study using electron microscopy noted the presence of some developing T-tubules associated with the SR (Caspi et al., 2007b).
Table 5. SR calcium release and uptake in hESC-CMs.
| Property | Cell Line(s) | Levela | Result | Reference |
|---|---|---|---|---|
| SR Ca uptake | H9.2, I3 | EB | SERCA inhibitor did not alter Ca2+ transient, contraction | (Binah et al., 2007; Dolnikov et al., 2006) |
| H1, HES-2 | Single | SERCA inhibitor slowed Ca2+ transient decay in caffeine-sensitive CMs | (Liu et al., 2007) | |
| H7 | Single | SERCA inhibitor reduced Ca2+ transient amplitude, slowed Ca2+ transient decay | (Zhu et al., 2009) | |
| H1 | Single | Faster Ca2+ transient decay in 90d vs 40d CMs | (Fu et al., 2010) | |
| SR Ca release | H9.2, I3 | EB | Caffeine and RyR did not alter Ca2+ transient, contraction | (Binah et al., 2007; Dolnikov et al., 2006) |
| H1, HES-2 | Single | Caffeine- and RyR-sensitive Ca2+ transient in ∼38% of CMs | (Liu et al., 2007) | |
| H9.2 | Single | Caffeine- and RyR-sensitive Ca2+ transient, SR load increased with maturation | (Satin et al., 2008) | |
| H7 | Single | Caffeine- and RyR-sensitive Ca2+ transient, SR load lower compared with fetal | (Zhu et al., 2009) | |
| H1 | Single | Larger Ca2+ transient amplitude, faster upstroke in 90d vs 40d CMs | (Fu et al., 2010) | |
| H9 | Single | RyR-sensitive Ca2+ transient, reduced amplitude | (Kim et al., 2010) | |
| HES-2 | EB | Caffeine-sensitive Ca2+ transient | (Gupta et al., 2010) | |
| H7, HES-3 | Single | Caffeine- and RyR-sensitive Ca2+ transient | (Lee et al., 2011) | |
| H9.2 | EB | No RyR-sensitive contraction | (Germanguz et al., 2011) |
Notes:
aLevel indicates whether contractility studies were performed in isolated cells (indicated by ‘Single’) or hEBs.
Other aspects of immature calcium cycling have been observed in hESC-CM studies. It was recently demonstrated that calcium transients in isolated hESC-CMs were partially driven by INCX, which has peak expression in fetal CMs and decreased expression in adult CMs (Fu et al., 2010). Additionally, activation of the IP3R pathway mediated release of calcium stores in hESC-CMs, in contrast with the situation in the adult ventricular myocyte, in which calcium release through IP3R is very low (Sedan et al., 2008).
On the other hand, one recent study found close co-localization between RyRs and L-type calcium channels and an excitation-contraction coupling “gain” (defined as the ratio of the calcium transient amplitude to the L-type calcium channel current) that increased at negative potentials (Zhu et al., 2009). This “gain” relationship is a hallmark of tight coupling between the L-type calcium channel and RyR in adult myocytes (Santana et al., 1996), suggesting mature calcium cycling in those isolated hESC-CMs.
Finally, another study found that agents that block RyR release or SERCA uptake had no effect on calcium transients in hESC-CMs, which suggests that SR calcium stores are not functional and that calcium transients and contractions depend mainly on transarcolemmal Ca2+ flux, despite SERCA2a protein expression at levels comparable to adult human myocardium (Binah et al., 2007). Thus, there is conflicting evidence as to the degree of calcium cycling maturity in hESC-CMs. Several review papers provide additional information regarding the maturity of calcium cycling in hESC-CMs (Itzhaki et al., 2006; Kong et al., 2010; Sedan and Binah, 2011; Siu et al., 2007; Zeevi-Levin et al., 2010).
5. Cellular heterogeneity and maturation
Procedures to differentiate hESCs into CMs yield heterogeneous populations of cell types that include non-CMs and immature CMs with varying electrophysiological phenotypes. These issues of heterogeneity and maturation must be addressed before these cells can be used for toxicity testing, drug discovery, disease modeling, or cellular therapy.
5.1. Heterogeneity of differentiated cells
The importance of cytokines, growth factors, cell-cell communication, and small molecules on stem cell differentiation to the cardiac lineage and development of electrophysiological and contractile activity is well established (Burridge et al., 2012; Chavakis et al., 2010). The differentiation of CMs generally falls along one of three methods (Burridge et al., 2012) – suspended embryoid bodies, forced aggregation, or cell monolayers, as noted in Tables 3 and 4. Various methods have been employed to enrich the fraction of hESC-CMs obtained from the heterogeneous population of cells. These include: density gradient centrifugation (Xu et al., 2006b; Xu et al., 2002), expression of a selection marker (generally, a fluorescent protein or antibiotic resistance gene) driven under the control of a CM-specific promoter (Gallo et al., 2008; Huber et al., 2007; Kita-Matsuo et al., 2009; Xu et al., 2008), activation of a suicide gene (Anderson et al., 2007), or antibody-based sorting on cell surface markers, which include ALCAM (Rust et al., 2009), EMILIN 2 (Van Hoof et al., 2010), SIRPA (Dubois et al., 2011) or VCAM1 (Uosaki et al., 2011). A novel alternative approach free of genetic modification is to stain cells with the mitochondrial dye, TMRM, which emits an optical signal that is largest for CMs (Hattori et al., 2010). New, label-free approaches to identify hESC-CMs include second harmonic generation optical signals (Awasthi et al., 2012) and Raman spectroscopy (Pascut et al., 2011). It might also be possible to separate hESC-CMs from their progenitors based on cellular dielectric properties, as has been done in mouse neural precursor cells (Flanagan et al., 2008).
5.2. Heterogeneity of electrophysiological phenotypes of hESC-CMs
The heterogeneity in cellular properties of hESC-CMs described in the earlier sections appears to be a universal finding and may arise in part because of intrinsic differences in the starting cell lines that are used (Moore et al., 2008; Osafune et al., 2008; Pekkanen-Mattila et al., 2009) and conditions under which the cells are differentiated and cultured (Pekkanen-Mattila et al., 2010). Heterogeneity becomes a critical issue if hESC-CMs are to be engrafted into recipient hearts for the purposes of cardiomyoplasty. Discrepancies in their electrophysiological and contractile properties with those of the host tissue can disturb the coordinated operation of the heart and disrupt physiological function. For example, hESC-CMs nearly always exhibit autonomic activity. While this makes them attractive as biological pacemakers when engrafted into the myocardium (Kehat et al., 2004; Xue et al., 2005), it also makes them intrinsic sources for ectopic activity that can be proarrhythmic in the atrium or ventricle.
Fortunately, new methods are on the horizon to purify for ventricular, atrial and nodal phenotypes. Controlled retinoid signaling regulates atrial versus ventricular differentiation (Zhang et al., 2011). MicroRNAs have been shown to regulate ventricular differentiation (Fu et al., 2011). Inhibition of neuregulin/ErbB signaling enhances the nodal phenotype in hESC-CMs (Zhu et al., 2010) while the addition of endothelin-1 to murine Nkx2.5+ cardiac progenitors promotes differentiation into pacemaking cells (Zhang et al., 2012). A substantial amount of work has drawn on an understanding of cardiogenic cues during development to establish methods for preferentially differentiating hESCs into cardiomyocytes. It is possible that the right combination of cues, which include cytokines (Chiriac et al., 2010), growth factors (Kattman et al., 2011; Laflamme et al., 2007a; Leschik et al., 2008; Pal and Khanna, 2007; Singla and Sun, 2005), non-CMs (Mummery et al., 2003), modulators of p38-MAPK (Gaur et al., 2010; Graichen et al., 2008; Kempf et al., 2011), Notch (Jang et al., 2008), Wnt/β-catenin (Paige et al., 2010; Qyang et al., 2007; Tran et al., 2009), and nitric oxide (Mujoo et al., 2008) signaling, extracellular matrix components (Battista et al., 2005; Chan et al., 2010; Gerecht et al., 2007), and other compounds such as ascorbic acid (Wang et al., 2010) and demethylating agents (Yoon et al., 2006) could also be applied at precise developmental timepoints to derive cardiomyocytes of a specified phenotype. The next section addresses the related issue of cell maturation.
5.3. Transcriptional Profiling of hESC-CMs and cellular maturation
Human ESC-CMs generated in vitro and destined for regenerative medicine should faithfully recapitulate the phenotype of adult ventricular cells, especially in relation to electrophysiological and functional development and maturation. Whole-genome expression profiling is one commonly employed approach used to compare and characterize different cell populations. When gene expression analysis is applied aptly, as was done in mouse (Fijnvandraat et al., 2003), it can stage differentiated cells with in vivo heart development to assess the degree of commitment and maturation. The first description of transcriptional profiling of hESC-CMs was reported in 2006 (Beqqali et al., 2006), and since then a number of microarray-based studies have been published (Cao et al., 2008; Gupta et al., 2010; Synnergren et al., 2008a; Synnergren et al., 2008b; Synnergren et al., 2012; Synnergren et al., 2011; Xu et al., 2009). Most of these reports relied on systems with preferential induction of CM differentiation (Beqqali et al., 2006), with partial enrichment of CMs by a Percoll gradient (Cao et al., 2008), or with manual enrichment of CMs by dissection of beating areas (Gupta et al., 2010; Synnergren et al., 2008a; Synnergren et al., 2008b; Synnergren et al., 2012). Only one study has employed a transgene construct utilizing the α-MHC promoter to isolate 99% purified hESC-CMs (Xu et al., 2009), but in no instance, has an analysis of chamber specific (i.e., atrial versus ventricular) hESC-CMs been reported. Molecular profiles have therefore been generated either from a mixed cell population consisting of hESC-CMs and non-CMs or from a mixed hESC-CM population containing atrial, ventricular, nodal and other contracting cardiac-like cells. In parallel, many of the published data include a developmental analysis of fetal or adult heart samples and comparison with time-dependent gene expression patterns as a function of differentiation, but most of these comparisons are complicated by the presence of non-CMs in cardiac biopsies and the use of un-staged samples (mixed and undefined samples from human heart). However, in three studies, developmentally defined heart samples were employed (aged 11-16 (Beqqali et al., 2006),19-21 (Cao et al., 2008), or 33-35 weeks (Synnergren et al., 2012) of development) for transcriptome analyses. These limitations have made it difficult to accurately gauge, based on transcriptomic data, the maturation and developmental states of hESC-CMs.
Despite these shortcomings, whole genome expression analyses have revealed several major findings. The first is that upon differentiation, markers of pluripotency (OCT4, SOX2, NANOG) are decreased, while those involving cardiac development (Brachyury T) and sarcomeric structures (including gene ontology (GO) sub-groups actin, cytoskeleton, myosin and sarcomere) are upregulated. Second, activation of individual genes occurs during distinct but variable stages of differentiation, as cardiac transcription factors and master genes like MEF2C, TBX2 and TBX5 are expressed prior to genes encoding cardiac-restricted structural and functional genes (Beqqali et al., 2006). These findings suggest unique molecular regulatory mechanisms that occur in a lineage- and time-dependent manner. Third, numerous gene expression profiles can be statistically identified that are conserved independently of differentiation protocols (Synnergren et al., 2008a). Fourth, a direct comparison of selected gene ontology (GO) terms between hESC-CMs and both fetal and adult hearts has revealed strong similarities among these cells. Fifth and perhaps most importantly, many of the hESC-CMs transcription profiles identified by microarrays are distinct from CMs generated in vivo. Some of these differences involve genes associated with heart development like BMP2, GATA5 and TGFβ2, which are highly expressed in hESC-CMs. Genes associated with ion transport like RYR2, PLN, KCNH2 show both similarities and differences with fetal and adult heart (Synnergren et al., 2012; Xu et al., 2009), while other genes implicated in cell communication, signal transduction and defense response are abundant in fetal and adult heart but are only poorly expressed in hESC-CMs. While some of these latter differences may be due to the presence of non-cardiac cells in the biopsies, it is also likely that the hESC-CMs have not experienced some in vivo signals necessary for gene regulation.
Based on these data, human ESC-CMs appear to be similar to, but unique from both fetal and adult heart cells. Although it is sometimes difficult to determine whether expression profiles are due to developmental signals or to maturation processes, the poor expression of genes encoding structural and force-generating proteins (eg. MYL2, MYH7, MYL3, MYH11 and TNNT2) in hESC-CMs relative to fetal heart samples suggest an absence of maturation signals in vitro such as the biomechanical stresses that are normally present during in vivo cardiac development. Similarly, GO processes associated with metabolic activity are less prevalent in hESC-CMs, consistent with the need for increased energy needs in forcefully contracting fetal and adult hearts (Cao et al., 2008). In contrast, KCNH2(HERG), the gene encoding a subunit of the voltage-gated potassium channel, is one of several genes that show comparable expression levels among hESC-CMs, fetal heart and adult heart (Xu et al., 2009), and these genes may not be informative for maturation. As these and other gene markers and profiles are examined experimentally, it is likely that some differential expression patterns will explain electrophysiological maturation states and differences in automaticity seen among various hESC-CM cell types. It is also hoped that the development of experimental systems more analogous to in vivo development will foster the maturation of hESC-CMs into therapeutically reliable ventricular cells.
5.4. Functional maturation of hESC-CMs
Maturation of embryonic heart cells during development is regulated by a complex set of spatially and temporally varying cues as discussed above; see also (Epstein, 2010). In culture, hESC-CMs are similar to fetal tissue in terms of their electrophysiological characteristics (Mummery et al., 2003), most notably, low expression of IK1 (Satin et al., 2004), high expression of INCX, and high expression of If. Electrophysiological criteria used for maturation include: hyperpolarization of membrane and increased expression of IK1, expression of HERG and its regulatory subunit MIRP1, reduction of If, and development of the ventricular phenotype (Asai et al., 2010; Caspi et al., 2009). Over time in culture, there is progressive development of ultrastructure, but notably, there is marked absence of the T-tubule system (Lieu et al., 2009; Snir et al., 2003) (although see Section 4.2). Furthermore, SR function is immature in terms of a modest Ca2+ transient and slow kinetics, moderate SERCA expression, absence of junctin, triadin and calsequestrin expression, and poorly organized and low expression of RyR2 (Liu et al., 2007; Satin et al., 2008), although these are not universal findings (see Section 4.2).
The immature state of hESC-CMs raises many issues. Immature cells may not give proper drug responses or serve as disease models faithful to post-natal, much less adult, myocardial cells. Engraftment of these cells into working myocardium could be problematic because of their mismatch in contractile force and electrophysiology with the recipient tissue. Nonuniform contraction might lead to triggered propagated contraction and reverse excitation-contraction coupling (ter Keurs, 2011). Disparity in contractile force can lead to mechanical dyssynchrony, which can cause decreased efficiency of contraction and detrimental mechanical and electrical remodeling (Bilchick et al., 2007; Tedrow et al., 2004). Furthermore, disparity may be accentuated at certain pacing rates, stretch, or rates of shortening, leading to mechanoelectrical feedback and arrhythmia (Tung and Thompson, 2011). Immaturity or abnormalities in calcium cycling can lead to afterdepolarizations and triggered activity (ter Keurs and Boyden, 2007). Mismatch in ion channel expression can lead to spatial gradients in action potential or calcium restitution properties, which can augment dynamic instabilities in electrophysiological function and produce wavebreaks and fibrillation (Weiss et al., 2005), and lead to potential anchoring sites for reentrant arrhythmia (Sekar et al., 2009). Deficient cell-cell coupling can lead to meandering activation wavefronts, discontinuous propagation and, ultimately, conduction failure (Rohr, 2004). Furthermore, by virtue of their electrical coupling to myocardial cells and presentation of abnormal electrical loads, engrafted hESC-CMs may introduce distributed regions of electrical heterogeneity that exacerbate coupling heterogeneities, leading to wavefront breakup and conduction block (Steinberg et al., 2006). On the other hand, hESC-CMs may also provide antiarrhythmic benefit, and were shown to improve conduction and reduce the incidence of reentry in an arrhythmogenic cell culture model (Thompson et al., 2012).
MicroRNAs have been shown to regulate electrophysiological maturation in terms of resting potential, APD and Ca2+ transients (Fu et al., 2011). Alternatively, cellular transplantation studies provide a reference point regarding the maturation of hESC-CMs in a natural but complex tissue environment. These studies are discussed below.
5.4.1. Native biological environment
Transplantation of hESC-CMs into host animal myocardium provides not only the opportunity to assess the therapeutic utility of these cells, but also places them in an environment with a multitude of cues that may be conducive to maturation. Studies are indicative of hESC-CM maturation with transplantation into murine hearts (van Laake et al., 2007; van Laake et al., 2010). At one week post transplantation, hESC-CMs had an immature phenotype indicated by the expression of only MLC2a and not MLC2v, although SMA was downregulated from pre-implantation levels (van Laake et al., 2007). Between 3 and 10 weeks, many cells also became positive for MLC2v until the majority became positive for either MLC2a or MLC2v. During this time period, increasing proportions of hESC-CMs became positive for α-actinin and tropomyosin, and the grafts became more aligned with host myocardium, developed more organized sarcomeres, and exhibited desmosomes and gap junctions. In even longer term grafts of 24 weeks in infarcted murine hearts (van Laake et al., 2010), hESC-CMS show loss of staining for cytokeratin 8 (found in immature hESC-CMs in vitro), increased sarcomeric organization, and numerous desmosomes. On the other hand, hESC-CMs have been shown to secrete collagen XVIII (van Laake et al., 2010), which is present in the fetal heart (Saarela et al., 1998). Transplanted hESC-CMs exhibited an elongated shape, the presence of cadherin and Cx43-positive cellular junctions, and some ANP-positive cells (Cui et al., 2007). Cellular grafts tend to vary in the levels of sarcomere organization present, from immature sarcomeric structures to organized structures similar to native myocardium (Dai et al., 2007). In regions containing hESC-CMs with more immature myofiber organization, positive Cx43 staining was observed within the cytoplasm and along the borders of transplanted cells, similar to that seen in fetal myocardium. In regions with more organized myofibers, clusters of Cx43 signal were present between bordering cells (Dai et al., 2007). Additionally, Cx43 is present at the border between the graft and host myocardium (Caspi et al., 2007a; Dai et al., 2007; Gepstein et al., 2010; Kehat et al., 2004), with more organized clusters of immunoreactivity observed when the graft consists of regions with more organized myofibrillar structure (Dai et al., 2007).
The finding that transplanted hESC-CMs increase their structural organization over time but do not attain fully mature sarcomeres has also been shown with hESC-CMs injected into chronically infarcted rat hearts (Fernandes et al., 2010). The immature phenotype of hESC-CMs was further demonstrated by the findings that transplanted cells were still proliferating 3 months after transplantation. Positive cadherin staining was present between transplanted hESC-CMs and, while in some cases it was present close to the border between the transplanted cells and host myocardium, in most cases hESC-CMs and host CMs were separated by scar tissue.
Cardiac tissue slices offer an attractive in vitro model for hESC-CM maturation since they retain the complex environment of the whole heart, provide convenient access to different parts of the tissue, and can be maintained in culture. Undifferentiated hESCs have been transplanted into neonatal rat ventricular slices and differentiated into CMs over 2 months in culture (Habeler et al., 2009). Pluripotency markers OCT4 and NANOG were downregulated over the first week, mesoderm differentiation markers GATA4 and MEF2C increased after day 10 and peaked at around 1 month, and cardiac markers MLC2a, β-MHC and ANP were expressed at 2 months after transplantation. A variety of other in vitro models are suitable assaying the functional interaction between stem cells and cardiomyocytes, including micropatterned cell pairs (Pedrotty et al., 2008) and engineered microtissues discussed in the following section (for a review of these methodologies, see (Bursac et al., 2010)). However, these have yet to be applied to hESC-CMs.
Finally, decellularized matrix can be used to provide the mixture of structural and biochemical cues encountered by cardiomyocytes in vivo. Rat CMs cultured on acellular porcine myocardial matrix infiltrate about 50μm into the scaffold, exhibit an elongated morphology, and develop concerted contractions after several days in culture (Eitan et al., 2010). Furthermore, murine EBs cultured on Matrigel, a basement membrane matrix from mouse sarcoma (Kleinman and Martin, 2005) or Cardiogel, matrix secreted by cardiac fibroblasts, had a greater response to isoproterenol and carbachol than did controls, although the reduction in beating rate with carbachol application was greater in Matrigel than in Cardiogel. Cardiomyocytes of EBs cultured on Cardiogel developed H-bands, M-bands, and T-tubules at earlier timepoints than those cultured on Matrigel or without any extracellular matrix components (Baharvand et al., 2005).
6. Bioengineering the cellular environment
The importance of the microenvironment was reviewed in the previous sections and is also reviewed in (Horton et al., 2009) and (Clause et al., 2010). A broad array of biomedical engineering approaches has been developed to control microenvironment in a rigorous manner (Ghafar-Zadeh et al., 2011) and influence stem cell differentiation (Burdick and Vunjak-Novakovic, 2009). These include the development of bioreactors (Figallo et al., 2007; Kehoe et al., 2010), use of biomaterials within the EB (Bratt-Leal et al., 2009), auxiliary use of hydrogels for cell delivery (Habib et al., 2011), microencapsulaton of cells (Jing et al., 2010), cylindrical and spherical microcarriers for cell attachment and transport (Lecina et al., 2010), and biomaterials as scaffolds (Chen et al., 2008) such as an elastomeric patch (Chen et al., 2010). Biomimetic platforms that recapitulate the mechanical and biochemical signals in the native environment may be effective in promoting maturation in hESC-CMs. To date, a few studies have focused on the effects of individual extracellular factors such as three-dimensional culture, mechanical stimulation, and the influence of non-CMs to augment maturation. These studies are discussed below.
6.1. Three-dimensional culture
The use of biologically-derived extracellular matrix components to surround hESC-CMs in a 3-dimensional culture environment has been shown to increase cellular maturity. Suspension of hESC-CMs within a PEGylated fibrinogen hydrogel resulted in a 3-dimensional network of randomly oriented cells exhibiting Cx43-positive gap junctions and α-sarcomeric actin striations typical of sarcomeric structures (Shapira-Schweitzer et al., 2009), although the levels of cell anisotropy and gap junctional connectivity did not reach those of similarly cultured neonatal rat CMs. Furthermore, hydrogels of solubilized porcine extracellular matrix (ECM) and collagen were used to encapsulate hEBs and promoted contractile function of hESC-CMs (Duan et al., 2011). A mixture of 75% ECM and 25% collagen increased the percent beating area and contraction amplitude of hEBs when compared to hydrogels with 25% ECM and 75% collagen or hydrogels with 100% collagen. Furthermore, cTnT expression in hEBs cultured in 75% ECM hydrogels was comparable to that in hEBs cultured in 100% collagen hydrogels supplemented with growth factors. Interestingly, the addition of growth factors to hydrogels containing ECM did not enhance differentiation, and only cells cultured in ECM hydrogels without growth factors exhibited mature cTnI striation patterns. Furthermore, Cx43 staining was most organized in 75% ECM cultures without supplemental growth factors and was localized both at the periphery of cells and at perinuclear sites. Thus, hydrogels containing a high percentage of native ECM were able to promote hEB differentiation and maturation without the addition of growth factors (Duan et al., 2011). Additionally, dissociated hEBs in a fibrinogen and thrombin gel have been used in the creation of engineered heart tissues (EHTs) capable of promoting more developed sarcomeric organization, Cx43 expression, and increased cellular alignment (Schaaf et al., 2011).
Synchronous beating has been observed in hydrogel-based hESC-CM constructs (Shapira-Schweitzer et al., 2009), with isoproterenol increasing and carbamylcholine decreasing the rate of contraction (Shapira-Schweitzer et al., 2009). However, contraction of these constructs had a lower average displacement amplitude than that of similar constructs which used neonatal rat CMs. Force of contraction in EHTs showed a trend toward increase with the addition of isoprenaline that could be reversed with the application of carbachol, and lowering extracellular calcium decreased the force (Schaaf et al., 2011). Increased maturation of hESC-CMs in EHTs compared with hEBs was indicated by increased levels of β-myosin heavy chain (MHC) transcripts (Schaaf et al., 2011). APDs of hESC-CMs in EHTs were approximately twice as long as those of hEB-cultured hESC-CMs, but varied greatly from 260 to 1200 ms, with longer APs having an increased upstroke velocity. However, maximum diastolic potential (MDP) was more positive in EHT hESC-CMs than in hEBs (Schaaf et al., 2011).
Porous scaffolds are another means of culturing hESC-CMs in a 3-dimensional environment (Caspi et al., 2007b; Lesman et al., 2010). hESC-CMs seeded onto scaffolds of poly-L-lactic acid and polylactic-glycolic acid varied in their levels of structural development (Caspi et al., 2007b). More mature hESC-CMs were characterized by increased cell alignment, abundant myofibrils in Z bands characteristic of organized sarcomeres, mitochondria surrounding the sarcomeres, developing T-tubules, and intercalated discs containing desmosomes and gap junctions. However, areas of immature hESC-CMs were also present (Caspi et al., 2007b). Spontaneous contractions of hESC-CMs within the construct were observed, and synchronous intracellular Ca2+ transients were visualized using laser confocal imaging. Additionally, hESC-CMs responded to positive and negative chronotropic agents.
6.2. Mechanical stimulation and topographic cues
The application of mechanical stress to hESC-CMs in 3-dimensional culture has been shown to influence cellular architecture, proliferation, and maturation (Tulloch et al., 2011). Constructs of hESC-CMs in a 3-dimensional type I collagen gel that were conditioned by uniaxial cyclic stress at 1Hz or static stress had more organized collagen fibers and increased cellular sarcomeric alignment than unconditioned constructs. However, the contractile, cytoskeletal and extracellular matrix organization were less developed than those of similar constructs using neonatal rat CMs. In addition, a combination of stress conditioning and endothelial cell coculture increased hESC-CM proliferation, and 3 weeks of static stress conditioning resulted in an up to 8-fold increase in active force when hESC-CM constructs were stretched up to 60% over slack length. The effects of modulating the frequency of applied stretch has been studied in murine ESC-CMs embedded in a type I collagen gel and stretched to 10% of their initial length (Shimko and Claycomb, 2008). Mechanical loading at 1Hz decreased cardiac gene expression while loading at 3Hz increased gene expression, sarcomeric organization, and gap junction formation.
Other approaches have attempted to incorporate topographical and mechanical cues (for a review of mechanotransduction in cardiomyocytes see (McCain and Parker, 2011)). Wrinkled PDMS substrates meant to mimic the heart's anisotropic architecture and accompanying mechanical cues are one such approach (Luna et al., 2011). hESC-CMs cultured on these substrates had organized sarcomeric structure and were able to align themselves with the substrate's principal axis. In the development of such substrates, the elasticity of engineered substrates is an important consideration and should be matched to that of the developing myocardium, as it influences CM beating, spreading, and changes in structural proteins (Engler et al., 2008).
Mechanical cues have also been imparted by devices incorporating hydrodynamic shear. Micro-bioreactor arrays can flow media directly over a culture of cells or in a plane above the cultured cells for reduced shear (Figallo et al., 2007). Although this device has been used only for vascular differentiation of 3-dimensional cultures of hESCs (Figallo et al., 2007), it may prove to be beneficial for cardiac differentiation as well.
6.3. Non-cardiomyocyte influences
Coculture of hESCs with cells from a mouse visceral-endoderm-like cell line, END-2, is known to instruct the differentiation of the hESCs into hESC-CMs and is part of many hESC-CM differentiation protocols (Mummery et al., 2003). The influence of non-CMs on the electrophysiological maturation of hESC-CMs has been studied using puromycin-selected hESC-CMs from early-stage (20 days or less) and late-stage (60 day) hEBs (Kim et al., 2010). In purified populations, application of the HCN channel blocker ZD7288 or the INa channel blocker TTX reduced beating rate to a greater extent in late-stage hESC-CMs than in early-stage hESC-CMs. Beating rate slowing in isolated early-stage hESC-CMs was not altered even if the hESC-CMs were cultured for an additional 40 days. Likewise, transcripts of NaV1.5, NaV1.1, CaV3.1, and Kir2.2 were increased in hESC-CMs from late-stage EBs compared with those selected from early-stage EBs and cultured for an equivalent amount of time. However, when hESC-CMs selected from early EBs were subsequently co-cultured with non-CMs from parental hESCs, beating rate slowing with the application of ZD7288 or TTX was observed and ion channel transcript levels were partially restored. This result suggests that non-CMs can influence the development of HCN and Na+ channel-dependent automaticity and of select ion channels in early-stage hESC-CMs. Furthermore, later-stage hESC-CMs could be stratified into an immature group and a more mature group exhibiting more negative MDP, larger AP amplitude, and faster Vmax. Early-stage hESC-CMs did not show such distinct populations unless in co-culture, corroborating the importance of non-CMs in the electrophysiological maturation of hESC-CMs.
Non-CMs have been combined with synthetic scaffolds in an effort to engineer environments that more closely recapitulate native myocardium. hESC-CMs were seeded onto porous scaffolds of poly-L-lactic acid (PLLA) and polylactic-glycolic acid, and the effects of various combinations of co-culture with human umbilical vein endothelial cells (HUVECs), hESC-derived endothelial cells (hESC-ECs), or murine embryonic fibroblasts (mEFs) were assessed (Caspi et al., 2007b; Lesman et al., 2010). The presence of HUVECs in scaffolds increased CM proliferation, while co-cultures of hESC-CMs with either HUVECs or hESC-ECs and tri-cultures of hESC-CMs with mEFs and either HUVECs or hESC-ECs upregulated both more mature (α-MHC and cTnI) and immature (atrial natriuretic factor and Nkx2.5 gene expression) CM markers (Caspi et al., 2007b). hESC-CMs co-seeded in scaffolds with hESC-ECs or HUVECs exhibit synchronous beating and intracellular Ca2+ transients along with the ability to respond to positive and negative chronotropic agents (Lesman et al., 2010). Active contraction, enhanced proliferation, and expression of β-MHC were also evident in similar tri-cell patches cultured without the scaffold. However, these constructs also expressed Nkx2.5, indicating their immature phenotype (Stevens et al., 2009).
6.4. Cell culture environment
The protocols and devices used to culture hESC-CMs are an important consideration since they incorporate different sets of cues and could potentially produce variations in cellular maturity. Numerous studies have indicated that hESC lines themselves have varying differentiation biases (Osafune et al., 2008; Pekkanen-Mattila et al., 2009), and there has been great interest in optimizing the culture of these cells for a specific lineage. The method of culture was found to be a significant factor, with increased expression of CM genetic markers when hEBs were cultured in suspension (Otsuji et al., 2010). Furthermore, combining suspension culture with replating can advance maturation, especially in pacemaker hESC-CMs (Otsuji et al., 2010). It is possible that cell lines biased toward CM differentiation could yield the most mature CMs when combined with an optimal CM differentiation protocol and culture conditions, although this is unknown at this time.
Platforms from synthetic materials show promise as potential culturing environments that allow for cardiac differentiation. Synthetic peptide-acrylate surfaces (PAS) incorporating biologically active peptides derived from extracellular matrix proteins have been developed (Melkoumian et al., 2010). hESCs cultured on PAS maintained pluripotency for over ten passages and could be differentiated into hESC-CMs.
A variety of engineering strategies employed with different cell types could also prove influential in maturing hESC-CMs. Hydrogels with confined 3-dimensional microarchitecture (Aubin et al., 2010) and patterned extracellular matrix islands of various shapes (Bray et al., 2008) have been shown to influence cellular elongation and alignment, as well as intracellular architecture, respectively.
Manipulation of the hEB itself has also yielded numerous culturing strategies (for a thorough review on EB engineering strategies, see (Bratt-Leal et al., 2009)). One such strategy employed by several investigators is control of hEB size (Mohr et al., 2010; Niebruegge et al., 2009), which has been demonstrated to affect cardiac differentiation (Mohr et al., 2010). One approach has been to use a 3-dimensional cuboidal microwell system to culture cell aggregates of varying dimensions and create uniformly-sized hEBs (Mohr et al., 2010). Intermediate 300μm microwells generated the highest percentage of contracting hEBs while the smallest 100μm microwells yielded the greatest percentage of cells that were CMs in contracting EBs. hEB size control has also been implemented with a micropatterned system incorporating dynamic stirred suspension culture and hypoxic conditions to increase cell output more than 3-fold (Niebruegge et al., 2009). Additionally, microfluidic trap systems show promise for providing tunable hEB growth conditions (Khoury et al., 2010).
7. Conclusion
A multitude of studies have characterized the electrophysiology and contractility of hESC-CMs in light of their great promise for studies of human myocardial function, toxicity testing, drug discovery and cardiac regeneration. In general, hESC-CMs possess intracellular structure, action potential, contractility, drug responses and intercellular coupling characteristic comparable to normal fetal tissue. Some aspects of the physiological function of hESC-CMs are now emerging, such as restitution of action potential duration, rate-staircase of contraction, intracellular calcium cycling and excitation-contraction coupling, but other aspects such as conduction velocity restitution, force-length and force-velocity relations are currently unknown. One major hurdle for the practical use of these cells is the substantial heterogeneity in their electrophysiological phenotype that occurs with current differentiation protocols. Our optical mapping data presented in this article show heterogeneity in AP shape among different hEBs that is much greater than that observed within individual hEBs. Another important obstacle is the present immaturity of the functional state of hESC-CMs, as evidenced by studies of myofibrillar organization, ion channel function, calcium cycling and contractile properties. Given that the properties of hESC-CMs vary widely with cell line, differentiation time, and culturing conditions, improved methods for the purification and selection of specific phenotypes from this heterogeneous population are needed. Bioengineering approaches to control the microenvironment, including cell culture devices, biomaterials, and direct manipulation of the hEB, show promise for achieving these goals.
Acknowledgments
Support for this work was provided by Maryland Stem Cell Research Fund grants 2008-MSCRFE-0084-00 (LT), 2011-MSCRFII-0008-00 (ETZ) and 2008-MSCRFII-0379-00 (ETZ), NIH grants S10 RR025544 (LT), R21 HL108210 (LT), U01HL099775 (ETZ) and U01HL100397 (ETZ), and the NIH NIA Intramural Research Program (KRB).
Footnotes
Note: Usage of the term embryoid body varies widely in the literature, ranging from natural aggregates of cells that co-assemble in hanging drops or other microenvironments, to dissected beating areas of the natural aggregates following plating on a dish. To avoid the clutter of detail, we have chosen to use the term loosely in this article to include both definitions, although in most cases it tends to be the latter. However, we will explicitly distinguish experiments on single hESC-CMs, hESC-CM monolayers and small cell aggregates of hESC-CMs.
Editors' Note: Please see also related communications in this issue by AUTHOR-1 et al. (2012) and AUTHOR-2 et al. (2012)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdul Kadir SH, Ali NN, Mioulane M, Brito-Martins M, Abu-Hayyeh S, Foldes G, Moshkov AV, Williamson C, Harding SE, Gorelik J. Embryonic stem cell-derived cardiomyocytes as a model to study fetal arrhythmia related to maternal disease. J Cell Mol Med. 2009;13:3730–41. doi: 10.1111/j.1582-4934.2009.00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D, Self T, Mellor IR, Goh G, Hill SJ, Denning C. Transgenic enrichment of cardiomyocytes from human embryonic stem cells. Mol Ther. 2007;15:2027–36. doi: 10.1038/sj.mt.6300303. [DOI] [PubMed] [Google Scholar]
- Apati A, Paszty K, Erdei Z, Szebeny K, Homolya L, Sarkadi B. Calcium signaling in pluripotent stem cells. Mol Cell Endocrinol. 2012;353:57–67. doi: 10.1016/j.mce.2011.08.038. [DOI] [PubMed] [Google Scholar]
- Asai Y, Tada M, Otsuji TG, Nakatsuji N. Combination of functional cardiomyocytes derived from human stem cells and a highly-efficient microelectrode array system: an ideal hybrid model assay for drug development. Curr Stem Cell Res Ther. 2010;5:227–32. doi: 10.2174/157488810791824502. [DOI] [PubMed] [Google Scholar]
- Aubin H, Nichol JW, Hutson CB, Bae H, Sieminski AL, Cropek DM, Akhyari P, Khademhosseini A. Directed 3D cell alignment and elongation in microengineered hydrogels. Biomaterials. 2010;31:6941–6951. doi: 10.1016/j.biomaterials.2010.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi S, Matthews DL, Li RA, Chiamvimonvat N, Lieu DK, Chan JW. Label-free identification and characterization of human pluripotent stem cell-derived cardiomyocytes using second harmonic generation (SHG) microscopy. J Biophotonics. 2012;5:57–66. doi: 10.1002/jbio.201100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baharvand H, Azarnia M, Parivar K, Ashtiani SK. The effect of extracellular matrix on embryonic stem cell-derived cardiomyocytes. J Mol Cell Cardiol. 2005;38:495–503. doi: 10.1016/j.yjmcc.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Battista S, Guarnieri D, Borselli C, Zeppetelli S, Borzacchiello A, Mayol L, Gerbasio D, Keene DR, Ambrosio L, Netti PA. The effect of matrix composition of 3D constructs on embryonic stem cell differentiation. Biomaterials. 2005;26:6194–207. doi: 10.1016/j.biomaterials.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Beqqali A, Kloots J, Ward-van Oostwaard D, Mummery C, Passier R. Genome-wide transcriptional profiling of human embryonic stem cells differentiating to cardiomyocytes. Stem Cells. 2006;24:1956–1967. doi: 10.1634/stemcells.2006-0054. [DOI] [PubMed] [Google Scholar]
- Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- Bettiol E, Sartiani L, Chicha L, Krause KH, Cerbai E, Jaconi ME. Fetal bovine serum enables cardiac differentiation of human embryonic stem cells. Differentiation. 2007;75:669–81. doi: 10.1111/j.1432-0436.2007.00174.x. [DOI] [PubMed] [Google Scholar]
- Bilchick KC, Helm RH, Kass DA. Physiology of biventricular pacing. Curr Cardiol Rep. 2007;9:358–65. doi: 10.1007/BF02938362. [DOI] [PubMed] [Google Scholar]
- Binah O, Dolnikov K, Sadan O, Shilkrut M, Zeevi-Levin N, Amit M, Danon A, Itskovitz-Eldor J. Functional and developmental properties of human embryonic stem cells-derived cardiomyocytes. J Electrocardiol. 2007;40:S192–6. doi: 10.1016/j.jelectrocard.2007.05.035. [DOI] [PubMed] [Google Scholar]
- Boudou T, Legant WR, Mu A, Borochin MA, Thavandiran N, Radisic M, Zandstra PW, Epstein JA, Margulies KB, Chen CS. A Microfabricated Platform to Measure and Manipulate the Mechanics of Engineered Cardiac Microtissues. Tissue Eng Part A. 2011 doi: 10.1089/ten.tea.2011.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braam SR, Mummery CL. Human stem cell models for predictive cardiac safety pharmacology. Stem Cell Res. 2010;4:155–6. doi: 10.1016/j.scr.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Braam SR, Tertoolen L, van de Stolpe A, Meyer T, Passier R, Mummery CL. Prediction of drug-induced cardiotoxicity using human embryonic stem cell-derived cardiomyocytes. Stem Cell Res. 2010;4:107–16. doi: 10.1016/j.scr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Bratt-Leal AM, Carpenedo RL, McDevitt TC. Engineering the embryoid body microenvironment to direct embryonic stem cell differentiation. Biotechnol Prog. 2009;25:43–51. doi: 10.1002/btpr.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray MA, Sheehy SP, Parker KK. Sarcomere alignment is regulated by myocyte shape. Cell Motil Cytoskeleton. 2008;65:641–51. doi: 10.1002/cm.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito-Martins M, Harding SE, Ali NN. beta(1)- and beta(2)-adrenoceptor responses in cardiomyocytes derived from human embryonic stem cells: comparison with failing and non-failing adult human heart. Br J Pharmacol. 2008;153:751–9. doi: 10.1038/sj.bjp.0707619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick JA, Vunjak-Novakovic G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng Part A. 2009;15:205–19. doi: 10.1089/ten.tea.2008.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge PW, Keller G, Gold JD, Wu JC. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell. 2012;10:16–28. doi: 10.1016/j.stem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge PW, Thompson S, Millrod MA, Weinberg S, Yuan X, Peters A, Mahairaki V, Koliatsos VE, Tung L, Zambidis ET. A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PLoS One. 2011;6:e18293. doi: 10.1371/journal.pone.0018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursac N, Kirkton RD, McSpadden LC, Liau B. Characterizing functional stem cell-cardiomyocyte interactions. Regen Med. 2010;5:87–105. doi: 10.2217/rme.09.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, Wagner RA, Wilson KD, Xie X, Fu JD, Drukker M, Lee A, Li RA, Gambhir SS, Weissman IL, Robbins RC, Wu JC. Transcriptional and functional profiling of human embryonic stem cell-derived cardiomyocytes. PLoS One. 2008;3:e3474. doi: 10.1371/journal.pone.0003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capi O, Gepstein L. Myocardial regeneration strategies using human embryonic stem cell-derived cardiomyocytes. J Control Release. 2006;116:211–8. doi: 10.1016/j.jconrel.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, Yankelson L, Aronson D, Beyar R, Gepstein L. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007a;50:1884–93. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- Caspi O, Itzhaki I, Kehat I, Gepstein A, Arbel G, Huber I, Satin J, Gepstein L. In vitro electrophysiological drug testing using human embryonic stem cell derived cardiomyocytes. Stem Cells Dev. 2009;18:161–72. doi: 10.1089/scd.2007.0280. [DOI] [PubMed] [Google Scholar]
- Caspi O, Lesman A, Basevitch Y, Gepstein A, Arbel G, Habib IH, Gepstein L, Levenberg S. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res. 2007b;100:263–72. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- Chan CK, Rolle MW, Potter-Perigo S, Braun KR, Van Biber BP, Laflamme MA, Murry CE, Wight TN. Differentiation of cardiomyocytes from human embryonic stem cells is accompanied by changes in the extracellular matrix production of versican and hyaluronan. J Cell Biochem. 2010;111:585–96. doi: 10.1002/jcb.22744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavakis E, Koyanagi M, Dimmeler S. Enhancing the outcome of cell therapy for cardiac repair: progress from bench to bedside and back. Circulation. 2010;121:325–35. doi: 10.1161/CIRCULATIONAHA.109.901405. [DOI] [PubMed] [Google Scholar]
- Chen QZ, Harding SE, N AN, Lyon AR, Boccaccini AR. Biomaterials in cardiac tissue engineering: ten years of research survey. Mat Sci Eng R. 2008;59:1–37. [Google Scholar]
- Chen QZ, Ishii H, Thouas GA, Lyon AR, Wright JS, Blaker JJ, Chrzanowski W, Boccaccini AR, Ali NN, Knowles JC, Harding SE. An elastomeric patch derived from poly(glycerol sebacate) for delivery of embryonic stem cells to the heart. Biomaterials. 2010;31:3885–93. doi: 10.1016/j.biomaterials.2010.01.108. [DOI] [PubMed] [Google Scholar]
- Chiriac A, Terzic A, Park S, Ikeda Y, Faustino R, Nelson TJ. SDF-1-enhanced cardiogenesis requires CXCR4 induction in pluripotent stem cells. J Cardiovasc Transl Res. 2010;3:674–82. doi: 10.1007/s12265-010-9219-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clause KC, Liu LJ, Tobita K. Directed stem cell differentiation: the role of physical forces. Cell Commun Adhes. 2010;17:48–54. doi: 10.3109/15419061.2010.492535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codina M, Elser J, Margulies KB. Current status of stem cell therapy in heart failure. Curr Cardiol Rep. 2010;12:199–208. doi: 10.1007/s11886-010-0098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Johkura K, Takei S, Ogiwara N, Sasaki K. Structural differentiation, proliferation, and association of human embryonic stem cell-derived cardiomyocytes in vitro and in their extracardiac tissues. J Struct Biol. 2007;158:307–17. doi: 10.1016/j.jsb.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Dai W, Field LJ, Rubart M, Reuter S, Hale SL, Zweigerdt R, Graichen RE, Kay GL, Jyrala AJ, Colman A, Davidson BP, Pera M, Kloner RA. Survival and maturation of human embryonic stem cell-derived cardiomyocytes in rat hearts. J Mol Cell Cardiol. 2007;43:504–16. doi: 10.1016/j.yjmcc.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Kloner RA. Myocardial regeneration by embryonic stem cell transplantation: present and future trends. Expert Rev Cardiovasc Ther. 2006;4:375–83. doi: 10.1586/14779072.4.3.375. [DOI] [PubMed] [Google Scholar]
- Davis RP, van den Berg CW, Casini S, Braam SR, Mummery CL. Pluripotent stem cell models of cardiac disease and their implication for drug discovery and development. Trends Mol Med. 2011;17:475–84. doi: 10.1016/j.molmed.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Dolnikov K, Shilkrut M, Zeevi-Levin N, Danon A, Gerecht-Nir S, Itskovitz-Eldor J, Binah O. Functional properties of human embryonic stem cell-derived cardiomyocytes. Ann N Y Acad Sci. 2005;1047:66–75. doi: 10.1196/annals.1341.006. [DOI] [PubMed] [Google Scholar]
- Dolnikov K, Shilkrut M, Zeevi-Levin N, Gerecht-Nir S, Amit M, Danon A, Itskovitz-Eldor J, Binah O. Functional properties of human embryonic stem cell-derived cardiomyocytes: intracellular Ca2+ handling and the role of sarcoplasmic reticulum in the contraction. Stem Cells. 2006;24:236–45. doi: 10.1634/stemcells.2005-0036. [DOI] [PubMed] [Google Scholar]
- Drouin E, Charpentier F, Gauthier C, Laurent K, Le Marec H. Electrophysiologic characteristics of cells spanning the left ventricular wall of human heart: evidence for presence of M cells. J Am Coll Cardiol. 1995;26:185–92. doi: 10.1016/0735-1097(95)00167-x. [DOI] [PubMed] [Google Scholar]
- Duan Y, Liu Z, O'Neill J, Wan LQ, Freytes DO, Vunjak-Novakovic G. Hybrid gel composed of native heart matrix and collagen induces cardiac differentiation of human embryonic stem cells without supplemental growth factors. J Cardiovasc Transl Res. 2011;4:605–15. doi: 10.1007/s12265-011-9304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois NC, Craft AM, Sharma P, Elliott DA, Stanley EG, Elefanty AG, Gramolini A, Keller G. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nat Biotechnol. 2011;29:1011–8. doi: 10.1038/nbt.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner DA, Kashimura T, O'Neill SC, Venetucci LA, Trafford AW. What role does modulation of the ryanodine receptor play in cardiac inotropy and arrhythmogenesis? J Mol Cell Cardiol. 2009;46:474–81. doi: 10.1016/j.yjmcc.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Eitan Y, Sarig U, Dahan N, Machluf M. Acellular cardiac extracellular matrix as a scaffold for tissue engineering: In-vitro cell support, remodeling and biocompatibility. Tissue Eng Part C Methods. 2010;16:671–83. doi: 10.1089/ten.TEC.2009.0111. [DOI] [PubMed] [Google Scholar]
- Endoh M. Force-frequency relationship in intact mammalian ventricular myocardium: physiological and pathophysiological relevance. European Journal of Pharmacology. 2004;500:73–86. doi: 10.1016/j.ejphar.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY, Speicher DW, Sanger JW, Sanger JM, Discher DE. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci. 2008;121:3794–802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JA. Franklin H. Epstein Lecture. Cardiac development and implications for heart disease. N Engl J Med. 2010;363:1638–47. doi: 10.1056/NEJMra1003941. [DOI] [PubMed] [Google Scholar]
- Fernandes S, Naumova AV, Zhu WZ, Laflamme MA, Gold J, Murry CE. Human embryonic stem cell-derived cardiomyocytes engraft but do not alter cardiac remodeling after chronic infarction in rats. J Mol Cell Cardiol. 2010;49:941–9. doi: 10.1016/j.yjmcc.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figallo E, Cannizzaro C, Gerecht S, Burdick JA, Langer R, Elvassore N, Vunjak-Novakovic G. Micro-bioreactor array for controlling cellular microenvironments. Lab Chip. 2007;7:710–9. doi: 10.1039/b700063d. [DOI] [PubMed] [Google Scholar]
- Fijnvandraat AC, van Ginneken ACG, de Boer PAJ, Ruijter JM, Christoffels VM, Moorman AFM, Deprez RHL. Cardiomyocytes derived from embryonic stem cells resemble cardiomyocytes of the embryonic heart tube. Cardiovascular Research. 2003;58:399–409. doi: 10.1016/s0008-6363(03)00282-7. [DOI] [PubMed] [Google Scholar]
- Flanagan LA, Lu J, Wang L, Marchenko SA, Jeon NL, Lee AP, Monuki ES. Unique dielectric properties distinguish stem cells and their differentiated progeny. Stem Cells. 2008;26:656–65. doi: 10.1634/stemcells.2007-0810. [DOI] [PubMed] [Google Scholar]
- Foldes G, Mioulane M, Wright JS, Liu AQ, Novak P, Merkely B, Gorelik J, Schneider MD, Ali NN, Harding SE. Modulation of human embryonic stem cell-derived cardiomyocyte growth: a testbed for studying human cardiac hypertrophy? J Mol Cell Cardiol. 2011;50:367–76. doi: 10.1016/j.yjmcc.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz MR. The electrical restitution curve revisited: steep or flat slope--which is better? J Cardiovasc Electrophysiol. 2003;14:S140–7. doi: 10.1046/j.1540.8167.90303.x. [DOI] [PubMed] [Google Scholar]
- Fu JD, Jiang P, Rushing S, Liu J, Chiamvimonvat N, Li RA. Na+/Ca2+ exchanger is a determinant of excitation-contraction coupling in human embryonic stem cell-derived ventricular cardiomyocytes. Stem Cells Dev. 2010;19:773–82. doi: 10.1089/scd.2009.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu JD, Rushing SN, Lieu DK, Chan CW, Kong CW, Geng L, Wilson KD, Chiamvimonvat N, Boheler KR, Wu JC, Keller G, Hajjar RJ, Li RA. Distinct roles of microRNA-1 and -499 in ventricular specification and functional maturation of human embryonic stem cell-derived cardiomyocytes. PLoS One. 2011;6:e27417. doi: 10.1371/journal.pone.0027417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo P, Grimaldi S, Latronico MV, Bonci D, Pagliuca A, Ausoni S, Peschle C, Condorelli G. A lentiviral vector with a short troponin-I promoter for tracking cardiomyocyte differentiation of human embryonic stem cells. Gene Ther. 2008;15:161–70. doi: 10.1038/sj.gt.3303017. [DOI] [PubMed] [Google Scholar]
- Gaur M, Ritner C, Sievers R, Pedersen A, Prasad M, Bernstein HS, Yeghiazarians Y. Timed inhibition of p38MAPK directs accelerated differentiation of human embryonic stem cells into cardiomyocytes. Cytotherapy. 2010;12:807–17. doi: 10.3109/14653249.2010.491821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepstein L. Cardiovascular therapeutic aspects of cell therapy and stem cells. Ann N Y Acad Sci. 2006;1080:415–25. doi: 10.1196/annals.1380.030. [DOI] [PubMed] [Google Scholar]
- Gepstein L, Ding C, Rahmutula D, Wilson EE, Yankelson L, Caspi O, Gepstein A, Huber I, Olgin JE. In vivo assessment of the electrophysiological integration and arrhythmogenic risk of myocardial cell transplantation strategies. Stem Cells. 2010;28:2151–61. doi: 10.1002/stem.545. [DOI] [PubMed] [Google Scholar]
- Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:11298–303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germanguz I, Sedan O, Zeevi-Levin N, Shtrichman R, Barak E, Ziskind A, Eliyahu S, Meiry G, Amit M, Itskovitz-Eldor J, Binah O. Molecular characterization and functional properties of cardiomyocytes derived from human inducible pluripotent stem cells. J Cell Mol Med. 2011;15:38–51. doi: 10.1111/j.1582-4934.2009.00996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghafar-Zadeh E, Waldeisen JR, Lee LP. Engineered approaches to the stem cell microenvironment for cardiac tissue regeneration. Lab Chip. 2011;11:3031–48. doi: 10.1039/c1lc20284g. [DOI] [PubMed] [Google Scholar]
- Gherghiceanu M, Barad L, Novak A, Reiter I, Itskovitz-Eldor J, Binah O, Popescu LM. Cardiomyocytes derived from human embryonic and induced pluripotent stem cells: comparative ultrastructure. J Cell Mol Med. 2011;15:2539–51. doi: 10.1111/j.1582-4934.2011.01417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh G, Self T, Barbadillo Munoz MD, Hall IP, Young L, Denning C. Molecular and phenotypic analyses of human embryonic stem cell-derived cardiomyocytes: opportunities and challenges for clinical translation. Thromb Haemost. 2005;94:728–37. doi: 10.1160/TH05-04-0268. [DOI] [PubMed] [Google Scholar]
- Graichen R, Xu X, Braam SR, Balakrishnan T, Norfiza S, Sieh S, Soo SY, Tham SC, Mummery C, Colman A, Zweigerdt R, Davidson BP. Enhanced cardiomyogenesis of human embryonic stem cells by a small molecular inhibitor of p38 MAPK. Differentiation. 2008;76:357–70. doi: 10.1111/j.1432-0436.2007.00236.x. [DOI] [PubMed] [Google Scholar]
- Grey C, Mery A, Puceat M. Fine-tuning in Ca2+ homeostasis underlies progression of cardiomyopathy in myocytes derived from genetically modified embryonic stem cells. Hum Mol Genet. 2005;14:1367–77. doi: 10.1093/hmg/ddi146. [DOI] [PubMed] [Google Scholar]
- Guo L, Abrams RM, Babiarz JE, Cohen JD, Kameoka S, Sanders MJ, Chiao E, Kolaja KL. Estimating the risk of drug-induced proarrhythmia using human induced pluripotent stem cell-derived cardiomyocytes. Toxicol Sci. 2011a;123:281–9. doi: 10.1093/toxsci/kfr158. [DOI] [PubMed] [Google Scholar]
- Guo L, Qian JY, Abrams R, Tang HM, Weiser T, Sanders MJ, Kolaja KL. The electrophysiological effects of cardiac glycosides in human iPSC-derived cardiomyocytes and in guinea pig isolated hearts. Cell Physiol Biochem. 2011b;27:453–62. doi: 10.1159/000329966. [DOI] [PubMed] [Google Scholar]
- Gupta MK, Illich DJ, Gaarz A, Matzkies M, Nguemo F, Pfannkuche K, Liang H, Classen S, Reppel M, Schultze JL, Hescheler J, Sarić T. Global transcriptional profiles of beating clusters derived from human induced pluripotent stem cells and embryonic stem cells are highly similar. BMC Dev Biol. 2010;10:98. doi: 10.1186/1471-213X-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeler W, Pouillot S, Plancheron A, Puceat M, Peschanski M, Monville C. An in vitro beating heart model for long-term assessment of experimental therapeutics. Cardiovasc Res. 2009;81:253–9. doi: 10.1093/cvr/cvn299. [DOI] [PubMed] [Google Scholar]
- Habib M, Caspi O, Gepstein L. Human embryonic stem cells for cardiomyogenesis. J Mol Cell Cardiol. 2008;45:462–74. doi: 10.1016/j.yjmcc.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Habib M, Shapira-Schweitzer K, Caspi O, Gepstein A, Arbel G, Aronson D, Seliktar D, Gepstein L. A combined cell therapy and in-situ tissue-engineering approach for myocardial repair. Biomaterials. 2011;32:7514–23. doi: 10.1016/j.biomaterials.2011.06.049. [DOI] [PubMed] [Google Scholar]
- Halbach M, Egert U, Hescheler J, Banach K. Estimation of action potential changes from field potential recordings in multicellular mouse cardiac myocyte cultures, Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2003;13:271–84. doi: 10.1159/000074542. [DOI] [PubMed] [Google Scholar]
- Hamlin RL. Animal models of ventricular arrhythmias. Pharmacol Ther. 2007;113:276–95. doi: 10.1016/j.pharmthera.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Hattori F, Chen H, Yamashita H, Tohyama S, Satoh YS, Yuasa S, Li W, Yamakawa H, Tanaka T, Onitsuka T, Shimoji K, Ohno Y, Egashira T, Kaneda R, Murata M, Hidaka K, Morisaki T, Sasaki E, Suzuki T, Sano M, Makino S, Oikawa S, Fukuda K. Nongenetic method for purifying stem cell-derived cardiomyocytes. Nat Methods. 2010;7:61–6. doi: 10.1038/nmeth.1403. [DOI] [PubMed] [Google Scholar]
- He JQ, January CT, Thomson JA, Kamp TJ. Human embryonic stem cell-derived cardiomyocytes: drug discovery and safety pharmacology. Expert Opin Drug Discov. 2007;2:739–753. doi: 10.1517/17460441.2.5.739. [DOI] [PubMed] [Google Scholar]
- He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003;93:32–9. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- Horton RE, Millman JR, Colton CK, Auguste DT. Engineering microenvironments for embryonic stem cell differentiation to cardiomyocytes. Regen Med. 2009;4:721–32. doi: 10.2217/rme.09.48. [DOI] [PubMed] [Google Scholar]
- Huber I, Itzhaki I, Caspi O, Arbel G, Tzukerman M, Gepstein A, Habib M, Yankelson L, Kehat I, Gepstein L. Identification and selection of cardiomyocytes during human embryonic stem cell differentiation. Faseb J. 2007;21:2551–63. doi: 10.1096/fj.05-5711com. [DOI] [PubMed] [Google Scholar]
- Itzhaki I, Schiller J, Beyar R, Satin J, Gepstein L. Calcium handling in embryonic stem cell-derived cardiac myocytes: of mice and men. Ann N Y Acad Sci. 2006;1080:207–15. doi: 10.1196/annals.1380.017. [DOI] [PubMed] [Google Scholar]
- Jang J, Ku SY, Kim JE, Choi K, Kim YY, Kim HS, Oh SK, Lee EJ, Cho HJ, Song YH, Lee SH, Suh CS, Kim SH, Moon SY, Choi YM. Notch inhibition promotes human embryonic stem cell-derived cardiac mesoderm differentiation. Stem Cells. 2008;26:2782–90. doi: 10.1634/stemcells.2007-1053. [DOI] [PubMed] [Google Scholar]
- Jing D, Parikh A, Tzanakakis ES. Cardiac cell generation from encapsulated embryonic stem cells in static and scalable culture systems. Cell Transplant. 2010;19:1397–412. doi: 10.3727/096368910X513955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson MK, Duker G, Tropp C, Andersson B, Sartipy P, Vos MA, van Veen TA. Quantified proarrhythmic potential of selected human embryonic stem cell-derived cardiomyocytes. Stem Cell Res. 2010;4:189–200. doi: 10.1016/j.scr.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Jonsson MK, Vos MA, Mirams GR, Duker G, Sartipy P, de Boer TP, van Veen TA. Application of human stem cell-derived cardiomyocytes in safety pharmacology requires caution beyond hERG. J Mol Cell Cardiol. 2012 doi: 10.1016/j.yjmcc.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Kang J, Chen XL, Ji J, Lei Q, Rampe D. Ca++ channel activators reveal differential L-type Ca++ channel pharmacology between native and stem cell-derived cardiomyocytes. J Pharmacol Exp Ther. 2012 doi: 10.1124/jpet.112.192609. [DOI] [PubMed] [Google Scholar]
- Kapucu FE, Pekkanen-Mattila M, Kujala V, Viik J, Aalto-Setela K, Kerkela E, Tanskanen JMA, Hyttinen J. Beating rate variability studies with human embryonic stem cell derived cardiomyocytes. IFMBE Proc. 2008;22:8–11. [Google Scholar]
- Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–40. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Kehat I, Gepstein A, Spira A, Itskovitz-Eldor J, Gepstein L. High-resolution electrophysiological assessment of human embryonic stem cell-derived cardiomyocytes: a novel in vitro model for the study of conduction. Circ Res. 2002;91:659–61. doi: 10.1161/01.res.0000039084.30342.9b. [DOI] [PubMed] [Google Scholar]
- Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407–14. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, Huber I, Satin J, Itskovitz-Eldor J, Gepstein L. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22:1282–9. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- Kehoe DE, Jing D, Lock LT, Tzanakakis ES. Scalable stirred-suspension bioreactor culture of human pluripotent stem cells. Tissue Eng Part A. 2010;16:405–21. doi: 10.1089/ten.tea.2009.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf H, Lecina M, Ting S, Zweigerdt R, Oh S. Distinct regulation of mitogen-activated protein kinase activities is coupled with enhanced cardiac differentiation of human embryonic stem cells. Stem Cell Res. 2011;7:198–209. doi: 10.1016/j.scr.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Khoury M, Bransky A, Korin N, Konak LC, Enikolopov G, Tzchori I, Levenberg S. A microfluidic traps system supporting prolonged culture of human embryonic stem cells aggregates. Biomed Microdevices. 2010;12:1001–8. doi: 10.1007/s10544-010-9454-x. [DOI] [PubMed] [Google Scholar]
- Kim C, Majdi M, Xia P, Wei KA, Talantova M, Spiering S, Nelson B, Mercola M, Chen HS. Non-cardiomyocytes influence the electrophysiological maturation of human embryonic stem cell-derived cardiomyocytes during differentiation. Stem Cells Dev. 2010;19:783–95. doi: 10.1089/scd.2009.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YY, Ku SY, Jang J, Oh SK, Kim HS, Kim SH, Choi YM, Moon SY. Use of long-term cultured embryoid bodies may enhance cardiomyocyte differentiation by BMP2. Yonsei Med J. 2008;49:819–27. doi: 10.3349/ymj.2008.49.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YY, Ku SY, Liu HC, Cho HJ, Oh SK, Moon SY, Choi YM. Cryopreservation of human embryonic stem cells derived-cardiomyocytes induced by BMP2 in serum-free condition. Reprod Sci. 2011;18:252–60. doi: 10.1177/1933719110385130. [DOI] [PubMed] [Google Scholar]
- Kita-Matsuo H, Barcova M, Prigozhina N, Salomonis N, Wei K, Jacot JG, Nelson B, Spiering S, Haverslag R, Kim C, Talantova M, Bajpai R, Calzolari D, Terskikh A, McCulloch AD, Price JH, Conklin BR, Chen HS, Mercola M. Lentiviral vectors and protocols for creation of stable hESC lines for fluorescent tracking and drug resistance selection of cardiomyocytes. PLoS One. 2009;4:e5046. doi: 10.1371/journal.pone.0005046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–86. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Kong CW, Akar FG, Li RA. Translational potential of human embryonic and induced pluripotent stem cells for myocardial repair: insights from experimental models. Thromb Haemost. 2010;104:30–8. doi: 10.1160/TH10-03-0189. [DOI] [PubMed] [Google Scholar]
- Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007a;25:1015–24. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- Laflamme MA, Zbinden S, Epstein SE, Murry CE. Cell-based therapy for myocardial ischemia and infarction: pathophysiological mechanisms. Annu Rev Pathol. 2007b;2:307–39. doi: 10.1146/annurev.pathol.2.010506.092038. [DOI] [PubMed] [Google Scholar]
- Layland J, Kentish JC. Positive force- and [Ca2+]i-frequency relationships in rat ventricular trabeculae at physiological frequencies. Am J Physiol. 1999;276:H9–H18. doi: 10.1152/ajpheart.1999.276.1.H9. [DOI] [PubMed] [Google Scholar]
- Lecina M, Ting S, Choo A, Reuveny S, Oh S. Scalable platform for human embryonic stem cell differentiation to cardiomyocytes in suspended microcarrier cultures. Tissue Eng Part C Methods. 2010;16:1609–19. doi: 10.1089/ten.TEC.2010.0104. [DOI] [PubMed] [Google Scholar]
- Lee YK, Ng KM, Lai WH, Chan YC, Lau YM, Lian Q, Tse HF, Siu CW. Calcium homeostasis in human induced pluripotent stem cell-derived cardiomyocytes. Stem Cell Rev. 2011;7:976–86. doi: 10.1007/s12015-011-9273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leschik J, Stefanovic S, Brinon B, Puceat M. Cardiac commitment of primate embryonic stem cells. Nat Protoc. 2008;3:1381–7. doi: 10.1038/nprot.2008.116. [DOI] [PubMed] [Google Scholar]
- Lesman A, Gepstein L, Levenberg S. Vascularization shaping the heart. Ann N Y Acad Sci. 2010;1188:46–51. doi: 10.1111/j.1749-6632.2009.05082.x. [DOI] [PubMed] [Google Scholar]
- Li SC, Wang L, Jiang H, Acevedo J, Chang AC, Loudon WG. Stem cell engineering for treatment of heart diseases: potentials and challenges. Cell Biol Int. 2009;33:255–67. doi: 10.1016/j.cellbi.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Liang H, Matzkies M, Schunkert H, Tang M, Bonnemeier H, Hescheler J, Reppel M. Human and murine embryonic stem cell-derived cardiomyocytes serve together as a valuable model for drug safety screening. Cell Physiol Biochem. 2010;25:459–66. doi: 10.1159/000303051. [DOI] [PubMed] [Google Scholar]
- Lieu DK, Liu J, Siu CW, McNerney GP, Tse HF, Abu-Khalil A, Huser T, Li RA. Absence of transverse tubules contributes to non-uniform Ca(2+) wavefronts in mouse and human embryonic stem cell-derived cardiomyocytes. Stem Cells Dev. 2009;18:1493–500. doi: 10.1089/scd.2009.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Fu JD, Siu CW, Li RA. Functional sarcoplasmic reticulum for calcium handling of human embryonic stem cell-derived cardiomyocytes: insights for driven maturation. Stem Cells. 2007;25:3038–44. doi: 10.1634/stemcells.2007-0549. [DOI] [PubMed] [Google Scholar]
- Liu J, Lieu DK, Siu CW, Fu JD, Tse HF, Li RA. Facilitated maturation of Ca2+ handling properties of human embryonic stem cell-derived cardiomyocytes by calsequestrin expression. Am J Physiol Cell Physiol. 2009;297:C152–9. doi: 10.1152/ajpcell.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell MJ, Mathur A. Cardiac stem cell therapy: progress from the bench to bedside. Heart. 2010;96:1531–7. doi: 10.1136/hrt.2009.192385. [DOI] [PubMed] [Google Scholar]
- Luna JI, Ciriza J, Garcia-Ojeda ME, Kong M, Herren A, Lieu DK, Li RA, Fowlkes CC, Khine M, McCloskey KE. Multiscale biomimetic topography for the alignment of neonatal and embryonic stem cell-derived heart cells. Tissue Eng Part C Methods. 2011;17:579–88. doi: 10.1089/ten.TEC.2010.0410. [DOI] [PubMed] [Google Scholar]
- Magyar J, Iost N, Kortvely A, Banyasz T, Virag L, Szigligeti P, Varro A, Opincariu M, Szecsi J, Papp JG, Nanasi PP. Effects of endothelin-1 on calcium and potassium currents in undiseased human ventricular myocytes. Pflugers Arch. 2000;441:144–9. doi: 10.1007/s004240000400. [DOI] [PubMed] [Google Scholar]
- Mandel Y, Weissman A, Schick R, Barad L, Novak A, Meiry G, Goldberg S, Lorber A, Rosen MR, Itskovitz-Eldor J, Binah O. Human Embryonic and Induced Pluripotent Stem Cells-Derived Cardiomyocytes Exhibit Beat Rate Variability and Power-Law Behavior. Circulation. 2012 doi: 10.1161/CIRCULATIONAHA.111.045146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandenius CF, Steel D, Noor F, Meyer T, Heinzle E, Asp J, Arain S, Kraushaar U, Bremer S, Class R, Sartipy P. Cardiotoxicity testing using pluripotent stem cell-derived human cardiomyocytes and state-of-the-art bioanalytics: a review. J Appl Toxicol. 2011;31:191–205. doi: 10.1002/jat.1663. [DOI] [PubMed] [Google Scholar]
- McCain ML, Parker KK. Mechanotransduction: the role of mechanical stress, myocyte shape, and cytoskeletal architecture on cardiac function. Pflugers Arch. 2011;462:89–104. doi: 10.1007/s00424-011-0951-4. [DOI] [PubMed] [Google Scholar]
- McCauley MD, Wehrens XH. Animal models of arrhythmogenic cardiomyopathy. Dis Model Mech. 2009;2:563–70. doi: 10.1242/dmm.002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta A, Chung YY, Ng A, Iskandar F, Atan S, Wei H, Dusting G, Sun W, Wong P, Shim W. Pharmacological response of human cardiomyocytes derived from virus-free induced pluripotent stem cells. Cardiovasc Res. 2011;91:577–86. doi: 10.1093/cvr/cvr132. [DOI] [PubMed] [Google Scholar]
- Melkoumian Z, Weber JL, Weber DM, Fadeev AG, Zhou Y, Dolley-Sonneville P, Yang J, Qiu L, Priest CA, Shogbon C, Martin AW, Nelson J, West P, Beltzer JP, Pal S, Brandenberger R. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat Biotechnol. 2010;28:606–10. doi: 10.1038/nbt.1629. [DOI] [PubMed] [Google Scholar]
- Milan DJ, MacRae CA. Animal models for arrhythmias. Cardiovasc Res. 2005;67:426–37. doi: 10.1016/j.cardiores.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Mohr JC, Zhang J, Azarin SM, Soerens AG, de Pablo JJ, Thomson JA, Lyons GE, Palecek SP, Kamp TJ. The microwell control of embryoid body size in order to regulate cardiac differentiation of human embryonic stem cells. Biomaterials. 2010;31:1885–93. doi: 10.1016/j.biomaterials.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JC, Fu J, Chan YC, Lin D, Tran H, Tse HF, Li RA. Distinct cardiogenic preferences of two human embryonic stem cell (hESC) lines are imprinted in their proteomes in the pluripotent state. Biochem Biophys Res Commun. 2008;372:553–8. doi: 10.1016/j.bbrc.2008.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujoo K, Sharin VG, Bryan NS, Krumenacker JS, Sloan C, Parveen S, Nikonoff LE, Kots AY, Murad F. Role of nitric oxide signaling components in differentiation of embryonic stem cells into myocardial cells. Proc Natl Acad Sci U S A. 2008;105:18924–9. doi: 10.1073/pnas.0810230105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, van der Heyden M, Opthof T, Pera M, de la Riviere AB, Passier R, Tertoolen L. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733–40. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- Nalos L, Varkevisser R, Jonsson M, Houtman M, Beekman J, van der Nagel R, Thomsen M, Duker G, Sartipy P, de Boer T, Peschar M, Rook M, van Veen T, van der Heyden M, Vos M. Comparison of I(Kr) blocking drugs Moxifloxacin and Dofetilide/E-4031 in 5 screening models of pro-arrhythmia reveals insufficient specificity of isolated cardiomyocytes. Br J Pharmacol. 2012;165:467–478. doi: 10.1111/j.1476-5381.2011.01558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanthakumar K, Jalife J, Masse S, Downar E, Pop M, Asta J, Ross H, Rao V, Mironov S, Sevaptsidis E, Rogers J, Wright G, Dhopeshwarkar R. Optical mapping of Langendorff-perfused human hearts: establishing a model for the study of ventricular fibrillation in humans. Am J Physiol Heart Circ Physiol. 2007;293:H875–80. doi: 10.1152/ajpheart.01415.2006. [DOI] [PubMed] [Google Scholar]
- Niebruegge S, Bauwens CL, Peerani R, Thavandiran N, Masse S, Sevaptisidis E, Nanthakumar K, Woodhouse K, Husain M, Kumacheva E, Zandstra PW. Generation of human embryonic stem cell-derived mesoderm and cardiac cells using size-specified aggregates in an oxygen-controlled bioreactor. Biotechnol Bioeng. 2009;102:493–507. doi: 10.1002/bit.22065. [DOI] [PubMed] [Google Scholar]
- Norstrom A, Akesson K, Hardarson T, Hamberger L, Bjorquist P, Sartipy P. Molecular and pharmacological properties of human embryonic stem cell-derived cardiomyocytes. Exp Biol Med (Maywood) 2006;231:1753–62. doi: 10.1177/153537020623101113. [DOI] [PubMed] [Google Scholar]
- O'Hara T, Virag L, Varro A, Rudy Y. Simulation of the undiseased human cardiac ventricular action potential: model formulation and experimental validation. PLoS Comput Biol. 2011;7:e1002061. doi: 10.1371/journal.pcbi.1002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osafune K, Caron L, Borowiak M, Martinez RJ, Fitz-Gerald CS, Sato Y, Cowan CA, Chien KR, Melton DA. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol. 2008;26:313–5. doi: 10.1038/nbt1383. [DOI] [PubMed] [Google Scholar]
- Otsuji TG, Minami I, Kurose Y, Yamauchi K, Tada M, Nakatsuji N. Progressive maturation in contracting cardiomyocytes derived from human embryonic stem cells: Qualitative effects on electrophysiological responses to drugs. Stem Cell Res. 2010;4:201–13. doi: 10.1016/j.scr.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Paige SL, Osugi T, Afanasiev OK, Pabon L, Reinecke H, Murry CE. Endogenous Wnt/beta-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PLoS One. 2010;5:e11134. doi: 10.1371/journal.pone.0011134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R, Khanna A. Similar pattern in cardiac differentiation of human embryonic stem cell lines, BG01V and ReliCellhES1, under low serum concentration supplemented with bone morphogenetic protein-2. Differentiation. 2007;75:112–22. doi: 10.1111/j.1432-0436.2006.00123.x. [DOI] [PubMed] [Google Scholar]
- Pascut FC, Goh HT, George V, Denning C, Notingher I. Toward label-free Raman-activated cell sorting of cardiomyocytes derived from human embryonic stem cells. J Biomed Opt. 2011;16:045002. doi: 10.1117/1.3570302. [DOI] [PubMed] [Google Scholar]
- Pedrotty DM, Klinger RY, Badie N, Hinds S, Kardashian A, Bursac N. Structural coupling of cardiomyocytes and noncardiomyocytes: quantitative comparisons using a novel micropatterned cell pair assay. Am J Physiol Heart Circ Physiol. 2008;295:H390–400. doi: 10.1152/ajpheart.91531.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekkanen-Mattila M, Chapman H, Kerkela E, Suuronen R, Skottman H, Koivisto AP, Aalto-Setala K. Human embryonic stem cell-derived cardiomyocytes: demonstration of a portion of cardiac cells with fairly mature electrical phenotype. Exp Biol Med (Maywood) 2010;235:522–30. doi: 10.1258/ebm.2010.009345. [DOI] [PubMed] [Google Scholar]
- Pekkanen-Mattila M, Kerkela E, Tanskanen JM, Pietila M, Pelto-Huikko M, Hyttinen J, Skottman H, Suuronen R, Aalto-Setala K. Substantial variation in the cardiac differentiation of human embryonic stem cell lines derived and propagated under the same conditions--a comparison of multiple cell lines. Ann Med. 2009;41:360–70. doi: 10.1080/07853890802609542. [DOI] [PubMed] [Google Scholar]
- Peng S, Lacerda AE, Kirsch GE, Brown AM, Bruening-Wright A. The action potential and comparative pharmacology of stem cell-derived human cardiomyocytes. J Pharmacol Toxicol Methods. 2010;61:277–86. doi: 10.1016/j.vascn.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Pillekamp F, Halbach M, Reppel M, Pfannkuche K, Nazzal R, Nguemo F, Matzkies M, Rubenchyk O, Hannes T, Khalil M, Bloch W, Sreeram N, Brockmeier K, Hescheler J. Physiological differences between transplanted and host tissue cause functional decoupling after in vitro transplantation of human embryonic stem cell-derived cardiomyocytes. Cell Physiol Biochem. 2009;23:65–74. doi: 10.1159/000204093. [DOI] [PubMed] [Google Scholar]
- Pillekamp F, Reppel M, Rubenchyk O, Pfannkuche K, Matzkies M, Bloch W, Sreeram N, Brockmeier K, Hescheler J. Force measurements of human embryonic stem cell-derived cardiomyocytes in an in vitro transplantation model. Stem Cells. 2007;25:174–80. doi: 10.1634/stemcells.2006-0094. [DOI] [PubMed] [Google Scholar]
- Poon E, Kong CW, Li RA. Human pluripotent stem cell-based approaches for myocardial repair: from the electrophysiological perspective. Mol Pharm. 2011;8:1495–504. doi: 10.1021/mp2002363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qyang Y, Martin-Puig S, Chiravuri M, Chen S, Xu H, Bu L, Jiang X, Lin L, Granger A, Moretti A, Caron L, Wu X, Clarke J, Taketo MM, Laugwitz KL, Moon RT, Gruber P, Evans SM, Ding S, Chien KR. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway. Cell Stem Cell. 2007;1:165–79. doi: 10.1016/j.stem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Ren Y, Lee MY, Schliffke S, Paavola J, Amos PJ, Ge X, Ye M, Zhu S, Senyei G, Lum L, Ehrlich BE, Qyang Y. Small molecule Wnt inhibitors enhance the efficiency of BMP-4-directed cardiac differentiation of human pluripotent stem cells. J Mol Cell Cardiol. 2011;51:280–7. doi: 10.1016/j.yjmcc.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppel M, Boettinger C, Hescheler J. Beta-adrenergic and muscarinic modulation of human embryonic stem cell-derived cardiomyocytes. Cell Physiol Biochem. 2004;14:187–96. doi: 10.1159/000080326. [DOI] [PubMed] [Google Scholar]
- Reppel M, Pillekamp F, Brockmeier K, Matzkies M, Bekcioglu A, Lipke T, Nguemo F, Bonnemeier H, Hescheler J. The electrocardiogram of human embryonic stem cell-derived cardiomyocytes. J Electrocardiol. 2005;38:166–70. doi: 10.1016/j.jelectrocard.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Rohr S. Role of gap junctions in the propagation of the cardiac action potential. Cardiovasc Res. 2004;62:309–22. doi: 10.1016/j.cardiores.2003.11.035. [DOI] [PubMed] [Google Scholar]
- Rust W, Balakrishnan T, Zweigerdt R. Cardiomyocyte enrichment from human embryonic stem cell cultures by selection of ALCAM surface expression. Regen Med. 2009;4:225–37. doi: 10.2217/17460751.4.2.225. [DOI] [PubMed] [Google Scholar]
- Saarela J, Rehn M, Oikarinen A, Autio-Harmainen H, Pihlajaniemi T. The short and long forms of type XVIII collagen show clear tissue specificities in their expression and location in basement membrane zones in humans. Am J Pathol. 1998;153:611–26. doi: 10.1016/S0002-9440(10)65603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana LF, Cheng H, Gomez AM, Cannell MB, Lederer WJ. Relation between the sarcolemmal Ca2+ current and Ca2+ sparks and local control theories for cardiac excitation-contraction coupling. Circ Res. 1996;78:166–71. doi: 10.1161/01.res.78.1.166. [DOI] [PubMed] [Google Scholar]
- Sartiani L, Bettiol E, Stillitano F, Mugelli A, Cerbai E, Jaconi ME. Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: a molecular and electrophysiological approach. Stem Cells. 2007;25:1136–44. doi: 10.1634/stemcells.2006-0466. [DOI] [PubMed] [Google Scholar]
- Satin J, Itzhaki I, Rapoport S, Schroder EA, Izu L, Arbel G, Beyar R, Balke Cw, Schiller J, Gepstein L. Calcium handling in human embryonic stem cell-derived cardiomyocytes. Stem Cells. 2008;26:1961–72. doi: 10.1634/stemcells.2007-0591. [DOI] [PubMed] [Google Scholar]
- Satin J, Kehat I, Caspi O, Huber I, Arbel G, Itzhaki I, Magyar J, Schroder EA, Perlman I, Gepstein L. Mechanism of spontaneous excitability in human embryonic stem cell derived cardiomyocytes. J Physiol. 2004;559:479–96. doi: 10.1113/jphysiol.2004.068213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf S, Shibamiya A, Mewe M, Eder A, Stohr A, Hirt MN, Rau T, Zimmermann WH, Conradi L, Eschenhagen T, Hansen A. Human engineered heart tissue as a versatile tool in basic research and preclinical toxicology. PLoS One. 2011;6:e26397. doi: 10.1371/journal.pone.0026397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schram G, Pourrier M, Melnyk P, Nattel S. Differential distribution of cardiac ion channel expression as a basis for regional specialization in electrical function. Circ Res. 2002;90:939–50. doi: 10.1161/01.res.0000018627.89528.6f. [DOI] [PubMed] [Google Scholar]
- Sedan O, Binah O. Excitation-contraction coupling, functional properties, and autonomic and hormonal regulation in human embryonic stem cell derived cardiomyocytes. In: Cohen IS, Gaudette GR, editors. Regenerating the Heart. Humana Press; New York: 2011. pp. 37–52. [Google Scholar]
- Sedan O, Dolnikov K, Zeevi-Levin N, Fleishmann N, Spiegel I, Berdichevski S, Amit M, Itskovitz-Eldor J, Binah O. Human embryonic stem cell-derived cardiomyocytes can mobilize 1,4,5-inositol trisphosphate-operated [Ca2+]i stores: the functionality of angiotensin-II/endothelin-1 signaling pathways. Ann N Y Acad Sci. 2010;1188:68–77. doi: 10.1111/j.1749-6632.2009.05085.x. [DOI] [PubMed] [Google Scholar]
- Sedan O, Dolnikov K, Zeevi-Levin N, Leibovich N, Amit M, Itskovitz-Eldor J, Binah O. 1,4,5-Inositol trisphosphate-operated intracellular Ca(2+) stores and angiotensin-II/endothelin-1 signaling pathway are functional in human embryonic stem cell-derived cardiomyocytes. Stem Cells. 2008;26:3130–8. doi: 10.1634/stemcells.2008-0777. [DOI] [PubMed] [Google Scholar]
- Sekar RB, Kizana E, Cho HC, Molitoris JM, Hesketh GG, Eaton BP, Marban E, Tung L. IK1 heterogeneity affects genesis and stability of spiral waves in cardiac myocyte monolayers. Circ Res. 2009;104:355–64. doi: 10.1161/CIRCRESAHA.108.178335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepac A, Sedlic F, Si-Tayeb K, Lough J, Duncan SA, Bienengraeber M, Park F, Kim J, Bosnjak ZJ. Isoflurane preconditioning elicits competent endogenous mechanisms of protection from oxidative stress in cardiomyocytes derived from human embryonic stem cells. Anesthesiology. 2010;113:906–16. doi: 10.1097/ALN.0b013e3181eff6b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira-Schweitzer K, Habib M, Gepstein L, Seliktar D. A photopolymerizable hydrogel for 3-D culture of human embryonic stem cell-derived cardiomyocytes and rat neonatal cardiac cells. J Mol Cell Cardiol. 2009;46:213–24. doi: 10.1016/j.yjmcc.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Shiels HA, White E. The Frank-Starling mechanism in vertebrate cardiac myocytes. J Exp Biol. 2008;211:2005–13. doi: 10.1242/jeb.003145. [DOI] [PubMed] [Google Scholar]
- Shimko VF, Claycomb WC. Effect of mechanical loading on three-dimensional cultures of embryonic stem cell-derived cardiomyocytes. Tissue Eng Part A. 2008;14:49–58. doi: 10.1089/ten.2007.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla DK, Sun B. Transforming growth factor-beta2 enhances differentiation of cardiac myocytes from embryonic stem cells. Biochem Biophys Res Commun. 2005;332:135–41. doi: 10.1016/j.bbrc.2005.04.098. [DOI] [PubMed] [Google Scholar]
- Siu CW, Moore JC, Li RA. Human embryonic stem cell-derived cardiomyocytes for heart therapies. Cardiovasc Hematol Disord Drug Targets. 2007;7:145–52. doi: 10.2174/187152907780830851. [DOI] [PubMed] [Google Scholar]
- Snir M, Kehat I, Gepstein A, Coleman R, Itskovitz-Eldor J, Livne E, Gepstein L. Assessment of the ultrastructural and proliferative properties of human embryonic stem cell-derived cardiomyocytes. Am J Physiol Heart Circ Physiol. 2003;285:H2355–63. doi: 10.1152/ajpheart.00020.2003. [DOI] [PubMed] [Google Scholar]
- Sobie EA, Song LS, Lederer WJ. Restitution of Ca(2+) release and vulnerability to arrhythmias. J Cardiovasc Electrophysiol. 2006;17(1):S64–S70. doi: 10.1111/j.1540-8167.2006.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg BE, Glass L, Shrier A, Bub G. The role of heterogeneities and intercellular coupling in wave propagation in cardiac tissue. Phil Trans R Soc Lond A. 2006;364:1299–311. doi: 10.1098/rsta.2006.1771. [DOI] [PubMed] [Google Scholar]
- Stevens KR, Kreutziger KL, Dupras SK, Korte FS, Regnier M, Muskheli V, Nourse MB, Bendixen K, Reinecke H, Murry CE. Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proc Natl Acad Sci U S A. 2009;106:16568–73. doi: 10.1073/pnas.0908381106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synnergren J, Adak S, Englund MC, Giesler TL, Noaksson K, Lindahl A, Nilsson P, Nelson D, Abbot S, Olsson B, Sartipy P. Cardiomyogenic gene expression profiling of differentiating human embryonic stem cells. J Biotechnol. 2008a;134:162–70. doi: 10.1016/j.jbiotec.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Synnergren J, Akesson K, Dahlenborg K, Vidarsson H, Ameen C, Steel D, Lindahl A, Olsson B, Sartipy P. Molecular signature of cardiomyocyte clusters derived from human embryonic stem cells. Stem Cells. 2008b;26:1831–40. doi: 10.1634/stemcells.2007-1033. [DOI] [PubMed] [Google Scholar]
- Synnergren J, Ameen C, Jansson A, Sartipy P. Global transcriptional profiling reveals similarities and differences between human stem cell-derived cardiomyocyte clusters and heart tissue. Physiological Genomics. 2012;44:245–258. doi: 10.1152/physiolgenomics.00118.2011. [DOI] [PubMed] [Google Scholar]
- Synnergren J, Ameen C, Lindahl A, Olsson B, Sartipy P. Expression of microRNAs and their target mRNAs in human stem cell-derived cardiomyocyte clusters and in heart tissue. Physiol Genomics. 2011;43:581–94. doi: 10.1152/physiolgenomics.00074.2010. [DOI] [PubMed] [Google Scholar]
- Tedrow U, Sweeney MO, Stevenson WG. Physiology of cardiac resynchronization. Curr Cardiol Rep. 2004;6:189–93. doi: 10.1007/s11886-004-0022-y. [DOI] [PubMed] [Google Scholar]
- te Keurs HE. Electromechanical coupling in the cardiac myocyte; stretch-arrhythmia feedback. Pflugers Arch. 2011;462:165–75. doi: 10.1007/s00424-011-0944-3. [DOI] [PubMed] [Google Scholar]
- ter Keurs HE, Boyden PA. Calcium and arrhythmogenesis. Physiol Rev. 2007;87:457–506. doi: 10.1152/physrev.00011.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SA, Burridge PW, Lipke EA, Shamblott M, Zambidis ET, Tung L. Engraftment of human embryonic stem cell derived cardiomyocytes improves conduction in an arrhythmogenic in vitro model. J Mol Cell Cardiol. 2012;53:15–23. doi: 10.1016/j.yjmcc.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TH, Wang X, Browne C, Zhang Y, Schinke M, Izumo S, Burcin M. Wnt3a-induced mesoderm formation and cardiomyogenesis in human embryonic stem cells. Stem Cells. 2009;27:1869–78. doi: 10.1002/stem.95. [DOI] [PubMed] [Google Scholar]
- Tulloch NL, Muskheli V, Razumova MV, Korte FS, Regnier M, Hauch KD, Pabon L, Reinecke H, Murry CE. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res. 2011;109:47–59. doi: 10.1161/CIRCRESAHA.110.237206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung L, Thompson S. Advantages and pitfalls of cell cultures as model systems to study cardiac mechanoelectric coupling. In: Kohl P, Sachs F, Franz MR, editors. Cardiac Mechano-Electric Coupling and Arrhythmias. Oxford University Press; Oxford, U.K: 2011. [Google Scholar]
- Uosaki H, Fukushima H, Takeuchi A, Matsuoka S, Nakatsuji N, Yamanaka S, Yamashita JK. Efficient and scalable purification of cardiomyocytes from human embryonic and induced pluripotent stem cells by VCAM1 surface expression. PLoS One. 2011;6:e23657. doi: 10.1371/journal.pone.0023657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoof D, Dormeyer W, Braam SR, Passier R, Monshouwer-Kloots J, Ward-van Oostwaard D, Heck AJ, Krijgsveld J, Mummery CL. Identification of cell surface proteins for antibody-based selection of human embryonic stem cell-derived cardiomyocytes. J Proteome Res. 2010;9:1610–8. doi: 10.1021/pr901138a. [DOI] [PubMed] [Google Scholar]
- van Laake LW, Passier R, Monshouwer-Kloots J, Verkleij AJ, Lips DJ, Freund C, den Ouden K, Ward-van Oostwaard D, Korving J, Tertoolen LG, van Echteld CJ, Doevendans PA, Mummery CL. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res. 2007;1:9–24. doi: 10.1016/j.scr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- van Laake LW, van Donselaar EG, Monshouwer-Kloots J, Schreurs C, Passier R, Humbel BM, Doevendans PA, Sonnenberg A, Verkleij AJ, Mummery CL. Extracellular matrix formation after transplantation of human embryonic stem cell-derived cardiomyocytes. Cell Mol Life Sci. 2010;67:277–290. doi: 10.1007/s00018-009-0179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkerk AO, Wilders R, van Borren MM, Peters RJ, Broekhuis E, Lam K, Coronel R, de Bakker JM, Tan HL. Pacemaker current (I(f)) in the human sinoatrial node. Eur Heart J. 2007;28:2472–8. doi: 10.1093/eurheartj/ehm339. [DOI] [PubMed] [Google Scholar]
- Wang K, Terrenoire C, Sampson K, Iyer V, Osteen JD, Lu J, Keller G, N D, Kass RS. Biophysical properties of slow potassium channels in human embryonic stem cell derived cardiomyocytes implicate subunit stoichiometry. J Physiol. 2011;589(24):6093–6104. doi: 10.1113/jphysiol.2011.220863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chen G, Song T, Mao G, Bai H. Enhancement of cardiomyocyte differentiation from human embryonic stem cells. Sci China Life Sci. 2010;53:581–9. doi: 10.1007/s11427-010-0111-7. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yue L, White M, Pelletier G, Nattel S. Differential distribution of inward rectifier potassium channel transcripts in human atrium versus ventricle. Circulation. 1998;98:2422–8. doi: 10.1161/01.cir.98.22.2422. [DOI] [PubMed] [Google Scholar]
- Weinberg S, Lipke EA, Tung L. In vitro electrophysiological mapping of stem cells. Methods Mol Biol. 2010;660:215–37. doi: 10.1007/978-1-60761-705-1_14. [DOI] [PubMed] [Google Scholar]
- Weiss JN, Chen PS, Qu Z, Karagueuzian HS, Lin SF, Garfinkel A. Electrical restitution and cardiac fibrillation. J Cardiovasc Electrophysiol. 2002;13:292–5. doi: 10.1046/j.1540-8167.2002.00292.x. [DOI] [PubMed] [Google Scholar]
- Weiss JN, Qu Z, Chen PS, Lin SF, Karagueuzian HS, Hayashi H, Garfinkel A, Karma A. The dynamics of cardiac fibrillation. Circulation. 2005;112:1232–40. doi: 10.1161/CIRCULATIONAHA.104.529545. [DOI] [PubMed] [Google Scholar]
- Wettwer E, Amos GJ, Posival H, Ravens U. Transient outward current in human ventricular myocytes of subepicardial and subendocardial origin. Circ Res. 1994;75:473–82. doi: 10.1161/01.res.75.3.473. [DOI] [PubMed] [Google Scholar]
- Xu C, He JQ, Kamp TJ, Police S, Hao X, O'Sullivan C, Carpenter MK, Lebkowski J, Gold JD. Human embryonic stem cell-derived cardiomyocytes can be maintained in defined medium without serum. Stem Cells Dev. 2006a;15:931–41. doi: 10.1089/scd.2006.15.931. [DOI] [PubMed] [Google Scholar]
- Xu C, Police S, Hassanipour M, Gold JD. Cardiac bodies: a novel culture method for enrichment of cardiomyocytes derived from human embryonic stem cells. Stem Cells Dev. 2006b;15:631–9. doi: 10.1089/scd.2006.15.631. [DOI] [PubMed] [Google Scholar]
- Xu C, Police S, Hassanipour M, Li Y, Chen Y, Priest C, O'Sullivan C, Laflamme MA, Zhu WZ, Van Biber B, Hegerova L, Yang J, Delavan-Boorsma K, Davies A, Lebkowski J, Gold JD. Efficient generation and cryopreservation of cardiomyocytes derived from human embryonic stem cells. Regen Med. 2011;6:53–66. doi: 10.2217/rme.10.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res. 2002;91:501–8. doi: 10.1161/01.res.0000035254.80718.91. [DOI] [PubMed] [Google Scholar]
- Xu XQ, Soo SY, Sun W, Zweigerdt R. Global expression profile of highly enriched cardiomyocytes derived from human embryonic stem cells. Stem Cells. 2009;27:2163–74. doi: 10.1002/stem.166. [DOI] [PubMed] [Google Scholar]
- Xu XQ, Zweigerdt R, Soo SY, Ngoh ZX, Tham SC, Wang ST, Graichen R, Davidson B, Colman A, Sun W. Highly enriched cardiomyocytes from human embryonic stem cells. Cytotherapy. 2008;10:376–89. doi: 10.1080/14653240802105307. [DOI] [PubMed] [Google Scholar]
- Xue T, Cho HC, Akar FG, Tsang SY, Jones SP, Marban E, Tomaselli GF, Li RA. Functional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: insights into the development of cell-based pacemakers. Circulation. 2005;111:11–20. doi: 10.1161/01.CIR.0000151313.18547.A2. [DOI] [PubMed] [Google Scholar]
- Yokoo N, Baba S, Kaichi S, Niwa A, Mima T, Doi H, Yamanaka S, Nakahata T, Heike T. The effects of cardioactive drugs on cardiomyocytes derived from human induced pluripotent stem cells. Biochem Biophys Res Commun. 2009;387:482–8. doi: 10.1016/j.bbrc.2009.07.052. [DOI] [PubMed] [Google Scholar]
- Yoon BS, Yoo SJ, Lee JE, You S, Lee HT, Yoon HS. Enhanced differentiation of human embryonic stem cells into cardiomyocytes by combining hanging drop culture and 5-azacytidine treatment. Differentiation. 2006;74:149–59. doi: 10.1111/j.1432-0436.2006.00063.x. [DOI] [PubMed] [Google Scholar]
- Zeevi-Levin N, Itskovitz-Eldor J, Binah O. Functional properties of human embryonic stem cell-derived cardiomyocytes. Crit Rev Eukaryot Gene Expr. 2010;20:51–9. doi: 10.1615/critreveukargeneexpr.v20.i1.40. [DOI] [PubMed] [Google Scholar]
- Zeevi-Levin N, Itskovitz-Eldor J, Binah O. Cardiomyocytes derived from human pluripotent stem cells for drug screening. Pharmacol Ther. 2012 doi: 10.1016/j.pharmthera.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, Kamp TJ. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30–41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Jiang J, Han P, Yuan Q, Zhang J, Zhang X, Xu Y, Cao H, Meng Q, Chen L, Tian T, Wang X, Li P, Hescheler J, Ji G, Ma Y. Direct differentiation of atrial and ventricular myocytes from human embryonic stem cells by alternating retinoid signals. Cell Res. 2011;21:579–87. doi: 10.1038/cr.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Guo JP, Chi YL, Liu YC, Zhang CS, Yang XQ, Lin HY, Jiang EP, Xiong SH, Zhang ZY, Liu BH. Endothelin-induced differentiation of Nkx2.5(+) cardiac progenitor cells into pacemaking cells. Mol Cell Biochem. 2012 doi: 10.1007/s11010-012-1309-8. [DOI] [PubMed] [Google Scholar]
- Zhu WZ, Santana LF, Laflamme MA. Local control of excitation-contraction coupling in human embryonic stem cell-derived cardiomyocytes. PLoS One. 2009;4:e5407. doi: 10.1371/journal.pone.0005407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WZ, Xie Y, Moyes KW, Gold JD, Askari B, Laflamme MA. Neuregulin/ErbB signaling regulates cardiac subtype specification in differentiating human embryonic stem cells. Circ Res. 2010;107:776–86. doi: 10.1161/CIRCRESAHA.110.223917. [DOI] [PMC free article] [PubMed] [Google Scholar]