Abstract
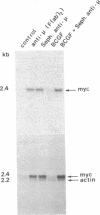
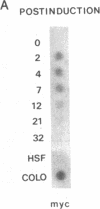
Resting human B cells can be activated to proliferate in the presence of both polyclonal antibodies to immunoglobulin mu heavy chains and B-cell growth factor (BCGF). This process appears to be temporally controlled in that the initial activation of the B cells and their responsiveness to BCGF is carried out by polyclonal anti-mu-chain antibodies alone. We have used this system to investigate the role of the c-myc gene in the cell cycle of normal human peripheral blood B cells. Our results show that the polyclonal anti-mu-chain antibody-induced B-cell activation is accompanied by a specific induction of c-myc gene expression without promoting subsequent entry into the S phase unless BCGF is added. Monoclonal antibodies to either mu chain or the pan-B-cell antigen Bp35 also revealed a similar G0-to-G1 transition and activation of c-myc gene expression. However, unlike activation with polyclonal anti-mu-chain antibodies, cells stimulated with these monoclonal antibodies do not acquire responsiveness to BCGF. The results imply that additional inducible functions must be present to potentiate the myc-specific function in order for the B cells to acquire the capacity to proliferate in response to BCGF. These findings are discussed in relation to the origin of B-cell malignancies.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Schwab M., Lin C. C., Varmus H. E., Bishop J. M. Homogeneously staining chromosomal regions contain amplified copies of an abundantly expressed cellular oncogene (c-myc) in malignant neuroendocrine cells from a human colon carcinoma. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1707–1711. doi: 10.1073/pnas.80.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armelin H. A., Armelin M. C., Kelly K., Stewart T., Leder P., Cochran B. H., Stiles C. D. Functional role for c-myc in mitogenic response to platelet-derived growth factor. Nature. 1984 Aug 23;310(5979):655–660. doi: 10.1038/310655a0. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Clark E. A., Shu G., Ledbetter J. A. Role of the Bp35 cell surface polypeptide in human B-cell activation. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1766–1770. doi: 10.1073/pnas.82.6.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Collins S., Groudine M. Amplification of endogenous myc-related DNA sequences in a human myeloid leukaemia cell line. Nature. 1982 Aug 12;298(5875):679–681. doi: 10.1038/298679a0. [DOI] [PubMed] [Google Scholar]
- Dalla-Favera R., Gelmann E. P., Martinotti S., Franchini G., Papas T. S., Gallo R. C., Wong-Staal F. Cloning and characterization of different human sequences related to the onc gene (v-myc) of avian myelocytomatosis virus (MC29). Proc Natl Acad Sci U S A. 1982 Nov;79(21):6497–6501. doi: 10.1073/pnas.79.21.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Evenson D. P., Staiano-Coico L., Sharpless T. K., Melamed M. L. Correlation between cell cycle duration and RNA content. J Cell Physiol. 1979 Sep;100(3):425–438. doi: 10.1002/jcp.1041000306. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Sharpless T., Staiano-Coico L., Melamed M. R. Subcompartments of the G1 phase of cell cycle detected by flow cytometry. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6696–6699. doi: 10.1073/pnas.77.11.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F., Hunkapiller M. W., Hood L. E., Devare S. G., Robbins K. C., Aaronson S. A., Antoniades H. N. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983 Jul 15;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Godal T., Davies C., Smeland E. B., Heikkilä R., Funderud S., Steen H. B., Hildrum K. Antibodies to surface IgM and IgD increase the expression of various class II antigens on human B cells. Eur J Immunol. 1985 Feb;15(2):173–177. doi: 10.1002/eji.1830150212. [DOI] [PubMed] [Google Scholar]
- Godal T., Ruud E., Heikkilä R., Funderud S., Michaelsen T., Jefferis R., Ling N. R., Hildrum K. Triggering of monoclonal human lymphoma B cells with antibodies to IgM heavy chains: differences of response obtained with monoclonal as compared to polyclonal antibodies. Clin Exp Immunol. 1983 Dec;54(3):756–764. [PMC free article] [PubMed] [Google Scholar]
- Goustin A. S., Betsholtz C., Pfeifer-Ohlsson S., Persson H., Rydnert J., Bywater M., Holmgren G., Heldin C. H., Westermark B., Ohlsson R. Coexpression of the sis and myc proto-oncogenes in developing human placenta suggests autocrine control of trophoblast growth. Cell. 1985 May;41(1):301–312. doi: 10.1016/0092-8674(85)90083-2. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H. Avian leukemia viruses: interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978 Nov 17;516(3):269–299. doi: 10.1016/0304-419x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Hann S. R., Thompson C. B., Eisenman R. N. c-myc oncogene protein synthesis is independent of the cell cycle in human and avian cells. 1985 Mar 28-Apr 3Nature. 314(6009):366–369. doi: 10.1038/314366a0. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Hemler M. E., Mann D. L., Eisenbarth G. S., Shelhamer J., Mostowski H. S., Thomas C. A., Strominger J. L., Fauci A. S. Characterization of a monoclonal antibody (4F2) that binds to human monocytes and to a subset of activated lymphocytes. J Immunol. 1981 Apr;126(4):1409–1414. [PubMed] [Google Scholar]
- Heikkilä R., Iversen J. G., Smeland E., Godal T. Intracellular Ca2+ buffering potentiates an 86Rb+ permeability response in human lymphocytes. Acta Physiol Scand. 1985 Jun;124(2):247–251. doi: 10.1111/j.1748-1716.1985.tb07658.x. [DOI] [PubMed] [Google Scholar]
- Heikkilä R., Ruud E., Funderud S., Godal T. Differences in modifications of cytoplasmic free Ca2+ concentration and 86Rb+ influx in human neoplastic B cells by antibodies to mu- relative to delta-Ig heavy chains. Clin Exp Immunol. 1985 Mar;59(3):695–702. [PMC free article] [PubMed] [Google Scholar]
- Howard M., Nakanishi K., Paul W. E. B cell growth and differentiation factors. Immunol Rev. 1984 Apr;78:185–210. doi: 10.1111/j.1600-065x.1984.tb00482.x. [DOI] [PubMed] [Google Scholar]
- Kehrl J. H., Muraguchi A., Butler J. L., Falkoff R. J., Fauci A. S. Human B cell activation, proliferation and differentiation. Immunol Rev. 1984 Apr;78:75–96. doi: 10.1111/j.1600-065x.1984.tb00477.x. [DOI] [PubMed] [Google Scholar]
- Kehrl J. H., Muraguchi A., Fauci A. S. Differential expression of cell activation markers after stimulation of resting human B lymphocytes. J Immunol. 1984 Jun;132(6):2857–2861. [PubMed] [Google Scholar]
- Kehrl J. H., Muraguchi A., Fauci A. S. Human B cell activation and cell cycle progression: stimulation with anti-mu and Staphylococcus aureus Cowan strain I. Eur J Immunol. 1984 Feb;14(2):115–121. doi: 10.1002/eji.1830140203. [DOI] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Klein G. Specific chromosomal translocations and the genesis of B-cell-derived tumors in mice and men. Cell. 1983 Feb;32(2):311–315. doi: 10.1016/0092-8674(83)90449-x. [DOI] [PubMed] [Google Scholar]
- Klein G. The role of gene dosage and genetic transpositions in carcinogenesis. Nature. 1981 Nov 26;294(5839):313–318. doi: 10.1038/294313a0. [DOI] [PubMed] [Google Scholar]
- Maizel A. L., Morgan J. W., Mehta S. R., Kouttab N. M., Bator J. M., Sahasrabuddhe C. G. Long-term growth of human B cells and their use in a microassay for B-cell growth factor. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5047–5051. doi: 10.1073/pnas.80.16.5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti G. E., Kuo M. C., Shaw S., Chang C. C., Demars R., Sogn J. A., Coligan J. E., Kindt T. J. A novel HLA-D/DR-like antigen specific for human B lymphoid cells. Biochemical evidence for similarity to but nonidentity with known HLA-D/DR antigens. J Exp Med. 1983 Dec 1;158(6):1924–1937. doi: 10.1084/jem.158.6.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers F., Andersson J. B cell activation: three steps and their variations. Cell. 1984 Jul;37(3):713–720. doi: 10.1016/0092-8674(84)90407-0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Pfeifer-Ohlsson S., Goustin A. S., Rydnert J., Wahlström T., Bjersing L., Stehelin D., Ohlsson R. Spatial and temporal pattern of cellular myc oncogene expression in developing human placenta: implications for embryonic cell proliferation. Cell. 1984 Sep;38(2):585–596. doi: 10.1016/0092-8674(84)90513-0. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Smeland E., Funderud S., Ruud E., Kiil Blomhoff H., Godal T. Characterization of two murine monoclonal antibodies reactive with human B cells. Their use in a high-yield, high-purity method for isolation of B cells and utilization of such cells in an assay for B-cell stimulating factor. Scand J Immunol. 1985 Mar;21(3):205–214. doi: 10.1111/j.1365-3083.1985.tb01422.x. [DOI] [PubMed] [Google Scholar]
- Steen H. B., Lindmo T. Cellular and nuclear volume during the cell cycle of NHIK 3025 cells. Cell Tissue Kinet. 1978 Jan;11(1):69–81. doi: 10.1111/j.1365-2184.1978.tb00876.x. [DOI] [PubMed] [Google Scholar]
- Stehelin D., Varmus H. E., Bishop J. M., Vogt P. K. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976 Mar 11;260(5547):170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- Stiles C. D., Capone G. T., Scher C. D., Antoniades H. N., Van Wyk J. J., Pledger W. J. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. B., Challoner P. B., Neiman P. E., Groudine M. Levels of c-myc oncogene mRNA are invariant throughout the cell cycle. 1985 Mar 28-Apr 3Nature. 314(6009):363–366. doi: 10.1038/314363a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]