Abstract
Background
In patients with heart failure (HF) and preserved ejection fraction (HFpEF), atrial fibrillation (AF) may predate, concur with, or develop after HFpEF diagnosis. We sought to define the temporal relationship between AF and HFpEF, identify factors associated with AF, and determine the prognostic impact of prevalent and incident AF in HFpEF.
Method and Results
From 1983 to 2010, 939 Olmsted County, MN residents (age 77±12years, 61% female) newly diagnosed with HFpEF (EF≥0.50) were evaluated. Baseline rhythm classification included: prior AF (>3 months before HFpEF diagnosis), concurrent AF (±3months), or sinus rhythm (SR). Incident AF (>3months after HFpEF diagnosis) and all-cause mortality were ascertained through February 2012. Prior (29%) and concurrent AF (23%) were associated with older age, higher BNP, and larger left atrial volume index at HFpEF diagnosis compared to SR. Of HFpEF patients in SR at diagnosis, 32% developed AF over a median (IQR) follow-up of 3.7(1.5–6.7) years (69 events per 1000 person-years). Age and diastolic dysfunction were positively, while statin use was inversely associated with incident AF. Using no AF as the referent, prior or concurrent AF (combined HR 1.3, 95%CI 1.0–1.6, p=0.03) and incident AF, modeled as a time-dependent covariate, (HR 2.1, 95%CI 1.4–3.0, p<0.001), were independently associated with death adjusting for pertinent covariates.
Conclusions
AF occurs in two-thirds of HFpEF patients at some point in the natural history and confers a poor prognosis. Further study is required to determine whether intervention for AF may improve outcomes or if statin use can prevent AF in HFpEF.
Keywords: diastolic heart failure, atrial fibrillation, prognosis, population
Introduction
There is an apparent collusion of three major trends: aging of the population, a virtual epidemic of atrial fibrillation (AF), and the emergence of heart failure (HF) with preserved ejection fraction (HFpEF) as the dominant form of HF, almost unique to the elderly1–3. Development of effective therapy for HFpEF has proved challenging, in part due to heterogeneous and incompletely understood pathophysiological mechanisms which occur in the setting of multiple comorbidities.
AF is a common comorbidity in HFpEF, reported in 25–39% of HFpEF patients, consistent across trial4, 5, community6, 7, registry8, and hospitalized9, 10 cohorts. AF may occur in patients destined to develop HFpEF due to similar risk factors including aging and hypertension and may precipitate HF in persons with milder impairment in cardiovascular function due to effects on heart rate or atrio-ventricular synchrony. Alternatively, HFpEF may predispose to AF as a result of chronic left atrial hypertension and atrial remodeling. Thus, AF may represent a consequence of HFpEF progression as occurs in HF with reduced ejection fraction (HFrEF)11, where AF is observed in patients with more severe functional impairment and systolic dysfunction12.
While previous studies have focused on AF present at time of recruitment or first HFpEF hospitalization5, 13–16, we examined a large community-based cohort of patients with incident HFpEF who had previous and subsequent ascertainment of AF as well as vital status. Thus, this cohort provided the unique ability to describe timing of AF occurrence in relation to HFpEF diagnosis. The objective of this study was to determine if the association of AF with cardiac dysfunction, HF severity or prognosis differed according to the temporal relationship of AF to HFpEF onset. We hypothesized that regardless of temporal association, the presence of AF is associated with worse cardiac dysfunction, HF severity and prognosis in HFpEF.
Methods
Study Setting
This population-based cohort study was conducted within Olmsted County, Minnesota using resources of the Rochester Epidemiology Project (REP) as previously described6, 17.
Identification of HFpEF Cohort
Olmsted County residents with a first diagnosis of HF between January 1, 1983 and December 31, 2010 (n=2852) were identified and HF validated as part of an on-going Olmsted County HF surveillance study6, 18. Patients who underwent echocardiography within 2 months of HF diagnosis, with an ejection fraction (EF) ≥0.5, were determined to have HFpEF and formed the final study cohort. This study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards. Informed consent for examination of medical records (or waiver prior to 1997) was obtained as appropriate.
AF Ascertainment
Prevalent AF was identified by documented AF on a clinically indicated ECG and/or ICD-9-CM code pertaining to AF (427.31), AF/flutter (427.3), or atrial flutter alone (427.32)19.
Prevalent AF was further sub-defined as AF occurring prior to (>3months) or concurrent with (±3months) HFpEF diagnosis. Patients in sinus rhythm (SR) at time of HFpEF diagnosis formed the referent population for baseline comparisons and ascertainment of incident AF during follow-up. Incident AF diagnosis was defined by date of first documentation of AF or atrial flutter on ECG or relevant ICD-9-CM code in the medical record >3 months following HFpEF diagnosis, during any hospitalization or outpatient visit. AF-related diagnostic codes were included as 12-lead ECG documentation alone has previously been reported to miss up to 10% of cases19. A random sample of 183 positively coded records were reviewed and ECG or other documentation (rhythm strip or Holter monitor) of AF confirmed in all cases.
Data Collection
Patient demographics, clinical diagnoses, and laboratory results, as well as CHADS2 and CHA2DS2-VASc scores20 were electronically abstracted from medical records (Supplemental methods). Echocardiographic data including EF, LV dimensions, diastolic function, pulmonary artery pressure, left atrial volume index (LAVI) and valvular disease within 2 months of HFpEF diagnosis were obtained from the Mayo Clinic Echocardiographic database.
Ascertainment of Vital Status
Vital status was determined through February 29th 2012 via REP procedures as previously described6.
Statistical Analysis
Group data are presented as frequencies (%), means (±SD) or median (interquartile range, IQR) as appropriate. Since eGFR, BNP and TSH distributions were skewed, values were log-transformed for analysis. Across-group comparisons were made using Pearson χ2 for categorical variables, and one-way analysis of variance (ANOVA) or Kruskal-Wallis test for continuous variables. Pairwise comparisons across groups were subject to Bonferroni correction for multiple comparisons. Cox proportional hazards regression was used to identify patient characteristics associated with incident AF. Unadjusted and age- and sex-adjusted Cox models were explored, with additional multivariable models adjusting for pertinent clinical baseline variables. Significant correlations and interactions (p<0.05) were assessed and accounted for.
Overall survival was estimated using the Kaplan-Meier method. Between-group survival was compared by the log-rank test. Cox proportional hazards regression was used to examine the association between all-cause death and AF status, controlling for pertinent covariates. Incident AF was modeled as a time-dependent covariate. All p-values were 2-tailed; p<0.05 was considered the threshold for statistical significance. Analyses were performed using JMP version 9.0 (SAS Institute, Cary, NC) and R version 2.14.1 (The R Foundation for Statistical Computing).
Results
Population characteristics
Over the study period, 2852 Olmsted County residents had a new HF diagnosis. In 872 patients echocardiographic confirmation of EF was unavailable. These patients were older and had more COPD but were otherwise comparable to patients with confirmed EFs (Supplemental Table 1). From the remaining 1980 patients, 939 (47.4%) had EF≥0.50 at HF diagnosis. Among these, 541/939 HFpEF patients (57.6%) were captured during a hospitalization, while 398/939 (42.4%) were outpatients. Of the inpatient cohort 23.8% were NYHA class III or IV, versus 12.5% of the outpatient cohort.
Prevalent AF and HFpEF
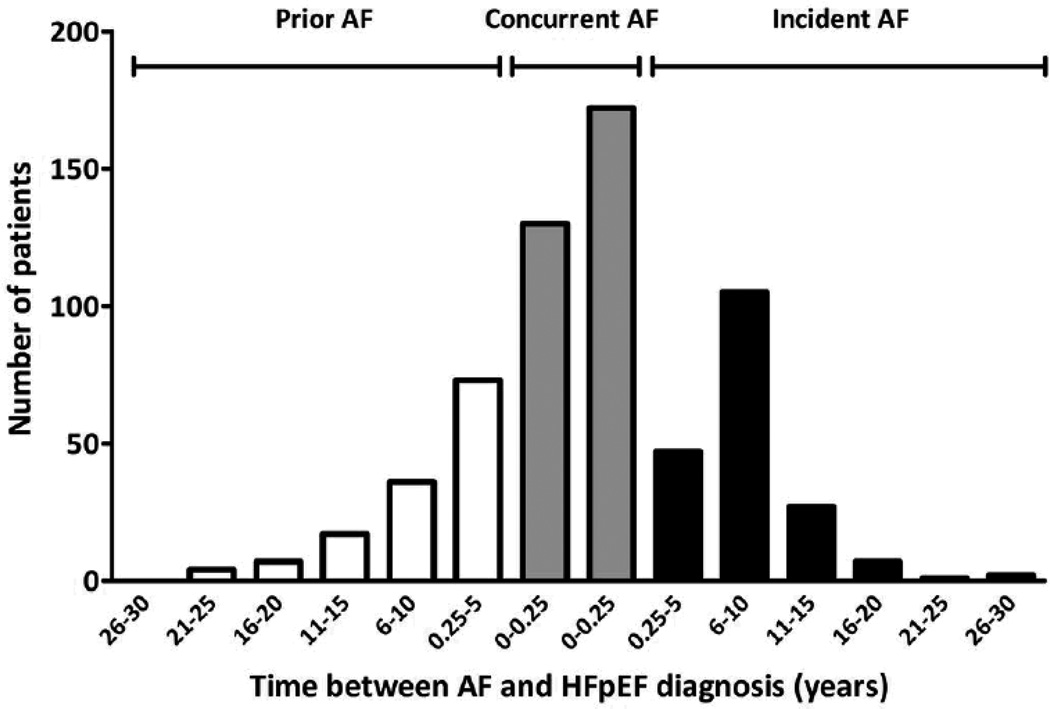
At HFpEF diagnosis, 270 (28.8%) patients had prior AF, 219 (23.3%) had concurrent AF, and 450 (47.9%) were in SR (Table 1, Figure 1). Among patients with prior AF, median (IQR) duration of AF was 5.1 (2.4–10.0) years. Prevalent AF was defined as prior and concurrent combined, and varied but did not appreciably increase over the study duration (1983–1990, 56%; 1991–2000, 45%; 2001–2010, 56%).
Table 1.
Baseline characteristics of all HFpEF patients by rhythm at HFpEF diagnosis
| Variables | N | Rhythm at HFpEF diagnosis | p-value | ||
|---|---|---|---|---|---|
| Prior AF (n=270) |
Concurrent AF (n=219) |
Sinus Rhythm (n=450) |
|||
| Demographics and clinical data | |||||
| Age, years | 939 | 79.0±10.1* | 77.6±11.1* | 74.4±13.9 | <0.0001 |
| Female sex, n (%) | 939 | 163 (60.4) | 137 (62.6) | 273 (60.7) | 0.87 |
| BMI, kg/m2 | 939 | 29.3±7.6 | 29.1±7.1 | 30.2±7.8 | 0.16 |
| Heart rate, bpm | 927 | 84±24 | 96±27*† | 83±19 | <0.0001 |
| Systolic BP, mmHg | 908 | 147±29 | 143±29* | 152±32 | 0.003 |
| Diastolic BP, mmHg | 908 | 77±17 | 79±17 | 77±17 | 0.56 |
| NYHA III-IV, n (%) | 796 | 50 (22) | 41 (23) | 61 (16) | 0.51 |
| Co-morbidities | |||||
| Previous MI, n (%) | 937 | 46 (17) | 20 (9)† | 65 (14) | 0.039 |
| Hypertension, n (%) | 934 | 191 (71) | 146 (67) | 290 (65) | 0.20 |
| Diabetes, n (%) | 938 | 62 (23) | 39 (18)* | 136 (30) | 0.0014 |
| Cerebrovascular disease, n (%) | 925 | 79 (30)* | 37 (17)† | 78 (18) | 0.0002 |
| COPD, n (%) | 921 | 53 (20) | 44 (20) | 79 (18) | 0.66 |
| Thyroid disease, n (%) | 912 | 19 (7) | 8 (4) | 26 (6) | 0.25 |
| Medication at HFpEF diagnosis | |||||
| ACEI or ARB, n (%) | 935 | 115 (43) | 85 (39) | 183 (41) | 0.66 |
| Beta-blocker, n (%) | 935 | 138 (52) | 105 (48) | 202 (45) | 0.25 |
| Diuretic, n (%) | 935 | 189 (70) | 143 (65) | 313 (70) | 0.39 |
| Statin, n (%) | 935 | 83 (31) | 46 (21)† | 112 (25) | 0.038 |
| AAD, n (%) | 935 | 34 (13)* | 32 (15)* | 8 (2) | <0.0001 |
| Laboratory data | |||||
| eGFR, ml/min/1.73m2φ | 926 | 53 (43–66) | 54(43–67) | 54 (42–68) | 0.73 |
| BNP, pg/ml‡φ | 328 | 485 (276–729)* | 485 (265–705)* | 327 (142–664) | 0.010 |
| TSH, mIU/Lφ | 358 | 2.2 (1.2–3.7) | 2.2 (1.5–3.6) | 2.1 (1.2–3.7) | 0.49 |
| LDL, g/dLφ | 527 | 79 (64–110)* | 84 (65–109) | 93 (71–118) | 0.019 |
| Echo data | |||||
| LVIDd, mm | 688 | 47.8±6.1 | 47.5±6.7 | 48.3±6.2 | 0.31 |
| LVEF, % | 939 | 60.1±6.4 | 60.8±7.2 | 60.9±7.0 | 0.33 |
| Deceleration time, ms | 625 | 187±56* | 186±48* | 216±57 | <0.0001 |
| TR velocity, m/s | 683 | 3.0±0.5 | 2.9±0.5 | 3.0±0.5 | 0.21 |
| LAVI, ml/m2§ | 304 | 54.7±22.5* | 47.0±13.5*† | 40.7±12.4 | <0.0001 |
| e’, ms‖φ | 364 | 0.06±0.02 | 0.06±0.03 | 0.06±0.02 | 0.10 |
| E/e’# | 354 | 18.7±9.0 | 18.2±8.8 | 16.7±7.6 | 0.11 |
Mean±SD shown [φmedian(IQR)]
p<0.05 vs. SR
p<0.05 vs. prior AF (both with Bonferroni correction)
Number of patients with BNP values available: Prior AF, n=103 (38.1%); concurrent AF, n=77 (35.2%); SR, n=148 (32.9%).
Number of patients with LAVI measurements available: Prior AF, n=95 (35.2%); concurrent AF, n=72 (32.9%); SR, n=137 (30.4%).
Number of patients with e’ measurements available: Prior AF, n=111 (41.1%); concurrent AF, n=81 (37.0%); SR, n=172 (38.2%).
Number of patients with E/e’ measurements available: Prior AF, n=110 (40.7%); concurrent AF, n=78 (35.6%); SR, n=166 (36.9%).
AAD, antiarrhythmic drug; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, brain-type natriuretic peptide; BP, blood pressure; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate (Modified Diet in Renal Disease formula); LAVI, left atrial volume index; LDL, low-density lipoprotein; LVEF, left ventricular ejection fraction; LVIDd, left ventricular internal dimension in end-diastole; NYHA, New York Heart Association Class; MI, myocardial infarction; TR, tricuspid regurgitation; TSH, thyroid stimulating hormone.
Figure 1.
Time between diagnosis of AF and HFpEF (truncated at ±30 years of HFpEF diagnosis). Zero represents HFpEF diagnosis (baseline).
As compared to patients in SR, HFpEF patients with prior or concurrent AF were older, had higher BNP and LAVI, shorter deceleration times and tended to have higher E/e’ (Table 1). Patients with concurrent AF had higher heart rates than patients with prior AF or SR, lower blood pressure and diabetes prevalence than HFpEF patients in SR, and lower LAVI and statin use than patients with prior AF. Patients with prior AF had more cerebrovascular disease than patients in SR or concurrent AF. Importantly, standard HF medications, EF, LV size and pulmonary pressures were similar regardless of AF status.
Incidence and risk factors for AF in HFpEF
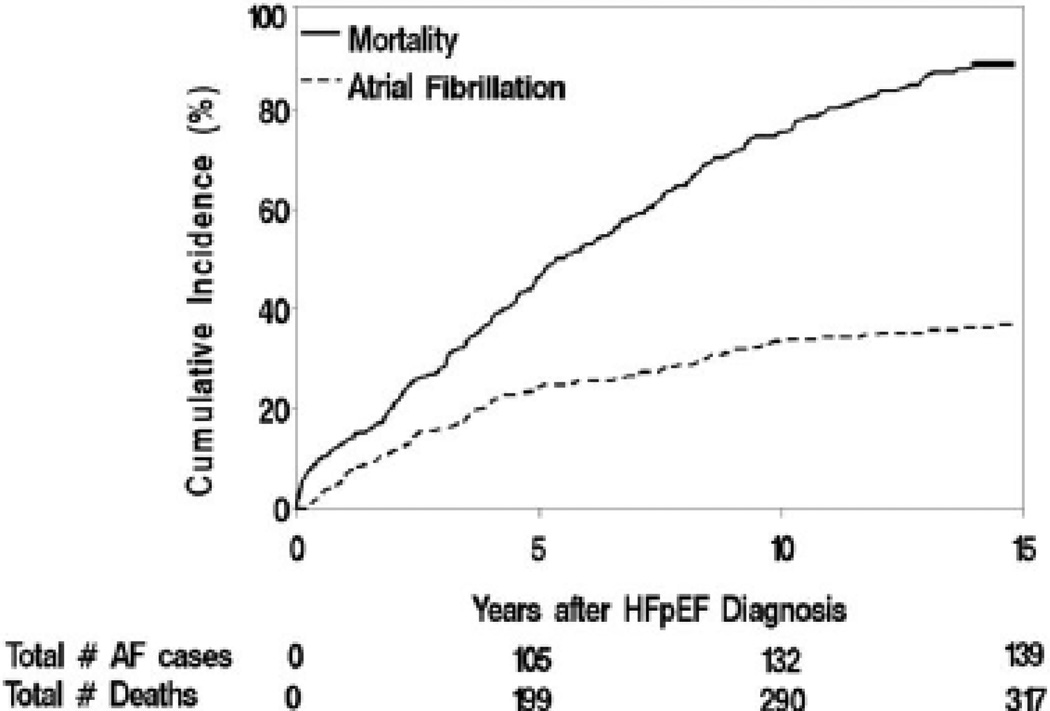
Over a median follow-up of 3.7 years, 142 (31.6%) patients in SR at HFpEF diagnosis were subsequently diagnosed with AF, giving an unadjusted incidence of 69 AF events per 1000 person-years. Median (IQR) time to AF development was 3.1(1.2–5.0) years (Figure 2). No significant secular trend in AF incidence was observed over the study duration (time period: unadjusted p=0.28; age/sex-adjusted p=0.18).
Figure 2.
Cumulative incidence of AF adjusting for death as a competing risk (dashed line) and cumulative incidence of death (solid line) among HFpEF patients presenting in sinus rhythm and no prior history of AF (truncated at 15 years after HFpEF diagnosis).
HFpEF patients who developed AF had less severe symptoms and lower rates of statin use at HF diagnosis than patients who did not, but were otherwise similar with respect to baseline clinical and echocardiographic characteristics (Table 2). Univariable associations with incident AF included older age, hypertension, lower eGFR, larger LAVI and higher filling pressures (estimated by E/e’, Table 320). LAVI and E/e’ were moderately correlated with each other (Spearman’s ρ 0.3, p=0.0005). In multivariable models, higher E/e’ remained associated with incident AF after adjusting for age, sex, hypertension, eGFR, and statin use (HR 1.47, 95%CI 1.00–2.12, p=0.05; Supplemental Table 2). Statin use was associated with a lower incidence of AF before and after adjustment for age and sex (Table 320), pertinent clinical variables (HR 0.60, 95%CI 0.38–0.92, p=0.02; Supplemental Table 2) and adjustment for low-density lipoprotein levels within 1 year of HFpEF diagnosis (HR 0.54, 95%CI 0.32–0.89, p=0.02; Supplemental Table 3). The CHADS2 and CHA2DS2-VASc scores were also associated with incident AF (Table 320).
Table 2.
Baseline characteristics of HFpEF patients presenting in sinus rhythm by future AF status.
| Variables | N | Overall (n=450) |
Rhythm status at last follow up | |
|---|---|---|---|---|
| Incident AF (n=142) |
No incident AF (n=308) |
|||
| Demographics and clinical data | ||||
| Age, years | 450 | 74.4±13.9 | 75.3±12.7 | 74.0±14.5 |
| Female sex, n (%) | 450 | 273 (60.7) | 84 (59.2) | 189 (61.4) |
| BMI, kg/m2 | 450 | 30.2±7.8 | 30.3±7.6 | 30.1±7.9 |
| Heart rate, bpm | 440 | 83±19 | 81±19 | 84±19 |
| Systolic BP, mmHg | 435 | 152±32 | 153±32 | 151±33 |
| Diastolic BP, mmHg | 435 | 77±17 | 77±15 | 77±18 |
| NYHA III-IV, n (%) | 382 | 61 (16) | 11(10) | 50 (19) |
| Co-morbidities | ||||
| Previous MI, n (%) | 448 | 65 (15) | 19 (14) | 46 (15) |
| Hypertension, n (%) | 448 | 290 (65) | 88 (62) | 202 (66) |
| Diabetes, n (%) | 449 | 136 (30) | 35 (25) | 101 (33) |
| Cerebrovascular disease, n (%) | 442 | 78 (18) | 23 (16) | 55 (18) |
| COPD, n (%) | 442 | 79 (18) | 21 (15) | 58 (19) |
| Thyroid disease, n (%) | 437 | 26 (6) | 10 (7) | 16 (5) |
| Medication at HFpEF diagnosis | ||||
| ACEI or ARB, n (%) | 448 | 183 (41) | 53 (37) | 130 (43) |
| Beta-blocker, n (%) | 448 | 202 (45) | 56 (39) | 146 (48) |
| Diuretic, n (%) | 448 | 313 (70) | 104 (73) | 209 (68) |
| Statin, n (%) | 448 | 112 (25) | 24 (17) | 88 (29) |
| AAD, n (%) | 448 | 8 (2) | 3 (2) | 5 (2) |
| Laboratory data | ||||
| eGFR, ml/min/1.73m2φ | 442 | 54 (43–68) | 53 (40–69) | 54 (43–68) |
| BNP, pg/mlφ | 148 | 327 (142–664) | 321 (152–561) | 332 (129–670) |
| TSH, mIU/Lφ | 145 | 2.1 (1.2–3.7) | 2.4 (0.9–4.5) | 2.1 (1.2–3.5) |
| LDL, g/dLφ | 233 | 93 (71–118) | 89 (71–113) | 104 (73–131) |
| Echo data | ||||
| LVIDd, mm | 321 | 48.3±6.2 | 49.1±6.6 | 48.0±5.9 |
| LVEF, % | 450 | 60.9±7.0 | 60.4±7.0 | 61.1±7.1 |
| Deceleration time, ms | 313 | 216±57 | 221±58 | 213±57 |
| TR velocity, m/s | 312 | 3.0±0.5 | 3.0±0.5 | 3.0±0.5 |
| LAVI, ml/m2 | 137 | 40.7±12.4 | 44.1±13.8 | 39.6±11.8 |
| e’, msφ | 172 | 0.06±0.02 | 0.06±0.02 | 0.06±0.02 |
| E/e’ | 166 | 16.7±7.6 | 18.4±8.9 | 16.0±7.0 |
Mean±SD shown [φmedian(IQR)]
AAD, antiarrhythmic drug; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, brain-type natriuretic peptide; BP, blood pressure; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate (Modified Diet in Renal Disease formula); LAVI, left atrial volume index; LDL, low-density lipoprotein; LVEF, left ventricular ejection fraction; LVIDd, left ventricular internal dimension in end-diastole; NYHA, New York Heart Association Class; MI, myocardial infarction; TR, tricuspid regurgitation; TSH, thyroid stimulating hormone.
Table 3.
Cox proportional hazards regression for prediction of incident atrial fibrillation in HFpEF.
| Variable | Univariable HR (95%CI) |
p-value | Age and sex-adjusted HR (95%CI) |
p-value |
|---|---|---|---|---|
| Age (per 10y) | 1.30 (1.14–1.50) | <0.0001 | - | - |
| Hypertension | 1.51 (1.0–2.16) | 0.019 | 1.41 (0.99–2.01) | 0.054 |
| Statin use | 0.59 (0.37–0.90) | 0.013 | 0.62 (0.39–0.95) | 0.027 |
| eGFR (per 10ml/min/1.73m2) | 0.89 (0.82–0.96) | 0.004 | 0.91 (0.83–0.99) | 0.027 |
| LAVI (per 10ml/m2) | 1.33 (1.02–1.70) | 0.033 | 1.30 (0.99–1.68) | 0.059 |
| e’ (per 0.01ms) | 0.83 (0.69–0.98) | 0.031 | 0.87 (0.72–1.05) | 0.15 |
| E/e’ ratio | 1.05 (1.01–1.09) | 0.012 | 1.04 (1.00–1.08) | 0.036 |
| CHADS2 | 1.15 (1.01–1.31) | 0.034 | n/a* | - |
| CHA2DS2-VASc | 1.12 (1.02–1.24) | 0.023 | n/a* | - |
CI, confidence interval; eGFR, estimated glomerular filtration rate (Modified Diet in Renal Disease formula); HR, hazard ratio; LAVI, left atrial volume index.
Age is a component of the CHADS2 score and age and sex are incorporated in the CHA2DS2-VASc score20.
Impact of AF on survival in HFpEF
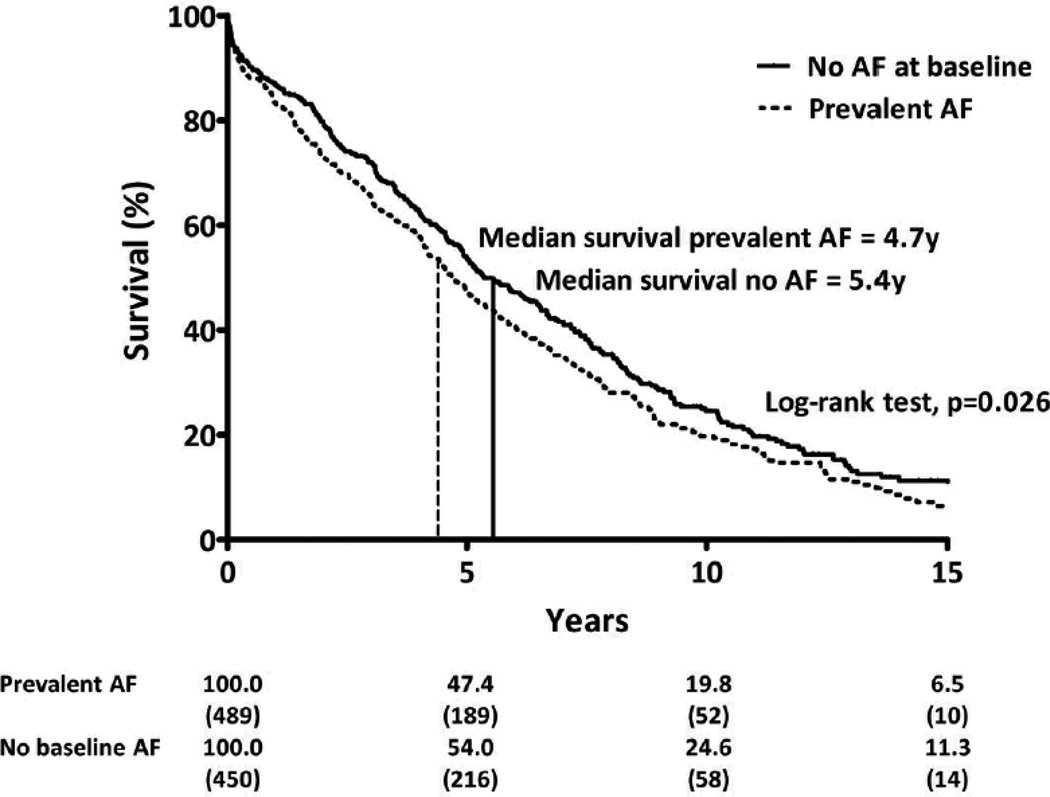
Survival data were available for all patients in this study. Median (IQR) follow-up was 4.3 (1.9–7.2) years or 3998 person-years after HFpEF diagnosis. There were 684 deaths overall (72.8% of the study population). Survival at two years was lower in subjects with prevalent AF as compared to SR at HFpEF diagnosis (73.2 vs. 79.8%, p=0.02) (Figure 3). Compared to HFpEF patients without prevalent or incident AF, prevalent AF was associated with reduced survival even after adjustment for age, sex, and pertinent clinical variables (Table 4). When stratified by AF group, prior AF was associated with reduced age- and sex-adjusted survival (HR for mortality 1.40, 95%CI 1.16–1.70, p<0.0006) while concurrent AF demonstrated a trend towards reduced age- and sex-adjusted survival (HR 1.18, 95%CI 0.96–1.45. p=0.11), compared to patients with no AF. The independent relationship between prior AF and mortality persisted after adjustment for pertinent covariates (Supplemental Table 4). Compared to HFpEF patients without prevalent or incident AF, incident AF was also independently associated with reduced survival after adjustment for pertinent covariates (Table 4). No sex-based differences were observed in AF incidence or HFpEF survival in this study.
Figure 3.
Kaplan-Meier survival analysis according to rhythm status at baseline. Survival curves shown for patients with prevalent AF (dashed line) and sinus rhythm (solid line) at HFpEF diagnosis. Median survival by group shown (vertical line). Numbers given below figure are survival rates (%) and number of patients at risk (in parentheses) at each 5-year interval, truncated at 15 years after HFpEF diagnosis.
Table 4.
Atrial fibrillation and risk of death in HFpEF.
| Variable | No AF (Referent) |
Prevalent AF | Incident AF |
|---|---|---|---|
| Unadjusted | 1.00 | 1.51 (1.27–1.78)** | 2.75 (2.17–3.49)** |
| Age and sex adjusted | 1.00 | 1.30 (1.09–1.54)* | 2.45 (1.93–3.11)** |
| Model 1† | 1.00 | 1.24 (1.04–1.47)* | 2.20 (1.72–2.81)** |
| Model 2‡ | 1.00 | 1.27 (1.06–1.51)* | 2.22 (1.73–2.84)** |
Data are reported as HR (95%CI)
p<0.001
p<0.05
Model 1 covariates include: age, sex, BMI, estimated GFR, hypertension, COPD, ARB, BB, statin.
Model 2 covariates include: Model 1 covariates and AAD use.
Discussion
In this large, population-based cohort study, over two-thirds of HFpEF patients had AF prior to, concurrent with, or subsequent to HFpEF diagnosis, underscoring the interplay of these two conditions. At HFpEF diagnosis, patients with prevalent (prior or concurrent) AF were older, had larger atria, worse DD and higher BNP than those in SR, consistent with more advanced HF. Development of incident AF was associated with older age, hypertension, renal dysfunction, LA dilatation and DD at HF diagnosis, but fewer patients treated with statins at HF diagnosis developed AF over time. Scores predictive of thromboembolic risk in AF were also associated with incident AF in HFpEF patients. Importantly, both prevalent and incident AF were associated with worse survival in HFpEF even after adjustment for potential confounders. These data suggest that AF may be a marker and potentially a mediator of increased mortality in HFpEF independent of other known risk factors.
Prevalence of AF in HFpEF
AF was present in over 50% of our community-based HFpEF patients at HF diagnosis, which greatly exceeds previous reported estimates from hospitalized8, 9 or clinical trial cohorts even with lower EF cut-offs4, 5, and prior population-based studies6, 7, 21. As there was no increase in AF prevalence over the study period, these data suggest that AF may be more prevalent among HFpEF patients than previously appreciated. Diagnosis of AF can incorporate a number of subtypes including paroxysmal AF (PAF), and a persistent or chronic form. Our inclusion of ‘any’ prior diagnosis of AF, specifically to include rigorous PAF ascertainment from medical records, may account for a higher prevalence compared to other studies determining AF status in HFpEF. In the CHARM-Preserved trial patients with an ECG demonstrating SR but with a history of AF were nonetheless categorized as no AF22. However, progression of PAF to persistent and then chronic AF is well recognized23 and the clinical associations and adverse prognostic implications of prevalent AF as classified here supports its clinical relevance.
AF and HFpEF share a number of common risk factors. In this study patients with prevalent AF were older, with more cerebrovascular disease (prior AF group) but otherwise had a similar clinical profile to HFpEF patients without AF at HF diagnosis. BNP was higher among patients with prevalent AF, though symptom severity (NYHA class) at presentation was similar between groups. The graded association between LAVI and duration of AF, i.e. larger in patients with prior than concurrent AF, and smallest in HFpEF patients with no AF, supports a link between LA remodeling in HFpEF and AF development. Several clinical studies have reported that DD is a risk factor for incident AF in the general population24, 25. Among markers of diastolic function, we also found a shorter E wave deceleration time was associated with prevalent AF in HFpEF.
Incidence of AF in HFpEF
The incidence rate of AF in this study was 69 cases per 1000 person-years. By comparison, AF incidence rates reported in the general population range from 3 to 6 cases per 1000 person-years26, 27, to 28.3 per 1000 person-years in US Medicare beneficiaries age 65 years and older28. In keeping with the notion that structural heart disease may promote atrial remodeling and maintenance of AF, a higher observed incidence among HFpEF patients is expected here. Interestingly however, the incidence rate also exceeds that reported among Framingham HFrEF patients (54 cases per 1000 person-years; mean age 73±11 years)29 and following myocardial infarction in Olmsted County subjects (42 cases per 1000 person-years; mean age 68±15 years)30 despite a comparable mean age to HFpEF patients presenting in SR. This is noteworthy from a public health perspective as recent studies in clinical trial cohorts suggest that prevalent AF imparts a greater relative risk of cardiovascular death or hospitalization for worsening HF in HFpEF patients compared to those with HFrEF5, 22.
An incidence rate for AF in HFpEF patients in the community has not been reported previously. In the CHARM-Preserved trial only 4.9% of the HFpEF (EF≥0.40) cohort in SR at recruitment developed AF by the end of the study (median follow-up 37.7 months) as compared to a crude incidence of 31.6% over 44 months in the current cohort. The CHARM cohort were younger at baseline (mean age 66.4±11.1 years) and healthy enough to enter a clinical trial. However, CHARM patients had prevalent rather than incident HFpEF and were required to have had a previous cardiovascular hospitalization for inclusion in the trial22. It is likely that our examination of the broader spectrum of HFpEF patients in the community setting accounts for the differences in AF incidence, further underscoring the difference between clinical trial and community cohorts.
Not unexpectedly we found increasing age, and degree of DD (E/e’) predicted incident AF in HFpEF patients presenting in SR, as has been observed in other clinical contexts24, 27. Our data confirm that these associations prevail in HFpEF and may be beneficial in risk stratification. Statin use displayed a significant and age-independent negative association with incident AF. A similar association has been shown in patients with coronary artery disease31,32, following cardiac surgery33, and between statin use and recurrent AF in patients with a history of AF34. However, the current data are the first to report an (inverse) association between statin use and AF incidence in HFpEF, which persists after multivariable adjustment including LDL levels. An antiarrhythmic effect of statins has been attributed to anti-inflammatory properties35, reduction of oxidative stress36, or modulation of the autonomic nervous system37. While the CORONA trial did not demonstrate a reduction in mortality with statin use in HFrEF38, a reduction in cardiovascular hospitalizations was observed which may be related to effects of statin use on AF incidence. Ancillary analysis of the GISSI-HF population (mixed HF etiology) also reported rosuvastatin therapy correlated with a decreased incidence of AF compared to placebo39. As no therapy has yet been shown to improve outcomes or reduce symptom burden in HFpEF, this novel finding warrants further investigation.
Association between AF and survival in HFpEF
Although previous studies have described the prognosis of patients with HFpEF and AF, most, if not all, concentrated on prevalent AF40, and largely compared outcomes to HFrEF patients rather than HFpEF patients without AF, as recently reviewed41. Importantly, we have demonstrated that incident AF after HFpEF diagnosis confers an independent and greater risk of death than either prevalent AF (prior or concurrent) or no AF among HFpEF patients in the community. As incident AF was not incorporated in most previous studies, the prognostic implications of AF in HFpEF may have been underestimated. Indeed, our findings are in agreement with a recent analysis of the Cardiovascular Research Network PRESERVE Study where incident AF was associated with a similar, albeit slightly lower, risk of all-cause death in HFpEF patients42. A less stringent definition of incident AF (AF occurring any time after recruitment) and shorter median follow up duration (1.8 years vs. 3.7 years herein) may explain the lower risk observed. Nevertheless, whether incident AF represents a marker and/or mediator of HFpEF progression remains unknown and difficult to confirm in an epidemiological study. Our multivariable analysis would suggest that the greater mortality more likely attributes to AF than other currently recognized (and adjusted for) prognostic factors including age, hypertension, and LV diastolic function.
As AF increases the rate of thromboembolic events and may worsen global cardiac performance and thus contribute to neurohumoral activation, prevention of AF may ameliorate the adverse outcomes associated with its presence. Though AFFIRM43 and AF-CHF44 failed to demonstrate an overall survival advantage associated with restoring and maintaining SR in a general AF or HFrEF population respectively, patients with HFpEF were excluded from the AF-CHF trial, thus a rhythm control strategy has not been tested in a dedicated HFpEF population.
Notably, this study also delineates an independent risk of death associated with concurrent AF in univariable analysis, though only a trend towards an association (p=0.11) after adjustment for age and sex (Supplemental Table 4). In these patients the chronology of events and permanency of AF is less clear. Additionally, fewer patients with concurrent AF had a history of MI compared to patients with prior AF, and fewer had diabetes compared to SR patients at HFpEF diagnosis. We speculate that this group represents a combination of patients with pre-existing but unrecognized AF as well as patients with less severe cardiovascular dysfunction in whom new-onset AF and its adverse hemodynamic sequelae may have been the triggering mechanism for emergence of HF.
Limitations
Rhythm classification was dependent upon clinical detection and documentation of AF in the medical record. Patients with asymptomatic AF may therefore be underrepresented in AF groups though any resulting survival bias would more likely underestimate AF incidence and AF-related risk. A portion of patients for whom echo EF data was unavailable may have been more likely to have HFpEF and AF. As a group patients without EF assessment were older, had a higher prevalence of COPD (Supplemental Table 1). Thus the impact of AF on outcomes in HFpEF may have been underestimated due to the exclusion of this particularly high risk subgroup. Observed associations with incident AF will generalize to HFpEF cases surviving beyond 3 months, as per the definition used. Detailed data on treatment regimens, adequacy of rate control and anticoagulation for AF patients, and cause of death were not ascertained. Similarly in mortality analyses, information for covariates besides rhythm were unavailable to update over time, including subsequent antiarrhythmic use, and may confound the risk of death observed. The population of Olmsted County is mainly white. Since the prevalence45 and incidence46 of AF are higher among Caucasians as compared to African Americans, these data may not be generalizable to non-Caucasian populations.
Conclusion
In this large population-based cohort of incident HFpEF, 66% of patients either had AF prior to, concurrent with, or subsequent to HFpEF diagnosis. Moreover, prevalent and incident AF were independently associated with increased mortality. In the absence of proven treatment strategies for HFpEF, further studies are required to determine whether AF represents a marker of HFpEF progression and/or a therapeutic target.
Supplementary Material
Clinical Perspective Summary.
Atrial fibrillation (AF) and heart failure with preserved ejection fraction (HFpEF) are burgeoning and commonly coexistent cardiovascular epidemics of an aging population. While previous studies have focused on the association between prevalent AF and outcomes in HFpEF patients, the predictors and clinical impact of incident AF remains unclear. We examined 939 community-based patients with incident HFpEF from Olmsted County, MN, and evaluated their clinical characteristics and mortality according to the temporal relationship of AF to HFpEF onset. AF occurred prior to (>3months) or concurrent with (±3 months) HFpEF diagnosis in 52% HFpEF patients and was associated with older age, higher brain natriuretic peptide, and larger left atrial volume index at HFpEF diagnosis compared to patients in sinus rhythm (SR). Of patients in SR at HFpEF diagnosis, 32% developed AF over a median follow-up of 3.7 years. Age and diastolic dysfunction were positively associated with incident AF, while HFpEF patients on statin therapy were significantly less likely to develop AF. Prevalent and incident AF were associated with a higher risk of all-cause mortality compared to HFpEF patients in SR. Incident AF was associated with the highest risk for death. AF is common in the natural history of HFpEF and confers a poor prognosis. Future studies are required to determine whether intervention for AF may improve outcomes or if statin use can prevent AF in HFpEF.
Acknowledgements
We would like to sincerely thank Susan A. Weston, MS and Ruoxiang Jiang for their assistance in data retrieval and study design.
Funding Sources: This study was supported by grants from the National Institutes of Health (RO1 HL072435) and the National Institute on Aging (R01 Ag034676). Dr. Zakeri is a Heart Failure Clinical Research Skills Development Core Fellow (U10 HL110262) and is supported by the Mayo Clinic CTSA [Grant Number TL1 TR000137 from the National Center for Advancing Translational Science (NCATS)]. Contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: None.
References
- 1.Braunwald E. Shattuck lecture--cardiovascular medicine at the turn of the millennium: Triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 2.Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: Treatment considerations for a dual epidemic. Circulation. 2009;119:2516–2525. doi: 10.1161/CIRCULATIONAHA.108.821306. [DOI] [PubMed] [Google Scholar]
- 3.Senni M, Tribouilloy CM, Rodeheffer RJ, Jacobsen SJ, Evans JM, Bailey KR, Redfield MM. Congestive heart failure in the community: A study of all incident cases in olmsted county, minnesota, in 1991. Circulation. 1998;98:2282–2289. doi: 10.1161/01.cir.98.21.2282. [DOI] [PubMed] [Google Scholar]
- 4.McMurray JJ, Carson PE, Komajda M, McKelvie R, Zile MR, Ptaszynska A, Staiger C, Donovan JM, Massie BM. Heart failure with preserved ejection fraction: Clinical characteristics of 4133 patients enrolled in the i-preserve trial. Eur J Heart Fail. 2008;10:149–156. doi: 10.1016/j.ejheart.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Linssen GC, Rienstra M, Jaarsma T, Voors AA, van Gelder IC, Hillege HL, van Veldhuisen DJ. Clinical and prognostic effects of atrial fibrillation in heart failure patients with reduced and preserved left ventricular ejection fraction. Eur J Heart Fail. 2011;13:1111–1120. doi: 10.1093/eurjhf/hfr066. [DOI] [PubMed] [Google Scholar]
- 6.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 7.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: Prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948–1955. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 8.Piccini JP, Hernandez AF, Zhao X, Patel MR, Lewis WR, Peterson ED, Fonarow GC. Quality of care for atrial fibrillation among patients hospitalized for heart failure. J Am Coll Cardiol. 2009;54:1280–1289. doi: 10.1016/j.jacc.2009.04.091. [DOI] [PubMed] [Google Scholar]
- 9.Lenzen MJ, Scholte op Reimer WJ, Boersma E, Vantrimpont PJ, Follath F, Swedberg K, Cleland J, Komajda M. Differences between patients with a preserved and a depressed left ventricular function: A report from the euroheart failure survey. Eur Heart J. 2004;25:1214–1220. doi: 10.1016/j.ehj.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Masoudi FA, Havranek EP, Smith G, Fish RH, Steiner JF, Ordin DL, Krumholz HM. Gender, age, heart failure with preserved left ventricular systolic function. J Am Coll Cardiol. 2003;41:217–223. doi: 10.1016/s0735-1097(02)02696-7. [DOI] [PubMed] [Google Scholar]
- 11.Dries DL, Exner DV, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: A retrospective analysis of the solvd trials. Studies of left ventricular dysfunction. J Am Coll Cardiol. 1998;32:695–703. doi: 10.1016/s0735-1097(98)00297-6. [DOI] [PubMed] [Google Scholar]
- 12.Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: Epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003;91:2D–8D. doi: 10.1016/s0002-9149(02)03373-8. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed A, Thornton P, Perry GJ, Allman RM, DeLong JF. Impact of atrial fibrillation on mortality and readmission in older adults hospitalized with heart failure. Eur J Heart Fail. 2004;6:421–426. doi: 10.1016/j.ejheart.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Ducharme A, Swedberg K, Pfeffer MA, Cohen-Solal A, Granger CB, Maggioni AP, Michelson EL, McMurray JJ, Olsson L, Rouleau JL, Young JB, Olofsson B, Puu M, Yusuf S. Prevention of atrial fibrillation in patients with symptomatic chronic heart failure by candesartan in the candesartan in heart failure: Assessment of reduction in mortality and morbidity (charm) program. Am Heart J. 2006;152:86–92. [PubMed] [Google Scholar]
- 15.Rusinaru D, Leborgne L, Peltier M, Tribouilloy C. Effect of atrial fibrillation on long-term survival in patients hospitalised for heart failure with preserved ejection fraction. Eur J Heart Fail. 2008;10:566–572. doi: 10.1016/j.ejheart.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Rivero-Ayerza M, Scholte Op Reimer W, Lenzen M, Theuns DA, Jordaens L, Komajda M, Follath F, Swedberg K, Cleland JG. New-onset atrial fibrillation is an independent predictor of in-hospital mortality in hospitalized heart failure patients: Results of the euroheart failure survey. Eur Heart J. 2008;29:1618–1624. doi: 10.1093/eurheartj/ehn217. [DOI] [PubMed] [Google Scholar]
- 17.Melton LJ., 3rd History of the rochester epidemiology project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 18.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 19.Chamberlain AM, Redfield MM, Alonso A, Weston SA, Roger VL. Atrial fibrillation and mortality in heart failure: A community study. Circ Heart Fail. 2011;4:740–746. doi: 10.1161/CIRCHEARTFAILURE.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: Results from the national registry of atrial fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 21.Gottdiener JS, McClelland RL, Marshall R, Shemanski L, Furberg CD, Kitzman DW, Cushman M, Polak J, Gardin JM, Gersh BJ, Aurigemma GP, Manolio TA. Outcome of congestive heart failure in elderly persons: Influence of left ventricular systolic function. The cardiovascular health study. Ann Intern Med. 2002;137:631–639. doi: 10.7326/0003-4819-137-8-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 22.Olsson LG, Swedberg K, Ducharme A, Granger CB, Michelson EL, McMurray JJ, Puu M, Yusuf S, Pfeffer MA. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: Results from the candesartan in heart failure-assessment of reduction in mortality and morbidity (charm) program. J Am Coll Cardiol. 2006;47:1997–2004. doi: 10.1016/j.jacc.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 23.Kerr CR, Humphries KH, Talajic M, Klein GJ, Connolly SJ, Green M, Boone J, Sheldon R, Dorian P, Newman D. Progression to chronic atrial fibrillation after the initial diagnosis of paroxysmal atrial fibrillation: Results from the canadian registry of atrial fibrillation. Am Heart J. 2005;149:489–496. doi: 10.1016/j.ahj.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 24.Tsang TS, Gersh BJ, Appleton CP, Tajik AJ, Barnes ME, Bailey KR, Oh JK, Leibson C, Montgomery SC, Seward JB. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol. 2002;40:1636–1644. doi: 10.1016/s0735-1097(02)02373-2. [DOI] [PubMed] [Google Scholar]
- 25.Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Risks for atrial fibrillation and congestive heart failure in patients >/=65 years of age with abnormal left ventricular diastolic relaxation. Am J Cardiol. 2004;93:54–58. doi: 10.1016/j.amjcard.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in olmsted county, minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 27.Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D'Agostino RB, Sr, Newton-Cheh C, Yamamoto JF, Magnani JW, Tadros TM, Kannel WB, Wang TJ, Ellinor PT, Wolf PA, Vasan RS, Benjamin EJ. Development of a risk score for atrial fibrillation (framingham heart study): A community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, Benjamin EJ, Curtis LH. Incidence and prevalence of atrial fibrillation and associated mortality among medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes. 2012;5:85–93. doi: 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The framingham heart study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 30.Jabre P, Jouven X, Adnet F, Thabut G, Bielinski SJ, Weston SA, Roger VL. Atrial fibrillation and death after myocardial infarction: A community study. Circulation. 2011;123:2094–2100. doi: 10.1161/CIRCULATIONAHA.110.990192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young-Xu Y, Jabbour S, Goldberg R, Blatt CM, Graboys T, Bilchik B, Ravid S. Usefulness of statin drugs in protecting against atrial fibrillation in patients with coronary artery disease. Am J Cardiol. 2003;92:1379–1383. doi: 10.1016/j.amjcard.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 32.Pellegrini CN, Vittinghoff E, Lin F, Hulley SB, Marcus GM. Statin use is associated with lower risk of atrial fibrillation in women with coronary disease: The hers trial. Heart. 2009;95:704–708. doi: 10.1136/hrt.2008.154054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patti G, Chello M, Candura D, Pasceri V, D'Ambrosio A, Covino E, Di Sciascio G. Randomized trial of atorvastatin for reduction of postoperative atrial fibrillation in patients undergoing cardiac surgery: Results of the armyda-3 (atorvastatin for reduction of myocardial dysrhythmia after cardiac surgery) study. Circulation. 2006;114:1455–1461. doi: 10.1161/CIRCULATIONAHA.106.621763. [DOI] [PubMed] [Google Scholar]
- 34.Fauchier L, Pierre B, de Labriolle A, Grimard C, Zannad N, Babuty D. Antiarrhythmic effect of statin therapy and atrial fibrillation a meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2008;51:828–835. doi: 10.1016/j.jacc.2007.09.063. [DOI] [PubMed] [Google Scholar]
- 35.Plenge JK, Hernandez TL, Weil KM, Poirier P, Grunwald GK, Marcovina SM, Eckel RH. Simvastatin lowers c-reactive protein within 14 days: An effect independent of low-density lipoprotein cholesterol reduction. Circulation. 2002;106:1447–1452. doi: 10.1161/01.cir.0000029743.68247.31. [DOI] [PubMed] [Google Scholar]
- 36.Rosenson RS. Statins in atherosclerosis: Lipid-lowering agents with antioxidant capabilities. Atherosclerosis. 2004;173:1–12. doi: 10.1016/S0021-9150(03)00239-9. [DOI] [PubMed] [Google Scholar]
- 37.Liu T, Li GP. Statins may prevent postoperative atrial fibrillation through autonomic modulation. Am J Cardiol. 2006;97:1266. doi: 10.1016/j.amjcard.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 38.Kjekshus J, Apetrei E, Barrios V, Bohm M, Cleland JG, Cornel JH, Dunselman P, Fonseca C, Goudev A, Grande P, Gullestad L, Hjalmarson A, Hradec J, Janosi A, Kamensky G, Komajda M, Korewicki J, Kuusi T, Mach F, Mareev V, McMurray JJ, Ranjith N, Schaufelberger M, Vanhaecke J, van Veldhuisen DJ, Waagstein F, Wedel H, Wikstrand J. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 39.Maggioni AP, Fabbri G, Lucci D, Marchioli R, Franzosi MG, Latini R, Nicolosi GL, Porcu M, Cosmi F, Stefanelli S, Tognoni G, Tavazzi L. Effects of rosuvastatin on atrial fibrillation occurrence: Ancillary results of the gissi-hf trial. Eur Heart J. 2009;30:2327–2336. doi: 10.1093/eurheartj/ehp357. [DOI] [PubMed] [Google Scholar]
- 40.Komajda M, Carson PE, Hetzel S, McKelvie R, McMurray J, Ptaszynska A, Zile MR, Demets D, Massie BM. Factors associated with outcome in heart failure with preserved ejection fraction: Findings from the irbesartan in heart failure with preserved ejection fraction study (i-preserve) Circ Heart Fail. 2011;4:27–35. doi: 10.1161/CIRCHEARTFAILURE.109.932996. [DOI] [PubMed] [Google Scholar]
- 41.Mamas MA, Caldwell JC, Chacko S, Garratt CJ, Fath-Ordoubadi F, Neyses L. A meta-analysis of the prognostic significance of atrial fibrillation in chronic heart failure. Eur J Heart Fail. 2009;11:676–683. doi: 10.1093/eurjhf/hfp085. [DOI] [PubMed] [Google Scholar]
- 42.McManus DD, Hsu G, Sung SH, Saczynski JS, Smith DH, Magid DJ, Gurwitz JH, Goldberg RJ, Go AS. Atrial fibrillation and outcomes in heart failure with preserved versus reduced left ventricular ejection fraction. J Am Heart Assoc. 2013;2:e005694. doi: 10.1161/JAHA.112.005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 44.Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, Bourassa MG, Arnold JM, Buxton AE, Camm AJ, Connolly SJ, Dubuc M, Ducharme A, Guerra PG, Hohnloser SH, Lambert J, Le Heuzey JY, O'Hara G, Pedersen OD, Rouleau JL, Singh BN, Stevenson LW, Stevenson WG, Thibault B, Waldo AL. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 45.Ruo B, Capra AM, Jensvold NG, Go AS. Racial variation in the prevalence of atrial fibrillation among patients with heart failure: The epidemiology, practice, outcomes, and costs of heart failure (epoch) study. J Am Coll Cardiol. 2004;43:429–435. doi: 10.1016/j.jacc.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 46.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and african-americans: The atherosclerosis risk in communities (aric) study. Am Heart J. 2009;158:111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.