Abstract
Susceptibility to oxygen toxicity was studied in three inbred and two hybrid strains of mice. Because in vitro studies have shown that the cytochrome P-450 enzymes can produce oxygen radicals and H2O2, we tested the hypothesis that inducibility of these enzymes might play a role in oxygen toxicity. Mice responsive to hepatic microsomal enzyme induction by aromatic hydrocarbons [C3H/HeJ, C3H/HeN, C3H/HeJ X DBA/2J (designated C3D2F1/J), C3H/HeN X DBA/2J (designated C3D2F1/N)] were more sensitive to the toxic effects of 100% oxygen exposure than were genetically unresponsive mice (DBA/2J). DBA/2J mice survived significantly longer exposure periods with less lung damage. Lung and liver cytochrome P-450 levels increased 2-to 3-fold in C3H and F1 mice during 100% oxygen exposure (maximum levels at 72-96 hr) and subsequently fell prior to death. No increases were seen in cytochrome P-450 levels in DBA/2J mice. Metabolic pathways involving cytochrome P-450 enzymes may initiate or modulate oxidative damage due to oxygen radicals. The difference in responsiveness of mice to microsomal enzyme induction may imply genetic differences in susceptibility to oxidative stress, may help to explain species differences in susceptibility, and may have long-term implications in therapeutics and patient care if similar inherited differences exist in humans.
Full text
PDF
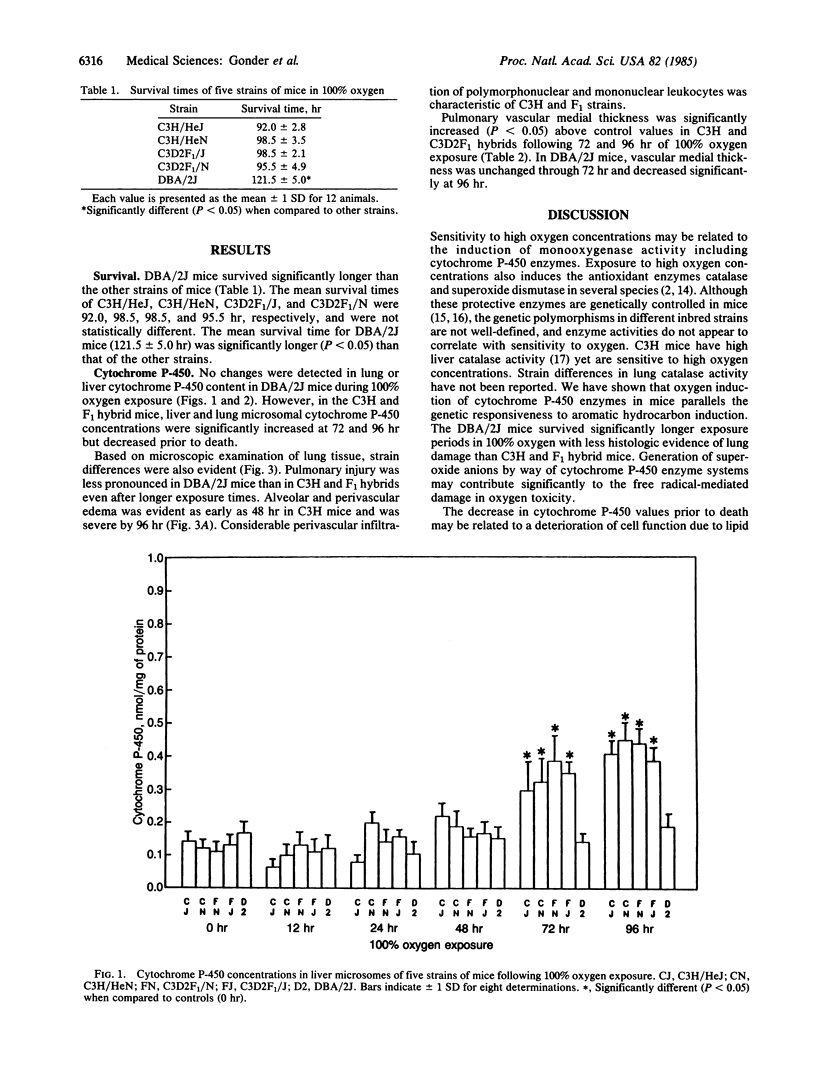
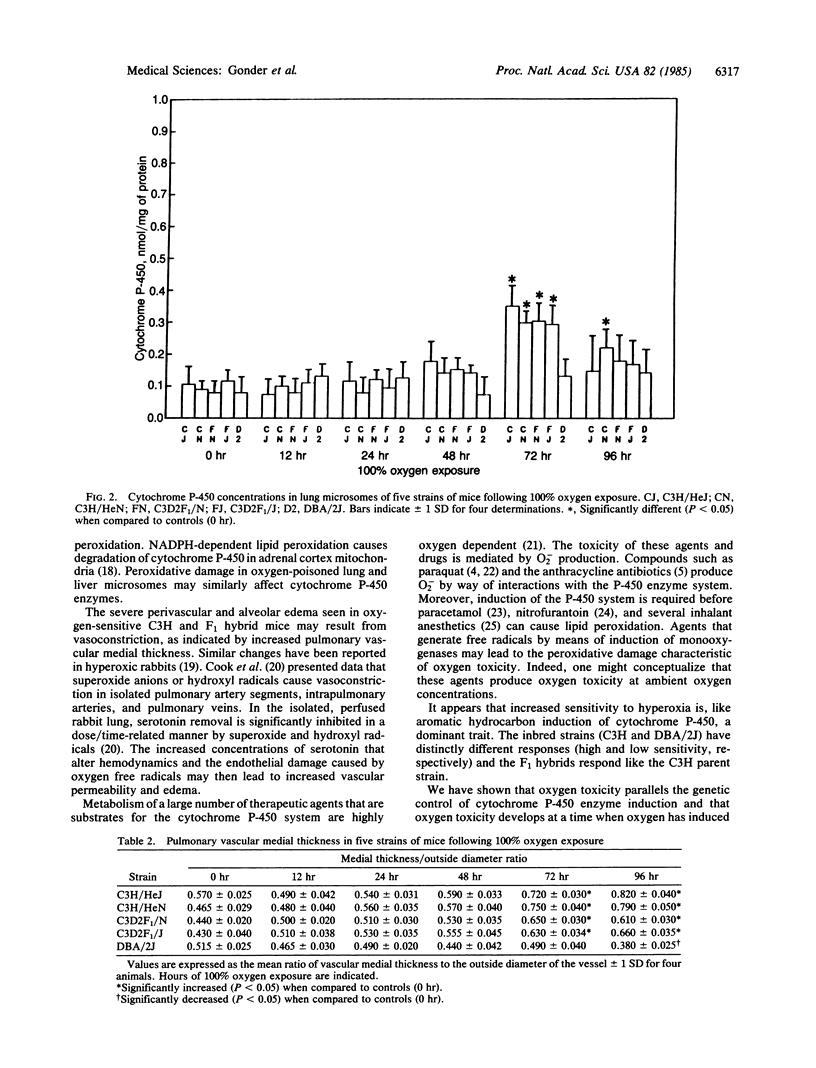
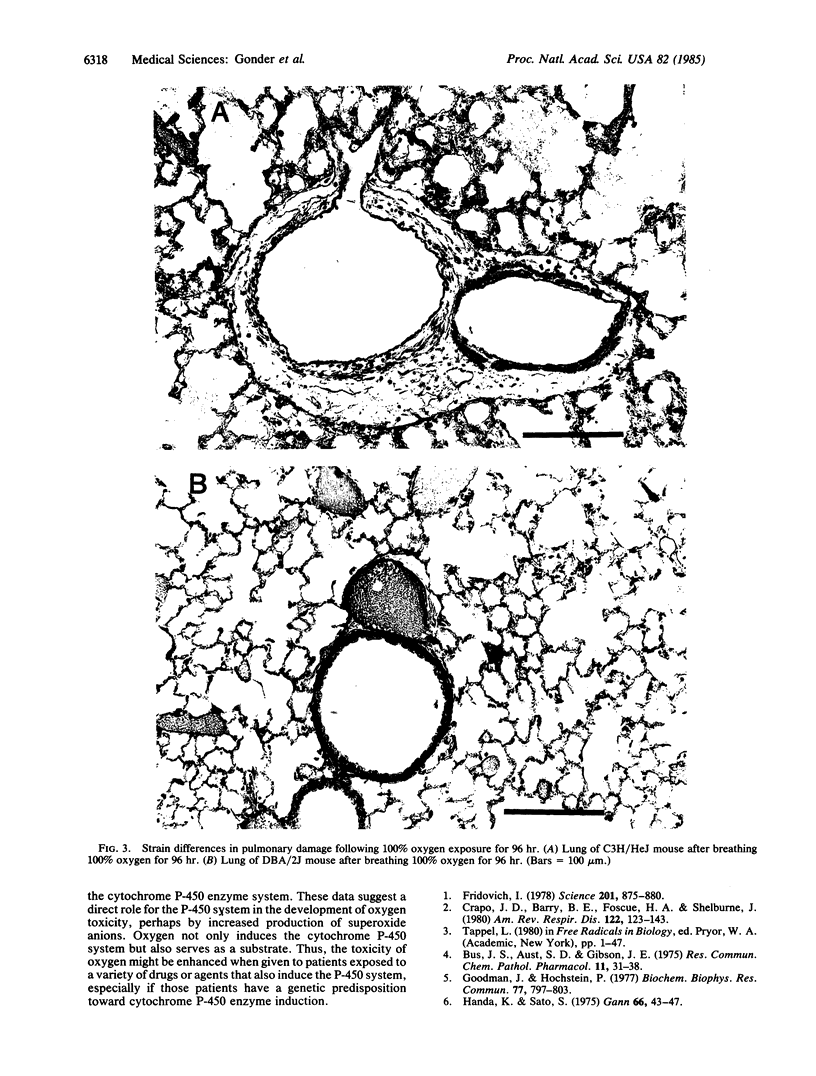

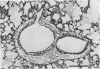
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auclair C., de Prost D., Hakim J. Superoxide anion production by liver microsomes from phenobarbital treated rat. Biochem Pharmacol. 1978 Feb 1;27(3):355–358. doi: 10.1016/0006-2952(78)90240-x. [DOI] [PubMed] [Google Scholar]
- Brown B. R., Jr Hepatic microsomal lipoperoxidation and inhalation anesthetics: a biochemical and morphologic study in the rat. Anesthesiology. 1972 May;36(5):458–465. doi: 10.1097/00000542-197205000-00008. [DOI] [PubMed] [Google Scholar]
- Bus J. S., Aust S. D., Gibson J. E. Lipid peroxidation: a possible mechanism for paraquat toxicity. Res Commun Chem Pathol Pharmacol. 1975 May;11(1):31–38. [PubMed] [Google Scholar]
- Bus J. S., Cagen S. Z., Olgaard M., Gibson J. E. A mechanism of paraquat toxicity in mice and rats. Toxicol Appl Pharmacol. 1976 Mar;35(3):501–513. doi: 10.1016/0041-008x(76)90073-9. [DOI] [PubMed] [Google Scholar]
- Clark J. M., Lambertsen C. J. Pulmonary oxygen toxicity: a review. Pharmacol Rev. 1971 Jun;23(2):37–133. [PubMed] [Google Scholar]
- Cook D. R., Howell R. E., Gillis C. N. Xanthine oxidase-induced lung injury inhibits removal of 5-hydroxytryptamine from the pulmonary circulation. Anesth Analg. 1982 Aug;61(8):666–670. [PubMed] [Google Scholar]
- Crapo J. D., Barry B. E., Foscue H. A., Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis. 1980 Jul;122(1):123–143. doi: 10.1164/arrd.1980.122.1.123. [DOI] [PubMed] [Google Scholar]
- Davisson M. T., Roderick T. H. Status of the linkage map of the mouse. Cytogenet Cell Genet. 1978;22(1-6):552–557. doi: 10.1159/000131022. [DOI] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Goodman J., Hochstein P. Generation of free radicals and lipid peroxidation by redox cycling of adriamycin and daunomycin. Biochem Biophys Res Commun. 1977 Jul 25;77(2):797–803. doi: 10.1016/s0006-291x(77)80048-x. [DOI] [PubMed] [Google Scholar]
- Handa K., Sato S. Generation of free radicals of quinone group-containing anti-cancer chemicals in NADPH-microsome system as evidenced by initiation of sulfite oxidation. Gan. 1975 Feb;66(1):43–47. [PubMed] [Google Scholar]
- Jones D. P. Hypoxia and drug metabolism. Biochem Pharmacol. 1981 May 15;30(10):1019–1023. doi: 10.1016/0006-2952(81)90436-6. [DOI] [PubMed] [Google Scholar]
- Keith I. M., Will J. A. Dynamics of the neuroendocrine cell--regulatory peptide system in the lung. Specific overview and new results. Exp Lung Res. 1982 Nov;3(3-4):387–402. doi: 10.3109/01902148209069665. [DOI] [PubMed] [Google Scholar]
- Klimek J., Schaap A. P., Kimura T. The relationship between NADPH-dependent lipid peroxidation and degradation of cytochrome P-450 in adrenal cortex mitochondria. Biochem Biophys Res Commun. 1983 Jan 27;110(2):559–566. doi: 10.1016/0006-291x(83)91186-5. [DOI] [PubMed] [Google Scholar]
- Longmuir I. S., Gottlieb S. F., Pashko L. L., Martin P. In vivo and in vitro induction of cytochrome P-450 synthesis in hyperoxia. Undersea Biomed Res. 1980 Sep;7(3):161–170. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Rowe H. A., Martin P. L., Longmuir I. S. Synthesis of cytochrome P-450 in isolated liver cells during exposure to hyperoxia. Undersea Biomed Res. 1984 Jun;11(2):185–192. [PubMed] [Google Scholar]
- Spielberg S. P., Gordon G. B. Nitrofurantoin cytotoxicity. In vitro assessment of risk based on glutathione metabolism. J Clin Invest. 1981 Jan;67(1):37–41. doi: 10.1172/JCI110030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. E., Kouri R. E., Hutton J. J. The genetics of aryl hydrocarbon hydroxylase induction in mice: a single gene difference between C57BL-6J and DBA-2J. Biochem Genet. 1972 Apr;6(2):157–168. doi: 10.1007/BF00486400. [DOI] [PubMed] [Google Scholar]
- Wendel A., Feuerstein S. Drug-induced lipid peroxidation in mice--I. Modulation by monooxygenase activity, glutathione and selenium status. Biochem Pharmacol. 1981 Sep 15;30(18):2513–2520. doi: 10.1016/0006-2952(81)90576-1. [DOI] [PubMed] [Google Scholar]
- White R. E., Coon M. J. Oxygen activation by cytochrome P-450. Annu Rev Biochem. 1980;49:315–356. doi: 10.1146/annurev.bi.49.070180.001531. [DOI] [PubMed] [Google Scholar]