Abstract
Objective
To determine whether statins affect type 1 interferon responses in relapsing-remitting multiple sclerosis (RRMS).
Design
Study effects of atorvastatin on type 1 interferon responses in Jurkat cells, mononuclear cells (MNCs) from therapy-naive patients with RRMS in vitro, and MNCs from interferon-treated RRMS patients in vivo in 4 conditions: no drug, statin only, interferon-beta only, and statin added on to interferon-beta therapy.
Patients
The study examined clinically stable patients with RRMS: 21 therapy-naive patients and 14 patients receiving interferon-beta with a statin.
Interventions
Statin effects on in vitro and in vivo interferon-beta–induced STAT1 transcription factor activation, expression of interferon-stimulated proteins in MNCs, and serum type 1 interferon activity.
Results
In vitro, atorvastatin dose dependently inhibited expression of interferon-stimulated P-Y-STAT1 by 44% (P< .001), interferon regulatory factor 1 protein by 30% (P= .006), and myxovirus resistance 1 protein by 32% (P=.004) compared with no-statin control in MNCs from therapy-naive RRMS patients. In vivo, 9 of 10 patients who received high-dose statins (80 mg) had a significant reduction in interferon-beta therapy–induced serum interferon-α/β activity, whereas only 2 of 4 patients who received medium-dose statins (40 mg) had reductions. High-dose add-on statin therapy significantly blocked interferon-beta function, with less P-Y-STAT1 transcription factor activation, and reduced myxovirus resistance 1 protein and viperin protein production. Medium doses of statins did not change STAT1 activation.
Conclusions
High-dose add-on statin therapy significantly reduces interferon-beta function and type 1 interferon responses in RRMS patients. These data provide a putative mechanism for how statins could counteract the beneficial effects of interferon-beta and worsen disease.
Multiple sclerosis (MS) is a chronic inflammatory disease in which autoreactive immune cells infiltrate the central nervous system (CNS), leading to demyelination and neurologic disability.1 Interferon-beta ameliorates MS by altering peripheral and CNS immune responses and reducing disease activity. A total of 80% of patients with relapsing-remitting MS (RRMS) benefit from interferon-beta, but patients with progressive MS have minimal response to interferon-beta therapy.2 Endogenous type 1 interferons (interferon-α and interferon-β) are important in disease progression and treatment response. Before therapy, interferon-α–related pathways are fundamentally dys-regulated in mononuclear cells (MNCs) from all forms of MS and are more abnormal than TH1, TH2, and other cytokine pathways.3 After transition of RRMS to progressive MS, interferon-β no longer can phosphorylate serine on STAT1 or induce certain genes in vitro.2
Statins ameliorate murine experimental autoimmune encephalomyelitis and are anti-inflammatory and neuroprotective.4,5 Atorvastatin and glatiramer acetate synergize in the treatment of CNS autoimmunity,6 so clinical trials in RRMS have combined interferon-beta with statins. In most placebo-controlled trials, combination therapy is safe and well tolerated but has no clinical or magnetic resonance imaging (MRI) benefit over interferon-beta monotherapy.7–9 However, in a smaller, placebo-controlled trial with MS patients who were stable while taking subcutaneous interferon-beta-1a for at least 1 year prior, adding high-dose atorvastatin caused clinical and MRI exacerbations in 10 of 17 patients. The interferon-beta–only group had fewer exacerbations (1 of 10 patients; P=.02), suggesting that statins antagonize interferon-beta therapy.10 In a 307-patient, randomized, placebo-controlled, double-blind, phase 4 study, high-dose simvastatin (80 mg) added to interferon-beta-1a therapy produced no additional benefit.8 There was actually a trend for higher relapse rates and disease activity in the comedication group compared with placebo, again suggesting antagonistic effects of add-on statin therapy.
How could statins impair interferon-beta therapy? Type 1 interferons bind to cell surface receptors, interferon-α receptor 1 and interferon-α receptor 2, and activate the JAK/STAT pathway, causing phosphorylation of tyrosine and serine residues on STAT1 and tyrosine on STAT2.11,12 Phosphorylated STAT1-STAT2 heterodimer together with interferon-regulated factor 9 forms a complex that binds to DNA of the interferon-stimulated response element.13,14 The activated P-Y-STAT1 transcription factor affects expression of 1000 genes; P-S-STAT1 enhances signaling in a subset of these genes. Interferon-beta induces myxovirus resistance 1 (MxA) and viperin proteins and endogenous interferon-β and interferon-α subtypes.15,16 Through this pathway, type 1 interferon alters TH1, TH2, and TH17 immunity, dendritic cell activation and maturation, cell cycle and apoptosis, and antigen presentation.17
We hypothesized that statins block the type 1 inter-feron pathway. We evaluated in vitro pharmacokinetic and dose effects of statins on interferon-induced phosphorylation of STAT1 and STAT2 transcription factors and downstream interferon-stimulated proteins, interferon regulatory factor 1 (IRF-1), MxA, and viperin. We also compared in vivo effects of high-dose statins plus interferon-beta therapy on interferon responses, induced proteins, and endogenous type 1 interferon activity.
METHODS
STUDY PARTICIPANTS
In Vitro Experiments
Twenty-one therapy-naive patients with RRMS, 12 women and 9 men, had a mean (SEM) age of 43.7 (2.2) years. None had been treated with immunomodulators for at least 3 months. None had ongoing infections.
In Vivo Experiments
Fourteen patients (4 black and 10 white; 64% female) had a mean (SEM) age of 54.2(2.6) years, an Expanded Disability Status Scale score of 4.10(0.49), an MS duration of 15.0(2.1) years, and interferon-beta treatment duration of 9.62 (1.66) years. Eleven patients were taking interferon-beta-1b, 2 patients were taking subcutaneous interferon beta-1a, and 1 patient was taking intramuscular interferon-beta-1a. There were 4 treatment groups in vivo: no drug, statin only, interferon-beta only, and statin added on to interferon-beta therapy. Serum and MNCs were obtained for all groups at various times.
Statin therapy was stopped for 5 to 7 days (>7 half-lives) to allow washout. Interferon-beta was stopped for 57 to 70 hours based on a prior study18 to allow washout and to reflect basal levels of interferon-induced genes. Before phlebotomy and re-administration of interferon-beta injections, patients undergoing continuous long-term statin therapy took 40-mg (n = 4) or 80-mg (n = 10) statins to maximize statin effects at a safe dose. After washouts, blood was drawn at 8 am for baseline and then 4 hours (within 5 minutes) after the interferon-beta injection.
A total of 5 ×106 MNCs were immediately lysed and stored for Western blotting. Another 5 × 106 cells were cultured for 24 hours after interferon injections for ex vivo induction of MxA and viperin proteins. Serum was assayed for endogenous basal and therapy-induced type 1 interferon activity at 0 and 4 hours. All participants gave written informed consent for the University of Chicago institutional review board–approved protocol.
DOSE RESPONSE AND KINETICS OF INTERFERONS VS STATINS
The MNCs were isolated with Ficoll-Hypaque density gradients. A total of 4 × 106 cells/mL were cultured in RPMI with 10% fetal calf serum (GIBCO 1640; Invitrogen) at 37°C in 5% carbon dioxide. Cells were preincubated for 15 minutes to 48 hours with 1-, 5-, 10-, or 20-μM atorvastatin (neat preparation; Anna Tallman, PharmD, Pfizer) and subsequently stimulated with interferon-beta-1b (0, 10, 20, 40, 80, 160, 320, and 500 U/mL) for 45 minutes to induce P-Y-STAT1 phosphorylation or for 24 hours to induce downstream proteins (MxA, IRF-1, and viperin; unphosphorylated STAT1 and STAT2). Stimulated cells were lysed and stored in 1×Laemmli buffer for Western blotting.2 Reversal of statin effects with 100-μM mevalonate (Sigma Chemical Co) confirmed that the 3-hydroxy-3-methylglutaryl coenzyme A pathway affects interferon signaling.18 In addition, in Jurkat T cells at 4 × 106 cells/mL, in vitro kinetics and dose-dependent inhibition with statins combined with induction by different forms of interferon-beta were assayed with Western blots.
SERUM INTERFERON-α/β ACTIVITY ASSAY
Serum samples from 14 RRMS patients were tested using a highly sensitive assay (limit of detection of 0.1 U/mL, well below typical 10- to 20-U/mL enzyme-linked immunosorbent assay [ELISA] thresholds). Moreover, ELISA can be less specific for serum interferon than this bioassay because ELISA detects cross-reacting but nonfunctional interferon-like proteins.19 Briefly, the epithelial-derived WISH cell line (CCL-25l, ATCC) was a reporter for responsiveness to interferon-α/β. Total cellular messenger RNA (mRNA) was purified, and complementary DNA was reverse transcribed and quantified by reverse transcription–polymerase chain reaction with primers for MxA-1, RNA-dependent protein kinase (protein kinase R), and interferon-induced protein with tetratricopeptide repeats 1 (IFIT-1). This bioassay was validated in large human populations and is specific for interferon-α/β activity.20–23 Pretreatment of serum samples from MS patients with antibodies to interferon-α and interferon-β abolishes interferon-induced gene expression in this assay.21,22,24
WESTERN BLOT ANALYSIS
A total of 4 × 106 MNCs/mL were induced with media alone or interferon-beta-1b at 160 U/mL for 45 minutes for assay of P-Y-STAT1 and P-Y-STAT2 or for 24 hours for STAT1, STAT2, IRF-1, MxA, and viperin.24 Interferon-beta–induced MxA mRNA25 is well correlated with MxA protein on Western blots.2 Nonetheless, protein was used to examine interferon-beta–induced MxA responses because fluctuations are more likely to be missed with short half-life mRNA. Antibodies were goat anti-Actin (sc-1615), goat anti–P-Y701-STAT1 (sc-7988), goat anti–P-Ser-STAT1 (sc-16570-R), rabbit anti–IRF-1 (sc-20) (all Santa Cruz Biotechnology), rat anti-MxA (Stefan Lanker, PhD, Biogen), mouse anti-viperin (Peter Cresswell, PhD, Yale University), rabbit anti-STAT1 (sc-346), and rabbit anti-STAT2 (sc-476, Santa Cruz).
STATISTICAL ANALYSIS
Values from washout vs treated experiments were compared with unpaired t tests. Baseline vs drug-induced values were analyzed with paired t tests in the same MS patients tested at all conditions.
RESULTS
INHIBITION OF TYPE 1 INTERFERON SIGNALING IN VITRO
Optimal conditions for interferon-stimulated STAT activation and downstream protein expression (MxA and IRF-1) were determined with different doses (0, 1, 5, 10, and 20 μM) and kinetics (0 and 15 minutes and 1, 3, and 24 hours) of atorvastatin before treatment in Jurkat T cells and RRMS MNCs.
Three interferon-beta forms (interferon-beta-1a for intramuscular and subcutaneous use, and interferon-beta-1b for subcutaneous use but tested in vitro here) induced tyrosine phosphorylation of STAT1 in Jurkat cells after 45 minutes in vitro (eFigure 1A; http://www.archneurol.com). Preincubation with atorvastatin for 24 hours inhibited interferon-beta–stimulated P-Y-STAT1 and MxA and IRF-1 protein expression in a dose-dependent manner for all 3 interferon-beta forms (160 U/mL) in Jurkat T cells in vitro (eFigures 1B). In human U937 monocytoid cells, all 3 interferon-beta forms exhibited similar dose responses and inhibition by statin before incubation (data not shown). Statins inhibited interferon-induced tyrosine phosphorylation on STAT1 but not on STAT2. P-S-STAT1 and nonphosphorylated STAT1 and STAT2 levels did not change (data not shown), indicating that atorvastatin specifically targets P-Y-STAT1 in Jurkat cells.
Blockade of interferon-stimulated P-Y-STAT1 began at 15 minutes and was maximal after 24 hours before incubation with high-dose atorvastatin (eFigure 2A). A total of 10 μM of atorvastatin markedly decreased interferon-beta-1b–stimulated MxA and IRF-1 production; lower statin doses were less inhibitory. High-dose atorvastatin inhibition of interferon-beta-1b–induced P-Y-STAT1 in MNCs from therapy-naive RRMS patients was confirmed with blockade of interferon γ, a strong inducer of P-Y-STAT1 (eFigure 2B).
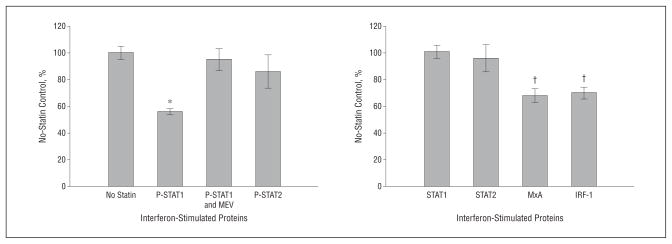
STAT1 and STAT2 must be phosphorylated for interferon-stimulated gene expression. After pretreatment for 24 hours with 10 μM of atorvastatin, MNCs from 21 therapy-naive RRMS patients were stimulated with 160 U/mL of interferon-beta-1b for 45 minutes. Atorvastatin reduced interferon-stimulated P-Y-STAT1 by 44% compared with no-statin control (P<.001) (Figure 1). One hour of 100-μM mevalonate before incubation reversed statin inhibition,26 indicating specificity of atorvastatin in inhibiting P-Y-STAT1. Atorvastatin did not block induction of type 1 interferon-stimulated P-S-STAT1 or unphosphorylated STAT1 and STAT2 in MNCs (Figure 1).
Figure 1.
In vitro atorvastatin reduces interferon-beta effects. Mononuclear cells from 21 therapy-naive patients with relapsing-remitting multiple sclerosis were pretreated at 24 hours with 10-μM atorvastatin, then induced with 160 U/mL of interferon-beta-1b for 45 minutes (phosphorylated/activated STAT transcription factors) and 24 hours (downstream proteins, STAT1, STAT2, interferon regulatory factor 1 [IRF-1], and myxovirus resistance 1 [MxA]). Proteins were quantified with Western blots and normalized with actin. *P<.001 vs no-statin control, †P<.05. MEV indicates 100-μM mevalonate. Error bars indicate SEM.
Pretreatment atorvastatin reduced downstream interferon-beta–stimulated IRF-1 (30% reduction, P=.006) and MxA protein (32% reduction, P= .004) compared with no-statin control in paired MNCs from the same therapy-naive RRMS patients (Figure 1). Pretreatment simvastatin (10 μM) also significantly inhibited type 1 interferon responses in MNCs from RRMS patients (data not shown).
HIGH-DOSE STATIN ADD-ON THERAPY AND IN VIVO INTERFERON-β SIGNALING IN RRMS PATIENTS
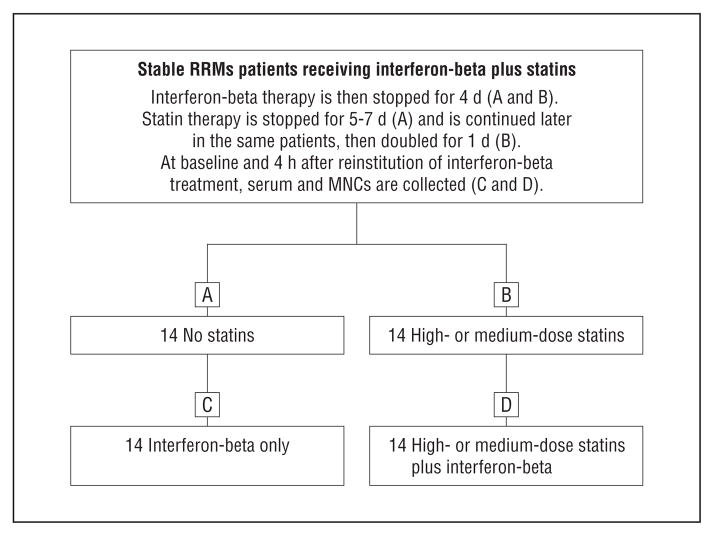
To determine whether statin add-on therapy impairs interferon-beta therapy induction of endogenous serum interferon-β activity, we measured serum type 1 interferon activity and interferon-induced proteins in 14 interferon-beta–treated RRMS patients under 4 different conditions (Figure 2). To determine the optimal time for measuring serum interferon activity, we first performed kinetics in stable RRMS patients receiving interferon-beta therapy but no statins. Blood was drawn at baseline and periodically from 10 minutes to 27 hours, and serum interferon activity was analyzed with a highly sensitive assay. Figure 3A shows representative kinetics from a stable RRMS patient given interferon-beta-1a (44 μg, 9 MU subcutaneously) after a 3-day interferon washout. Serum type 1 interferon activity was elevated by 30 minutes after interferon-beta injection and remained high until 6 hours later, then declined by 27 hours. Interferon-β averaged a 3-fold induction of P-Y-STAT1 from baseline. This interferon activity initially reflects administered interferon-beta and later is from therapy-induced endogenous interferon-β and interferon-α, based on blocking experiments with specific anti–interferon-α and anti–interferon-β antibodies. We used the 4-hour point for in vivo interferon-β stimulation because interferon-α/β induction was still high 4 hours after interferon-beta injection, and many interferon-stimulated genes are induced within 4 hours.27
Figure 2.
Fourteen clinically stable patients with relapsing-remitting multiple sclerosis (RRMS) receiving interferon-beta plus statin therapy stopped interferon-beta therapy and stopped (A and C) or continued medium- or high-dose (B and D) statin therapy. Serum type 1 interferon activity and Western blots of STAT1 and STAT2 phosphorylation were performed at 0 and 4 hours after interferon-beta injection; in vivo–induced myxovirus resistance 1 and viperin proteins were measured with Western blots at 24 hours. MNCs indicates mononuclear cells.
Figure 3.
Statins reduce interferon-beta therapy induction of serum type 1 interferon activity in 14 stable patients with relapsing-remitting multiple sclerosis. A, In vivo Rebif kinetics after a 3-day washout. B and C, Statin add-on therapy blocks interferon-beta therapy induction of serum interferon-α/β activity in 14 patients with relapsing-remitting multiple sclerosis. Serum samples were obtained at 8 am after statin washout or long-term statin alone and then exactly 4 hours after interferon-beta injections or high-dose statins plus 4 hours of interferon-beta therapy. *P <.001 vs interferon alone (paired t test). Error bars indicate SEM.
Statin add-on therapy significantly reduced serum type 1 interferon activity compared with interferon-beta monotherapy (Figure 3B). Nine of 10 patients who received high-dose statins (80 mg of atorvastatin or simvastatin) had significant reduction in serum interferon- α/β activity. Two of these patients had undetectable levels of serum interferon activity at all conditions even with this sensitive bioassay (Figure 3C). However, 2 of 4 patients who received medium-dose statins (40 mg) had no reduction in serum interferon-α/β activity. These data indicate that high-dose statin add-on therapy inhibits interferon-β activity in most patients, whereas moderate doses have lesser inhibitory effects.
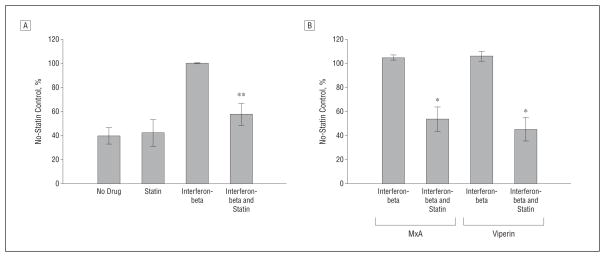
Ex vivo MNCs were studied in 4 different treatment conditions to confirm the in vitro effects of statins on interferon-β responses. There was a significant in vivo reduction in tyrosine phosphorylation of STAT1 in the high-dose statin add-on group compared with interferon-beta monotherapy, whereas medium-dose statins did not affect STAT1 phosphorylation (Figure 4A). In addition, 6 of 14 RRMS patients receiving combination therapy had significant reduction in interferon-stimulated MxA and viperin proteins compared with interferon-beta monotherapy (Figure 4B). These results demonstrate that high-dose statin add-on therapy blocks interferon-β responses in vivo.
Figure 4.
Comparison of interferon and interferon with statin therapy. The addition of a statin blocks interferon-beta therapy induction of P-Y-STAT1 (A) and myxovirus resistance 1 (MxA) and viperin proteins (B) in 14 patients with relapsing-remitting multiple sclerosis. Mononuclear cell lysates from no drug, statin alone, interferon-beta alone at 4 hours, and 4 hours of interferon-beta plus statin conditions are shown. Proteins were quantified with Western blots and normalized with actin. *P <.001 vs interferon-beta (paired t test). Error bars indicate SEM.
Together, our data from cell culture, in vitro studies in therapy-naive RRMS patients, and in vivo studies in interferon-beta–treated RRMS patients receiving statin add-on therapy reveal that high-dose statins inhibit interferon-α/β activity by blocking tyrosine phosphorylation on STAT1 and preventing interferon responses.
COMMENT
We demonstrate that high-dose statins inhibit interferon signaling. Atorvastatin dose dependently inhibits interferon-β induction of P-Y-STAT1 and downstream proteins. Preincubation in vitro with statins in Jurkat T cells and MNCs blocked interferon responses within 15 minutes and reached maximal inhibition at 24 hours (eFigures 1 and 2). This finding is consistent with other dose and pharmacokinetic studies.26,28 Statins inhibit cholesterol synthesis but are also anti-inflammatory and thus are a potential therapy for MS and other neuroinflammatory diseases.29–32
Statins suppress proinflammatory TH1 and TH17 responses in experimental autoimmune encephalomyelitis and MS lymphocytes.28,33–35 In 30 RRMS patients, monotherapy with 80 mg of simvastatin appeared to reduce the volume and number of gadolinium-positive MRI lesions by 44% from baseline in patients with active disease.36 Treatment with high-dose atorvastatin for 9 months reduced MRI contrast-enhancing lesions (CELs).37 In these uncontrolled studies with significant baseline MRI activity, the decrease in activity could have arisen from regression to the mean.38
Potential mechanisms of statin benefit in MS include (1) regulating extracellular kinase ERK and p38 phosphorylation through Rac and Rho pathways, which would block TH1 activation and induce a TH2 shift39; (2) impairing activation of Ras superfamily GTPases to inhibit the major histocompatibility class II antigen presentation pathway40; (3) blocking STAT activation to inhibit interleukin 17 production34; and (4) disturbing formation of cholesterol-containing microdomains (lipid rafts), thereby inhibiting function of the T-cell receptor and major histocompatibility class I and II.41–44 However, MRI and clinical effects may be complex in humans because simvastatin inhibits CNS remyelination by blocking oligodendrocyte progenitor differentiation,45 and atorvastatin promotes some proinflammatory TH1 responses by raising interleukin 12p70.46
High-dose atorvastatin in vitro specifically blocks formation of P-Y-STAT1 but not P-S-STAT1 or P-Y-STAT2 in MNCs from therapy-naive RRMS patients (Figure 2). High-dose statins in vivo also block interferon-beta–induced transcription factor activation and expression of interferon-induced proteins in RRMS; moderate-dose statins were less inhibitory (Figures 3 and 4). Our results may explain why some clinical studies with high-dose statins (80 mg/d) added to interferon-beta therapy found loss of clinical benefit or worsening of MRI, whereas studies with relatively low-dose statins (20 mg/d) are more variable.7,8,10,37,47,48
In the interferon-beta–only group of the SENTINEL trial (intramuscular interferon-beta-1awith or without natalizumab), a subgroup of 40 RRMS patients with ongoing disease activity while taking interferon-beta received low to high doses of various statins. No differences were found in clinical activity, CELs, or new T2 lesions.7 In another study,47 the total relapse rate was lower with 40 mg of simvastatin added on to intramuscular interferon-beta-1a, but the MRI results did not favor simvastatin. With low-dose atorvastatin added on (20 mg/d) to patients with active disease while receiving subcutaneous interferon-beta-1a therapy, CELs and relapses were reduced compared with baseline in the combination group vs the interferon monotherapy group.48 In 16 RRMS patients with consistent baseline MRI activity, 80 mg of atorvastatin added on to 22 μg of interferon-beta-1a or to interferon-beta-1b therapy nonsignificantly reduced the number and volume of CELs vs baseline but increased T2 lesions for 9 months.38 A parallel atorvastatin-only group showed similar effects, so regression to the mean is possible. A large phase 4 study (307 RRMS patients) demonstrated that 80 mg of simvastatin added on to weekly interferon-beta-1a did not benefit clinical and MRI activity and suggested that simvastatin should not be added as treatment for RRMS.8 Simvastatin (80 mg) (n = 21) and placebo (n = 16) groups had no difference in expression of interferon-β–inducible genes IL10, TNFSF10, MX1, and IRF7 in PAX gene–collected whole blood, appropriately obtained 9 to 12 hours after injection of interferon-beta-1a intramuscularly.
Our in vivo study design differed from other studies7,8 that found no changes in interferon responses. We used statistically powerful, paired, within-subject analysis to minimize variability between patients receiving or not receiving statin therapy vs cross-sectional comparisons between placebo and statin groups. We measured more stable protein production instead of mRNA and used MNCs instead of whole blood to eliminate the up to 15-fold higher signals from polymorphonuclear leukocytes and reticulocytes in whole blood.27 Moreover, our serum interferon activity assay is much more sensitive than ELISA.24
We tested only 14 RRMS patients, but statistical significance was found for multiple measures. We did not study long-term statin effects on clinical and MRI activity in these 14 RRMS patients because prolonged block of interferon therapy could allow recurrence of clinical activity.10 Different statins may have various effects on interferon-beta therapy based on their half-lives, pharmacokinetics, and blood-brain barrier penetration based on hydrophobicity vs hydrophilicity.28,49,50 Divergent results among clinical studies could be due to various doses and forms of statins,51 weekly vs every-other-day interferon-beta, effects on oligodendroglia and immune cells,9 and wide pharmacogenomic divergence in response to statins.52
In conclusion, high-dose statin add-on therapy impaired the ability of interferon-beta to activate STAT1 and, in turn, to induce IRF-1, serum type 1 interferons, and MxA and viperin proteins. More important, subtle shifts in immune cell activation or expression of regulatory proteins can disproportionately increase an ordinarily small percentage of autoreactive cells.53 This study provides evidence that high-dose statins (80 mg/d) inhibit interferon effects by targeting STAT1 activation in vitro and during interferon-beta therapy. This finding suggests that MS patients who have high cholesterol levels should be cautious when combining high-dose statin therapy with interferon-beta.
Supplementary Material
Acknowledgments
Funding/Support: This research was supported by research grant PP1556 from the National MS Society, the Brain Research Foundation, Serono (Drs Feng and Reder), and grants K08 AI083790 and P30 DK42086 from the National Institutes of Health (Dr Niewold).
Footnotes
Financial Disclosure: None reported.
Online-Only Material: The eFigures are available at http://www.archneurol.com.
Additional Contributions: Thanks to Joy Derwenskas, DO, Northwestern University, for patient referral and Adil Javed, MD, PhD, for critical reading.
Author Contributions: Study concept and design: Feng and Reder. Acquisition of data: Feng, Han, Kilaru, Franek, Niewold, and Reder. Analysis and interpretation of data: Feng, Han, Kilaru, Franek, Niewold, and Reder. Drafting of the manuscript: Feng, Kilaru, Franek, and Reder. Critical revision of the manuscript for important intellectual content: Feng, Han, Niewold, and Reder. Statistical analysis: Feng, Kilaru, Franek, and Reder. Obtained funding: Feng and Reder. Administrative, technical, and material support: Feng, Han, Niewold, and Reder. Study supervision: Feng and Reder.
References
- 1.Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17(2):210–218. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng X, Petraglia AL, Chen M, Byskosh PV, Boos MD, Reder AT. Low expression of interferon-stimulated genes in active multiple sclerosis is linked to subnormal phosphorylation of STAT1. J Neuroimmunol. 2002;129(1–2):205–215. doi: 10.1016/s0165-5728(02)00182-0. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi KD, Ruderman DL, Croze E, et al. IFN-β–regulated genes are abnormally expressed in therapy-naive MS mononuclear cells: unbiased gene expression analysis parallels literature on signaling pathways. J Neuroimmunol. 2008;195:116–120. doi: 10.1016/j.jneuroim.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Youssef S, Stüve O, Patarroyo JC, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420(6911):78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 5.Greenwood J, Steinman L, Zamvil SS. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nat Rev Immunol. 2006;6 (5):358–370. doi: 10.1038/nri1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stüve O, Youssef S, Weber MS, et al. Immunomodulatory synergy by combination of atorvastatin and glatiramer acetate in treatment of CNS autoimmunity. J Clin Invest. 2006;116(4):1037–1044. doi: 10.1172/JCI25805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudick RA, Pace A, Rani MR, et al. Effect of statins on clinical and molecular responses to intramuscular interferon beta-1a. Neurology. 2009;72(23):1989–1993. doi: 10.1212/WNL.0b013e3181a92b96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorensen PS, Lycke J, Erälinna JP, et al. SIMCOMBIN study investigators. Simvastatin as add-on therapy to interferon β-1a for relapsing-remitting multiple sclerosis (SIMCOMBIN study): a placebo-controlled randomised phase 4 trial. Lancet Neurol. 2011;10(8):691–701. doi: 10.1016/S1474-4422(11)70144-2. [DOI] [PubMed] [Google Scholar]
- 9.Sellner J, Weber MS, Vollmar P, Mattle HP, Hemmer B, Stüve O. The combination of interferon-beta and HMG-CoA reductase inhibition in multiple sclerosis: enthusiasm lost too soon? CNS Neurosci Ther. 2010;16(6):362–373. doi: 10.1111/j.1755-5949.2010.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birnbaum G, Cree B, Altafullah I, Zinser M, Reder AT. Combining beta interferon and atorvastatin may increase disease activity in multiple sclerosis. Neurology. 2008;71(18):1390–1395. doi: 10.1212/01.wnl.0000319698.40024.1c. [DOI] [PubMed] [Google Scholar]
- 11.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 12.Takaoka A, Yanai H. Interferon signalling network in innate defence. Cell Microbiol. 2006;8(6):907–922. doi: 10.1111/j.1462-5822.2006.00716.x. [DOI] [PubMed] [Google Scholar]
- 13.Ghislain JJ, Fish EN. Application of genomic DNA affinity chromatography identifies multiple interferon-α–regulated Stat2 complexes. J Biol Chem. 1996;271(21):12408–12413. doi: 10.1074/jbc.271.21.12408. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Leung S, Qureshi S, Darnell JE, Jr, Stark GR. Formation of STAT1-STAT2 heterodimers and their role in the activation of IRF-1 gene transcription by interferon-α. J Biol Chem. 1996;271(10):5790–5794. doi: 10.1074/jbc.271.10.5790. [DOI] [PubMed] [Google Scholar]
- 15.Reder AT. MxA: a biomarker for predicting multiple sclerosis disease activity. Neurology. 2010;75(14):1222–1223. doi: 10.1212/WNL.0b013e3181f6466f. [DOI] [PubMed] [Google Scholar]
- 16.Hinson ER, Joshi NS, Chen JH, et al. Viperin is highly induced in neutrophils and macrophages during acute and chronic lymphocytic choriomeningitis virus infection. J Immunol. 2010;184(10):5723–5731. doi: 10.4049/jimmunol.0903752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vosslamber S, van Baarsen LG, Verweij CL. Pharmacogenomics of IFN-βin multiple sclerosis: towards a personalized medicine approach. Pharmacogenomics. 2009;10(1):97–108. doi: 10.2217/14622416.10.1.97. [DOI] [PubMed] [Google Scholar]
- 18.Ghittoni R, Patrussi L, Pirozzi K, et al. Simvastatin inhibits T-cell activation by selectively impairing the function of Ras superfamily GTPases. FASEB J. 2005;19(6):605–607. doi: 10.1096/fj.04-2702fje. [DOI] [PubMed] [Google Scholar]
- 19.Jabs WJ, Hennig, Zawatzky R, Kirchner H. Failure to detect antiviral activity in serum and plasma of healthy individuals displaying high activity in ELISA for IFN-alpha and IFN-beta. J Interferon Cytokine Res. 1999;19(5):463–469. doi: 10.1089/107999099313901. [DOI] [PubMed] [Google Scholar]
- 20.Niewold TB, Kelly JA, Flesch MH, Espinoza LR, Harley JB, Crow MK. Association of the IRF5 risk haplotype with high serum interferon-α activity in systemic lupus erythematosus patients. Arthritis Rheum. 2008;58(8):2481–2487. doi: 10.1002/art.23613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hua J, Kirou K, Lee C, Crow MK. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti-RNA binding protein autoantibodies. Arthritis Rheum. 2006;54(6):1906–1916. doi: 10.1002/art.21890. [DOI] [PubMed] [Google Scholar]
- 22.Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK. High serum IFN-α activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007;8(6):492–502. doi: 10.1038/sj.gene.6364408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niewold TB, Kariuki SN, Morgan GA, Shrestha S, Pachman LM. Elevated serum interferon-α activity in juvenile dermatomyositis: associations with disease activity at diagnosis and after thirty-six months of therapy. Arthritis Rheum. 2009;60(6):1815–1824. doi: 10.1002/art.24555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng X, Reder NP, Yanamandala M, et al. Type I interferon signature is high in lupus and neuromyelitis optica but low in multiple sclerosis. J Neurol Sci. 2012;313(1–2):48–53. doi: 10.1016/j.jns.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Voort LF, Vennegoor A, Visser A, et al. Spontaneous MxA mRNA level predicts relapses in patients with recently diagnosed MS. Neurology. 2010;75(14):1228–1233. doi: 10.1212/WNL.0b013e3181f6c556. [DOI] [PubMed] [Google Scholar]
- 26.Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6(12):1399–1402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 27.Reder AT, Velichko S, Yamaguchi KD, et al. IFN-β1b induces transient and variable gene expression in relapsing-remitting multiple sclerosis patients independent of neutralizing antibodies or changes in IFN receptor RNA expression. J Interferon Cytokine Res. 2008;28(5):317–331. doi: 10.1089/jir.2007.0131. [DOI] [PubMed] [Google Scholar]
- 28.Youssef S, Stüve O, Patarroyo JC, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420(6911):78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 29.Zamvil SS, Steinman L. Cholesterol-lowering statins possess anti-inflammatory activity that might be useful for treatment of MS. Neurology. 2002;59(7):970–971. doi: 10.1212/wnl.59.7.970. [DOI] [PubMed] [Google Scholar]
- 30.Stüve O, Prod’homme T, Slavin A, et al. Statins and their potential targets in multiple sclerosis therapy. Expert Opin Ther Targets. 2003;7(5):613–622. doi: 10.1517/14728222.7.5.613. [DOI] [PubMed] [Google Scholar]
- 31.Neuhaus O, Stüve O, Archelos JJ, Hartung HP. Putative mechanisms of action of statins in multiple sclerosis: comparison to interferon-β and glatiramer acetate. J Neurol Sci. 2005;233(1–2):173–177. doi: 10.1016/j.jns.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 32.Peng X, Jin J, Giri S, et al. Immunomodulatory effects of 3-hydroxy-3-methylglutaryl coenzyme-A reductase inhibitors, potential therapy for relapsing remitting multiple sclerosis. J Neuroimmunol. 2006;178(1–2):130–139. doi: 10.1016/j.jneuroim.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Aktas O, Waiczies S, Smorodchenko A, et al. Treatment of relapsing paralysis in experimental encephalomyelitis by targeting Th1 cells through atorvastatin. J Exp Med. 2003;197(6):725–733. doi: 10.1084/jem.20021425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X, Jin J, Peng X, Ramgolam VS, Markovic-Plese S. Simvastatin inhibits IL-17 secretion by targeting multiple IL-17-regulatory cytokines and by inhibiting the expression of IL-17 transcription factor RORC in CD4+ lymphocytes. J Immunol. 2008;180(10):6988–6996. doi: 10.4049/jimmunol.180.10.6988. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Tao Y, Troiani L, Markovic-Plese S. Simvastatin inhibits IFN regulatory factor 4 expression and Th17 cell differentiation in CD4+ T cells derived from patients with multiple sclerosis. J Immunol. 2011;187(6):3431–3437. doi: 10.4049/jimmunol.1100580. [DOI] [PubMed] [Google Scholar]
- 36.Vollmer T, Key L, Durkalski V, et al. Oral simvastatin treatment in relapsing-remitting multiple sclerosis. Lancet. 2004;363(9421):1607–1608. doi: 10.1016/S0140-6736(04)16205-3. [DOI] [PubMed] [Google Scholar]
- 37.Paul F, Waiczies S, Wuerfel J, et al. Oral high-dose atorvastatin treatment in relapsing-remitting multiple sclerosis. PLoS One. 2008;3(4):e1928. doi: 10.1371/journal.pone.0001928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Y, Traboulsee A, Petkau AJ, Li D. Regression of new gadolinium enhancing lesion activity in relapsing-remitting multiple sclerosis. Neurology. 2008;70(13 pt 2):1092–1097. doi: 10.1212/01.wnl.0000285426.73143.f7. [DOI] [PubMed] [Google Scholar]
- 39.Dunn SE, Youssef S, Goldstein MJ, et al. Isoprenoids determine Th1/Th2 fate in pathogenic T cells, providing a mechanism of modulation of autoimmunity by atorvastatin. J Exp Med. 2006;203(2):401–412. doi: 10.1084/jem.20051129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghittoni R, Napolitani G, Benati D, et al. Simvastatin inhibits the MHC class II pathway of antigen presentation by impairing Ras superfamily GTPases [published correction appears in Eur J Immunol. 2006;36(12):3381] Eur J Immunol. 2006;36(11):2885–2893. doi: 10.1002/eji.200636567. [DOI] [PubMed] [Google Scholar]
- 41.Feng X, Heyden NV, Ratner L. Alpha interferon inhibits human T-cell leukemia virus type 1 assembly by preventing Gag interaction with rafts. J Virol. 2003;77(24):13389–13395. doi: 10.1128/JVI.77.24.13389-13395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuipers HF, Biesta PJ, Groothuis TA, Neefjes JJ, Mommaas AM, van den Elsen PJ. Statins affect cell-surface expression of major histocompatibility complex class II molecules by disrupting cholesterol-containing microdomains. Hum Immunol. 2005;66(6):653–665. doi: 10.1016/j.humimm.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Ehrenstein MR, Jury EC, Mauri C. Statins for atherosclerosis: as good as it gets? N Engl J Med. 2005;352(1):73–75. doi: 10.1056/NEJMe048326. [DOI] [PubMed] [Google Scholar]
- 44.Fessler MB, Parks JS. Intracellular lipid flux and membrane microdomains as organizing principles in inflammatory cell signaling. J Immunol. 2011;187(4):1529–1535. doi: 10.4049/jimmunol.1100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miron VE, Zehntner SP, Kuhlmann T, et al. Statin therapy inhibits remyelination in the central nervous system. Am J Pathol. 2009;174(5):1880–1890. doi: 10.2353/ajpath.2009.080947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sellner J, Greeve I, Findling O, et al. Effect of interferon-beta and atorvastatin on Th1/Th2 cytokines in multiple sclerosis. Neurochem Int. 2008;53(1–2):17–21. doi: 10.1016/j.neuint.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Togha M, Karvigh SA, Nabavi M, et al. Simvastatin treatment in patients with relapsing-remitting multiple sclerosis receiving interferon beta 1a: a double-blind randomized controlled trial. Mult Scler. 2010;16(7):848–854. doi: 10.1177/1352458510369147. [DOI] [PubMed] [Google Scholar]
- 48.Lanzillo R, Orefice G, Quarantelli M, et al. Atorvastatin Combined to Interferon to Verify the Efficacy (ACTIVE) in relapsing-remitting active multiple sclerosis patients: a longitudinal controlled trial of combination therapy. Mult Scler. 2010;16(4):450–454. doi: 10.1177/1352458509358909. [DOI] [PubMed] [Google Scholar]
- 49.Corsini A, Bellosta S, Baetta R, Fumagalli R, Paoletti R, Bernini F. New insights into the pharmacodynamic and pharmacokinetic properties of statins. Pharmacol Ther. 1999;84(3):413–428. doi: 10.1016/s0163-7258(99)00045-5. [DOI] [PubMed] [Google Scholar]
- 50.Butterfield DA, Barone E, Mancuso C. Cholesterol-independent neuroprotective and neurotoxic activities of statins: perspectives for statin use in Alzheimer disease and other age-related neurodegenerative disorders. Pharmacol Res. 2011;64(3):180–186. doi: 10.1016/j.phrs.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neuvonen PJ, Niemi M, Backman JT. Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006;80(6):565–581. doi: 10.1016/j.clpt.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 52.Kajinami K, Akao H, Polisecki E, Schaefer EJ. Pharmacogenomics of statin responsiveness. Am J Cardiol. 2005;96(9A):65K–70K. doi: 10.1016/j.amjcard.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 53.Germain RN. The art of the probable: system control in the adaptive immune system. Science. 2001;293(5528):240–245. doi: 10.1126/science.1062946. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.