The nuclear transport machinery is a common target of several viruses that disrupt nuclear transport pathways to facilitate viral replication and avert the immune response. In addition, viral interactions with transport factors led to the identification of several important constituents of nuclear transport pathways. This review explores the importance of nucleocytoplasmic trafficking to viral pathogenesis and discusses how the relationship of viruses with the host transport machinery has led to new antiviral therapeutic strategies and exposed previously unknown cellular mechanisms.
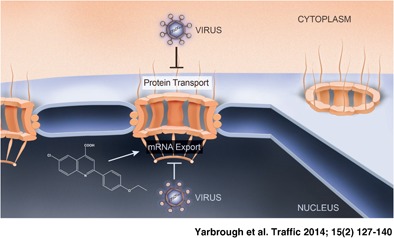
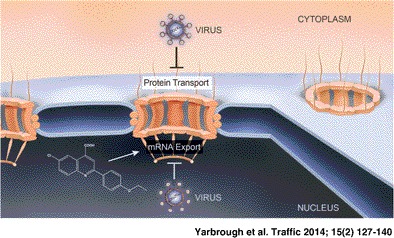
Keywords: mRNA export, nuclear import, nuclear pore complex, nuclear transport, nucleocytoplasmic trafficking, virus
Abstract
Trafficking of proteins and RNA into and out of the nucleus occurs through the nuclear pore complex (NPC). Because of its critical function in many cellular processes, the NPC and transport factors are common targets of several viruses that disrupt key constituents of the machinery to facilitate viral replication. Many viruses such as poliovirus and severe acute respiratory syndrome (SARS) virus inhibit protein import into the nucleus, whereas viruses such as influenza A virus target and disrupt host mRNA nuclear export. Current evidence indicates that these viruses may employ such strategies to avert the host immune response. Conversely, many viruses co‐opt nucleocytoplasmic trafficking to facilitate transport of viral RNAs. As viral proteins interact with key regulators of the host nuclear transport machinery, viruses have served as invaluable tools of discovery that led to the identification of novel constituents of nuclear transport pathways. This review explores the importance of nucleocytoplasmic trafficking to viral pathogenesis as these studies revealed new antiviral therapeutic strategies and exposed previously unknown cellular mechanisms. Further understanding of nuclear transport pathways will determine whether such therapeutics will be useful treatments for important human pathogens.
The nucleus is a double membrane‐bound organelle in eukaryotic cells, which contains the cell's genetic material. The inner lipid bilayer delineates the nuclear contents and the outer layer is continuous with the endoplasmic reticulum (ER). This physical segregation provides a layer of regulation that allows for selection of molecules to be transported into and out of the nucleus, which is critical for proper gene expression and cell survival. Trafficking of material between the nucleus and cytoplasm occurs through the nuclear pore complex (NPC), which is the gateway between these compartments 1.
The NPC is a large, multisubunit complex of approximately 100 MDa in vertebrates that consists of about 30 different proteins, termed nucleoporins (Nups) 1, 2, 3. Structurally, the NPC is made up of an eightfold symmetrical framework that surrounds a central transport channel that spans the inner and outer membranes of the nuclear envelope 1. Nups in this channel have unfolded domains containing FG repeats, which are docking sites for transport receptors 1. Peripheral Nups interact with the central core, form rings at the nuclear and cytoplasmic sides of the NPC and also bind Nups embedded in the nuclear envelope. In the nuclear side, Nups form filaments that connect to generate the nuclear basket. At the cytoplasmic side, the Nup filaments protrude into the cytoplasm. The composition of Nups at the NPC was thought to be universal. However, distinct combinations of Nups have been found in NPCs of different cell types, indicating that some NPCs may have distinct functions depending on their composition (reviewed in 4).
NPCs allow free passage of ions and small molecules, but molecules larger than approximately 20–40 kDa are actively transported through the NPC by the mobile nuclear transport machinery 5, 6, 7, 8, 9, 10. Distinct nuclear transport pathways regulate the movement of these macromolecules into and out of the nucleus. For example, proteins with nuclear functions can be imported via the NPC by various pathways depending on their nuclear localization signals, whereas nuclear RNAs are actively exported after transcription via different mechanisms to be translated or to function in the cytoplasm. Proper gene expression is critical for the cell to adapt to its constantly changing needs 11. Therefore, efficient transport through the NPC must be a selective and highly regulated process. Partly because of the high level of regulation, viruses often exploit nucleocytoplasmic transport pathways in order to facilitate viral replication and evade the host antiviral response. In this review, we focus on discoveries that shed light onto virus–host interactions that occur at the level of nucleocytoplasmic trafficking. In addition, we highlight recent efforts to develop new antiviral therapeutic strategies that target host nuclear transport pathways that are crucial for viral propagation and that are revealing novel cellular processes.
Nuclear Transport of Proteins
Most facilitated transport of proteins through the NPC requires soluble transport receptors and a transport signal. Proteins targeted into or out of the nucleus interact with transport receptors via a short amino acid motif, named nuclear localization signal (NLS) or nuclear export signal (NES), respectively 1. Soluble transport receptors termed karyopherins (also known as importins, exportins, transportins and snurportin) are multidomain transport factors that bind to cargo through its cargo‐binding domain. Karyopherin α (Kapα) recognizes so‐called classical NLS motifs, whereas karyopherin β (Kapβ) interacts with non‐classical NLS sequences such as the PY‐NLS motif, which is found in structurally disordered regions of proteins and contains invariant proline and tyrosine residues in the Kapβ recognition sequence 1, 12. Karyopherins also contain an NPC‐binding domain(s) and an N‐terminal‐binding domain for the small GTPase Ran, which interacts with the karyopherin to regulate the association and dissociation of the transport receptor–cargo complex. Ran acts a molecular switch and alternates between GDP‐ and GTP‐bound states. In its GTP form, interaction of Ran with import complexes results in their dissociation. On the other hand, formation of export complexes requires Ran in its GTP‐bound state 1. Conversion between the GDP‐ and GTP‐bound forms of Ran is regulated by the cytoplasmic GTPase‐activating protein (RanGAP) and the guanine nucleotide exchange factor (RanGEF), which is located at the nucleoplasmic side of the NPC 1, 5, 13. Thus, RanGDP is found in the cytoplasm, whereas RanGTP is primarily nuclear. The asymmetric distribution of these regulatory factors creates a Ran gradient across the nuclear envelope that is fundamental for the directionality of nucleocytoplasmic transport. During import, karyopherins facilitate the transport of cargo across the NPC. Once in the nucleus, RanGTP stimulates the dissociation of the karyopherin–cargo complex. For export, karyopherin–cargo complex formation is stimulated by RanGTP and results in the translocation of the complex through the NPC. In the cytoplasm, hydrolysis of RanGTP to its GDP state results in the dissociation of the karyopherin–cargo complex 1. Here, we discuss how viruses disrupt these protein transport pathways to manipulate cellular processes and inhibit host antiviral mechanisms to facilitate viral replication (Figure 1).
Figure 1.
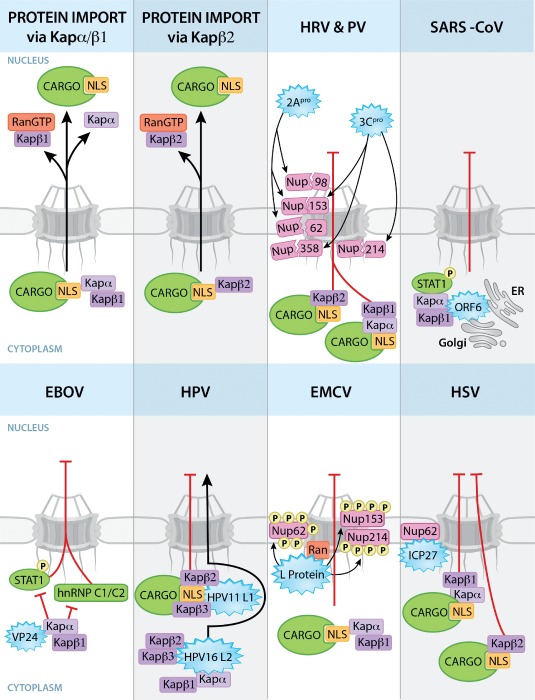
Viral strategies to disrupt nucleocytoplasmic trafficking of proteins. Nuclear import pathways mediated by Kapα/Kapβ1 or Kapβ2 are shown. Kapα and Kapβ2 bind proteins or cargos with specific NLSs. Kapβ1 and β2 translocate the import complexes through the NPC via interactions with Nups. The import complexes are dissociated by RanGTP at the nucleoplasmic side of the NPC. Viral proteins (blue starbursts) interact with the depicted host factors to disrupt nuclear transport pathways. 2Apro and 3Cpro of HRV and PV degrade Nups and block nuclear import of proteins via the Kapα/β1 and Kapβ2 pathways. SARS‐CoV ORF6 protein effectively disrupts nuclear import of phosphorylated STAT1 by tethering PY‐STAT1–Kapα/Kapβ complex to ER/Golgi membranes. Alternatively, EBOV‐VP24 binds Kapα preventing its interaction with phosphorylated STAT1 and hnRNP C1/C2, which accumulate in the cytoplasm. In HPV, while HPV11 L1 binds Kapβ2/β3 and disrupts cargo import, the viral HPV16 L2 protein gets imported into nucleus by binding to Kapβ2, Kapβ3 and Kapα/Kapβ1 complex. To inhibit protein import, L protein of EMCV hyperphosphorylates Nups and binds Ran. ICP27 protein of HSV interacts with Nup62 and blocks nuclear import of proteins via Kapα/Kapβ1 and Kapβ2 pathways. Disruption of nuclear import of proteins by other viruses is discussed in the text.
Viral Disruption of Nucleocytoplasmic Transport of Proteins
Poliovirus and human rhinovirus disrupt nucleocytoplasmic trafficking of proteins by viral‐mediated proteolytic cleavage of specific Nups
Poliovirus (PV) and human rhinovirus (HRV) are positive‐stranded RNA viruses from the Picornaviridae family that replicate entirely in the cytoplasm of the host cell. Infection of mammalian cells with PV and HRV results in the cytoplasmic mislocalization of cellular proteins bearing a classical NLS, which requires the Kapα/Kapβ1 import pathway 14, 15. In addition, cytoplasmic relocalization of M9 NLS‐containing host proteins, which requires the Kapβ2/transportin1 nuclear import pathway, was also observed during PV and HRV infection 14, 15. These and other studies revealed that infection with PV and HRV resulted in the proteolytic degradation of several nucleoporins, including Nup62, Nup98 and Nup153, and possibly other factors, by the viral‐expressed 2A protease (2Apro) 14, 15, 16, 17, 18, 19, 20. In fact, inhibiting the PV 2Apro suppressed the relocalization of cellular proteins, demonstrating that this effect is 2Apro‐dependent 17. An in‐depth analysis of different rhinovirus species revealed that 2Apro targeted Nup62, Nup98 and Nup153 for proteolysis, albeit the rate and sites of cleavage were different 20. Moreover, the HRV 3C protease (3Cpro) and its precursor form, 3CD, also target nucleoporins such as Nup153, Nup214 and Nup358 for degradation, leading to disruption of NPC permeability and nucleocytoplasmic trafficking and mislocalization of nuclear proteins 21. Thus, both PV and HRV target nucleoporins to alter the composition of the NPC and trafficking of proteins into and out of the nucleus, which may function as a mechanism to inhibit host antiviral defense pathways by disrupting the translocation of important cellular proteins involved in immunity.
Viral antagonism of the host immune response by blocking STAT nuclear import
The severe acute respiratory syndrome coronavirus (SARS‐CoV) is a positive‐stranded RNA virus responsible for the potentially deadly human disease SARS. SARS‐CoV may disrupt specific cellular pathways to suppress host immune responses, resulting in disease spreading. Host invasion by pathogens elicits a number of immune responses to facilitate pathogen clearance. During infection, activated signal transducer and activator of transcription 1 (STAT1) is imported into the nucleus to bind to interferon‐stimulated response elements (ISRE) found on the promoter region of interferon (IFN)‐inducible genes 22. During SARS‐CoV infection, the viral open reading frame 6 (ORF6) protein blocks STAT1 nuclear translocation without affecting its phosphorylation 23. To prevent nuclear import of STAT1, SARS‐CoV ORF6 binds and tethers the nuclear import factors Kapα2 and Kapβ1 to the ER/Golgi membrane, effectively blocking STAT1 transport into the nucleus 24 and the C‐terminus of ORF6 is essential for its import block activity 25. Thus, SARS‐CoV blocks nucleocytoplasmic trafficking of host immune signaling proteins, which is an effect that likely contributes to viral infection.
Ebola virus (EBOV) is a negative‐stranded RNA virus that primarily replicates in the cytoplasm of the host cell and is responsible for hemorrhagic fever in humans. The EBOV VP24 viral protein blocks the nuclear translocation of tyrosine‐phosphorylated STAT1 (PY‐STAT1) to inhibit host IFN‐α/β and IFN‐γ signaling 26. Truncation mutants of the VP24 protein revealed that amino acids 26–50 are important for its ability to block IFN‐β expression 26. Mechanistically, VP24 specifically binds to the PY‐STAT1 nuclear transport receptor Kapα1 and disrupts the formation of the Kapα‐PY‐STAT1 transport complex, preventing PY‐STAT1 nuclear translocation 27. Importantly, the VP24 viral protein of the mouse‐adapted Zaire and Reston EBOV species also interacted with Kapα1 and disrupted PY‐STAT1 nuclear import 28. Further characterization of VP24 from Zaire, mouse‐adapted Zaire and Reston EBOV revealed that VP24 also interacts with karyopherins α5 and α6 and inhibits the binding of karyopherin to PY‐STAT1 28. In addition, EBOV VP24 interaction with Kapα1 has also been shown to disrupt nuclear import of hnRNP C1/C2, which interacts with the same region of Kapα1 as PY‐STAT1 29. Redistribution of hnRNP C1/C2 to the cytoplasm may facilitate EBOV replication similar to hnRNP C cytoplasmic relocalization by PV, which has been shown to stabilize PV RNA to promote viral replication 30.
Other globally important human pathogens inhibit host immune signaling by disrupting nuclear transport of proteins. For example, measles virus nucleocapsid (N) protein inhibits host IFN signaling pathways by preventing import of active STAT to the nucleus without affecting Jak and STAT phosphorylation or STAT degradation 31. In addition, rotavirus antagonizes IFN signaling pathways via inhibition of transcription of host antiviral factors by blocking nuclear import, but not activation, of STAT1, STAT2 and NF‐κB 32. In sum, blockage of nuclear import of immune signaling factors by associating with and disrupting soluble transport factors represents an effective viral strategy to inhibit the host antiviral response.
Encephalomyocarditis virus alters nucleocytoplasmic trafficking as a tactic to achieve IFN suppression and viral replication
Encephalomyocarditis virus (EMCV) is a positive‐stranded RNA virus from the Picornaviridae family that manipulates nucleocytoplasmic transport to propagate in the cytoplasm of infected cells and suppress the host immune response. The EMCV leader (L) protein, a non‐enzymatic viral component, disrupts nuclear transport of proteins by binding and suppressing the activity of Ran‐GTPase, thus altering the Ran gradient that is essential for the regulation of nucleocytoplasmic transport 33, 34. In addition, EMCV infection results in Nup62, Nup153 and Nup214 hyperphosphorylation in an L protein‐dependent manner, which may alter the integrity of and/or transport receptor–cargo complex binding to the NPC 34. The hyperphosphorylation of Nups and disruption of the RanGTP gradient represent an alternative mechanism by which EMCV effectively interferes with nucleocytoplasmic trafficking compared to the proteolytic activity associated with other picornaviruses discussed above 34. Similarly, infection with mengovirus, a positive‐stranded RNA picornavirus, results in the hyperphosphorylation of Nup62 in an L protein‐dependent manner, which may also alter host nuclear import and export pathways 35. Moreover, EMCV infection results in increased NPC permeability and enhanced relocalization of nuclear proteins to the cytoplasm of infected cells 36. These effects on nucleocytoplasmic transport were shown to require the zinc finger motif and phosphorylation of the threonine 47 (Thr47) residue of the EMCV leader protein 36. Thus, EMCV utilizes several different mechanisms to alter nuclear transport of host cell factors to block host IFN response and promote viral replication.
Theiler's murine encephalomyelitis virus disrupts IRF3 function by altering its subcellular localization
Theiler's murine encephalomyelitis virus (TMEV) is a single‐stranded RNA virus known to disrupt nucleocytoplasmic trafficking of cellular factors to interfere with the expression of host antiviral mechanisms 37, 38. The TMEV leader (L) protein promotes the redistribution of host proteins, such as the polypyrimidine tract‐binding (PTB) protein and IFN regulatory factor 3 (IRF3) 37. By interfering with IRF3 subcellular localization, TMEV may prevent transcription of host genes, such as IFNs, involved in antiviral response 37. In addition, TMEV infection results in Nup98 hyperphosphorylation 38. Thus, to effectively replicate in the host cell, TMEV alters the cellular distribution of host defense factors via disruption of nucleocytoplasmic trafficking.
Venezuelan equine encephalitis virus and nuclear pores
Venezuelan equine encephalitis virus (VEEV) is a positive‐stranded RNA virus that has the ability to cause fatal disease in humans and equine species. Subcellular localization studies revealed that a fraction of the VEEV capsid protein localizes to the nuclear envelope, where it may regulate nucleocytoplasmic trafficking 39. In fact, the capsid protein blocks several nuclear transport pathways without affecting the diffusion of small proteins through the NPC 39. This block is mediated by a complex formed with VEEV capsid protein, the nuclear export receptor CRM1 and karyopherin/importin α/β 40. This complex blocks nuclear transport of cargos, which accumulate in the central channel of the NPC 40. However, the physiological consequences of such a blockage require further examination.
Human papillomavirus inhibits Kap β2‐ and Kap β3‐mediated nuclear import
Human papillomavirus (HPV) is a DNA virus known to be involved in various human diseases ranging from skin warts and some cardiovascular diseases to cervical and other cancers. HPV types 11 (HPV11) and 16 (HPV16) have been shown to associate with host nuclear import receptors 41, 42. The HPV11 L1 major capsid protein binds to karyopherins β2 and β3 and disrupts nuclear import through these pathways 41. This inhibitory function was also observed in the L1 capsid protein of high‐risk HPV16 41. Although HPV11 L1 capsid protein blocks nuclear protein import, the functional consequences of such a block are unknown. Similarly, the HPV16 L2 minor capsid protein directly interacts with the nuclear import receptors Kapβ2 and Kapβ3, and also forms a complex with the Kapα2/Kapβ1 heterodimer by interacting with Kapα2 42. By binding different soluble import receptors, HPV16 L2 protein efficiently translocates to the nucleus of infected cells to facilitate assembly of HPV virions 42.
Herpes simplex virus inhibits nuclear import of proteins via interaction with the NPC
The important human pathogen herpes simplex virus (HSV) is a DNA virus that interferes with host nuclear transport pathways. The HSV‐1 ICP27 protein blocks nuclear import of proteins via the Kapα/β1 and Kapβ2 (transportin) nuclear import pathways by associating to the NPC through direct interactions with Nup62 43. Although HSV‐1 blocks host nuclear trafficking via ICP27, HSV‐1 can still export its viral mRNAs by directly interacting with the nuclear export factor Aly/Ref, followed by recruitment of the mRNA export factor NXF1/TAP 44, as will be discussed below. These functions of ICP27 may ensure the translation of viral mRNAs while the expression and trafficking of host proteins is drastically affected.
In sum, both RNA and DNA viruses have developed varied strategies to regulate the NPC and nuclear transport of proteins to promote an environment more favorable to viral replication. To respond to these challenges, the host cell may secrete antiviral cytokines, such as IFNs. In fact, studies have shown that IFNs upregulate the expression of certain nucleoporins 16, 45, which illustrates a host strategy to restore balance to nucleocytoplasmic trafficking.
Nuclear Export of mRNA
Most DNA and even a few RNA viruses replicate within the nucleus of a host cell, and these viruses utilize nuclear export pathways to express their genes 46. In fact, studies of viral mechanisms to export viral RNA revealed two proteins that are important nuclear export factors of host RNAs, CRM1 (chromosome region maintenance 1, also known as exportin 1, XPO1) and NXF1 (nuclear export factor 1, also known as TIP‐associated protein, TAP) 47, 48, 49. These proteins are exploited by several viruses to promote viral mRNA export and/or inhibit host mRNA trafficking in order to prevent a proper host immune response (Figure 2).
Figure 2.
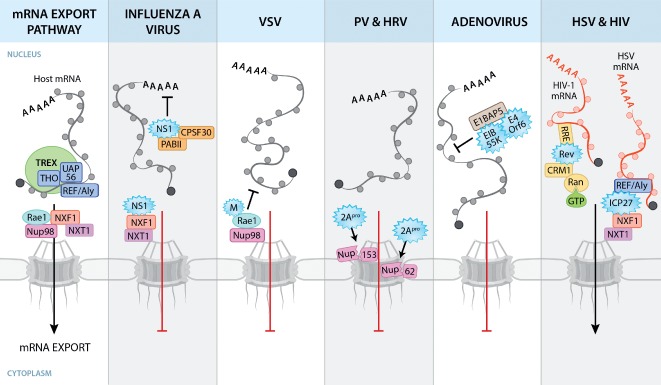
Viral disruption of host mRNA nuclear export pathways. Host mRNA export is coordinated by the TREX complex, which consists of THO, UAP56 and REF/Aly. The association of REF with mRNA recruits the mRNA export receptor heterodimer NXF1‐NXT1, which mediates export of mRNAs by interacting with Nups at the NPC. Circles surrounding mRNAs depict RNA‐binding proteins. Viral proteins (depicted as blue starbursts) disrupt mRNA nuclear export by interacting with host factors. IAV NS1 binds and disrupts factors involved in cellular mRNA processing and export. VSV M protein interacts with Rae1 and Nup98, resulting in mRNA nuclear export block. 2Apro of PV and HRV cleaves Nups to disrupt NPC architecture. AdV E1B 55K and E4orf6 proteins disrupt NXF1‐mediated host mRNA export by binding to E1B‐AP5. Other viruses, such as herpesviruses and HIV, utilize cellular transport pathways to promote viral mRNA export. The herpesvirus protein ICP27 facilitates preferential export of viral mRNAs through interaction with REF/Aly and NXF1. The HIV‐1 Rev protein facilitates nuclear export of unspliced or partially spliced viral mRNAs through the Rev‐responsive element (RRE), an RNA signature on these viral mRNAs. Rev‐bound viral RNA binds CRM1 and RanGTP and is translocated through the NPC.
Before being exported from the nucleus, mRNAs must be properly processed into a mature mRNA by undergoing capping, splicing and polyadenylation 50, 51. After processing, mRNAs are loaded with mRNA export factors, which recruit export receptors to the mRNAs. The transcription–export (TREX) complex coordinates nuclear export of mRNAs with transcription and processing 52, 53, 54. This complex is comprised of the multisubunit THO complex that functions in transcription elongation 53, 55, 56, 57 and the export factors UAP56 (also known as HEL) and REF (also known as ALY). UAP56 is an ATP‐dependent RNA helicase that is also involved in splicing and it is required for assembly of the TREX complex. In the ATP‐bound form, UAP56 binds REF, stimulating the association of REF with mRNA 58, 59, 60. Subsequently, REF interacts with the mRNA export receptor heterodimer NXF1‐NXT1 (also known as TAP‐p15), which mediates nuclear export of the mRNAs by binding phenylalanine–glycine (FG) repeat domains on Nups at the NPC 49, 61, 62, 63. FG‐containing Nups, such as Nup98, mediate translocation of the mRNP (messenger ribonucleoprotein) complex through the NPC 63, 64. Another mRNA export factor involved in this process is Rae1 (also known as Mrnp41), a nucleocytoplasmic shuttling protein that interacts with mRNA, NXF1 and Nup98 65, 66, 67, 68, 69, 70, 71, 72, 73. Rae1 may recruit NXF1 to Nup98 67. A more detailed discussion on Rae1 is found in another review 74. Upon reaching the cytoplasmic side of the NPC, the mRNP is released by the ATPase activity of Dbp5, which is stimulated by Gle1 and IP6 75, 76, 77, 78.
While NXF1 is the main mRNA export receptor in humans, CRM1 is an important receptor required for export of proteins with a leucine‐rich NES and is also known to export certain classes of RNA, including subsets of mRNAs 79, 80, 81. Nuclear export of RNA via CRM1 requires an adaptor protein bound to cargo RNA that interacts with CRM1 via an NES 82.
Viruses Are Tools for Discovery of Host Pathways
The identification of CRM1 as a nuclear export receptor highlights the use of viruses as important tools for discovery. CRM1 was originally identified as a result of investigating the mechanism by which complex retroviruses such as human immunodeficiency virus 1 (HIV‐1) were able to subvert host mechanisms that prevent export of unspliced or incompletely spliced RNA transcripts 47, 48. HIV‐1 encodes the Rev protein that interacts with these viral RNAs via the Rev response element (RRE) 83, 84, 85. This interaction exposes the NES of Rev, which binds CRM1 and promotes export of viral RNAs from the nucleus via the NPC 47.
NXF1 was also discovered through the study of viral interactions with host proteins. Simple retroviruses such as Mason‐Pfizer monkey virus (MPMV) do not encode a Rev protein. Thus, nuclear export of unspliced RNAs from this virus occurs via a constitutive transport element (CTE), a cis‐acting stem loop that contains the RNA sequence that interacts with NXF1/TAP to facilitate export 49, 86. Importantly, microinjection of excess CTE‐containing RNA into Xenopus oocytes was shown to block export of cellular mRNAs, while export of CRM1‐dependent molecules was unaffected. This established NXF1/TAP as the major mRNA export receptor in eukaryotes 63.
Recently, a novel mechanism for export of large mRNPs was discovered that was functionally similar to the budding of herpesvirus virions from the nucleus of host cells 87, 88, 89. This process is independent of NPC activity but dependent upon type A lamins, which along with type B lamins make up a fibrous lattice that forms the nuclear lamina associated with the inner nuclear membrane 90. The authors noted the presence of poly(A) RNA within large foci that appeared to invaginate and bud through the inner and outer nuclear membranes and proposed that herpesviruses may have hijacked this endogenous eukaryotic mechanism to facilitate budding of large viral particles that are too large to pass through the NPC 88. The similarity between these two processes highlights yet another way in which the study of viruses can lead to important discoveries that increase our understanding of eukaryotic cellular mechanisms.
Viral Inhibition of Host mRNA Export
RNA viruses promote viral replication via disruption of mRNA export pathways
In addition to exploiting host mRNA nuclear export pathways, viruses also block export of host mRNAs. The expression of mRNAs encoding cellular defense proteins is critical for the host to mount a proper immune response to invading pathogens. Therefore, mRNA export pathways are an enticing target for viruses to block host expression of antiviral genes. Similar to most viral infections, influenza A virus (IAV) replication in vertebrate cells is recognized by the innate immune system. Upon recognition, the innate immune system triggers signal transduction pathways that lead to production of type I IFNs, which are antiviral cytokines that induce the expression of mRNAs encoding antiviral factors 91, including nucleoporins 16, 45. IAV has evolved several mechanisms to inhibit this response, mainly through non‐structural protein 1 (NS1), a multifunctional protein with activities in both the nucleus and cytoplasm 91, 92.
In the nucleus, NS1 inhibits mRNA processing and export 74. The steps of mRNA processing and export are closely linked, as some proteins that interact with mRNAs remain bound throughout both processes, whereas others are exchanged for factors specific for each step 93. NS1 inhibits pre‐mRNA splicing by binding to the U6 snRNA component of the spliceosome and/or through interactions with the putative splicing protein NS1‐BP 94, 95. NS1 further disrupts host mRNA processing by binding to the 30‐kDa subunit of the cleavage and polyadenylation specificity factor (CPSF30) and the poly(A)‐binding protein II (PABII), which are involved in binding the polyadenylation signal and in the elongation of the poly(A) chain of mRNAs, respectively 96, 97. The interaction of NS1 with these proteins inhibits 3′‐end processing of host mRNAs and contributes to inhibition of host gene expression. However, production of viral transcripts is unaffected by NS1‐mediated disruption of mRNA processing because poly(A) tail synthesis on viral mRNAs is carried out by the viral polymerase complex 98, 99. Additionally, NS1 may facilitate splicing of the viral M1 mRNA segment by interacting with the host mRNA‐binding proteins, NS1‐BP (NS1‐binding protein) and hnRNP K 100. This permits efficient processing of viral mRNA transcripts before nuclear export occurs. In addition to disrupting host mRNA processing, IAV further disrupts expression of host antiviral genes via NS1 interactions with the host mRNA export machinery. NS1 interacts with the mRNA export factors NXF1‐NXT1, which form a complex with Rae1 and E1B‐AP5, preventing nuclear export of poly(A) RNA 101. Altogether, these studies show that NS1 of IAV employs several mechanisms to inhibit the connected and highly regulated processes of mRNA processing and export. Interestingly, mRNAs encoding antiviral factors are retained in the nucleus owing to NS1‐mediated inhibition of mRNA nuclear export 102, indicating that disruption of these pathways likely represents a viral strategy to promote viral replication and avoid the host immune response.
Certain RNA viruses that replicate in the cytoplasm also inhibit host mRNA nuclear export. Vesiculoviruses, such as vesicular stomatitis virus (VSV), are negative‐stranded RNA viruses that prevent proper mRNA export through the action of the VSV matrix (M) protein 45, 69, 102, 103, 104, 105, 106, resulting in inhibition of host gene expression. This effect decreases competition of VSV mRNAs with host mRNAs for use of the translational machinery. Similar to IAV infection, blockage of mRNA export by VSV also prevents expression of mRNAs that encode antiviral factors 102. The M protein contains NLSs that allow its import into the nucleus, where it exerts its inhibitory function on mRNA export 104, 107. Once inside the nucleus, M protein interacts with the mRNA export factor Rae1, which is in complex with Nup98 69, 105. This prevents export of bulk poly(A) mRNAs during VSV infection. It has been reported that the interaction of M protein with Rae1 and Nup98 inhibits host transcription 108. However, high levels of polyadenylated RNA are retained inside the nucleus in the presence of M protein indicating complete mRNA synthesis, as shown by various methods including nucleocytoplasmic fractionation followed by microarray analysis and real‐time reverse transcriptase polymerase chain reaction 102, oligo‐dT in situ hybridization 45, 69, 105, 106 and mRNA nuclear export assays in Xenopus oocytes 103, 104, 105, 106. These results indicate that M protein utilizes post‐transcriptional mechanisms to inhibit gene expression. Transcriptional studies are necessary to investigate whether M protein regulates expression of certain subsets, rather than the general population, of mRNAs. This is possible, as Nup98 has been shown to regulate transcription of subsets of genes 109, 110.
The inhibition of bulk poly(A) mRNA export by M protein may occur via several mechanisms. The VSV M–Rae1–Nup98 complex may inhibit the export of a subset of mRNAs that include major regulators of gene expression, thereby indirectly triggering a shutoff of host gene expression. Another possibility is that Rae1–Nup98 may facilitate export of bulk NXF1‐mediated mRNA export. Therefore, M protein inhibition of Rae1–Nup98 would lead to retention of the majority of mRNAs in the nucleus. Interestingly, M protein‐mediated block of mRNA export can be antagonized by IFN, which upregulates the expression of Nup98, Nup96 and Rae1 16, 45. Genome‐wide studies that identify mRNAs that are directly targeted by the Rae1–Nup98 complex at early stages of infection as well as additional biochemical studies on the interaction of M protein with the mRNA export machinery will further reveal the mechanism of action of VSV M protein.
Interestingly, both Rae1 and Nup98 function during mitosis to regulate spindle assembly 111, 112. VSV M protein interaction with this complex inhibited mitotic progression and triggered substantial cell death 113. This has several implications for VSV, which is an oncolytic virus that is currently being developed as a cancer therapeutic 114, 115, 116. As tumor cells have an increased mitotic index, VSV‐mediated mitotic cell death likely contributes to its oncolytic activity.
As discussed above, picornaviruses are RNA viruses that replicate within the host cell cytoplasm and regulate nucleocytoplasmic trafficking. Many picornaviruses, including the important human pathogens PV and HRV, inhibit nucleocytoplasmic trafficking of host proteins and mRNAs to promote viral protein synthesis and disrupt host expression of antiviral factors. As discussed above, PV and HRV infection results in the mislocalization or degradation of several important nuclear export factors such as Nup62, Nup98 and Nup153 14, 15, 16, 19, 20. Cleavage and subsequent degradation or mislocalization of these proteins is mediated by the viral 2Apro, which leads to changes in NPC architecture that affects both host protein and mRNA transport 14, 15, 16, 17, 19, 20. Overall, RNA viruses employ a multitude of strategies to inhibit nucleocytoplasmic trafficking, which suppresses the host innate immune response and enhances viral replication.
Disruption of host gene expression by DNA viruses facilitates export of viral RNA
Not only RNA viruses disrupt nucleocytoplasmic trafficking of mRNA. Many DNA viruses selectively inhibit host mRNA export, while ensuring that viral mRNAs are efficiently exported after transcription. Adenoviruses (AdVs) are double‐stranded DNA viruses that infect many vertebrate species, including humans. Two adenoviral protein products, E1B‐55K and Ad E4 open reading frame 6 (E4orf6), have been shown to mediate the degradation of cellular proteins that may have a deleterious effect on viral propagation 117. The interaction of E1B‐55K and E4orf6 with host proteins results in the formation of a complex with E3 ubiquitin ligase activity that may contribute to inhibition of host mRNA export and promotion of late viral mRNA export from the nucleus 118, 119. In one model, it was proposed that the E1B‐55K and E4orf6 ubiquitin ligase activity promotes the degradation of an as of yet unidentified cellular protein involved in host mRNA export 118. Another possibility is that E1B‐55K disrupts NXF1‐mediated host mRNA export by binding to E1B‐AP5, a member of the RNP family that interacts with NXF1 63. While it is clear that AdV is able to regulate cellular mRNA export to favor export of late AdV mRNAs, more studies are needed to establish how AdV infection promotes nuclear accumulation of host mRNAs.
As discussed above, herpesviruses are experts at hijacking host cell functions to ensure viral replication. These viruses replicate within the nucleus and therefore must export viral transcripts to the cytoplasm for protein synthesis. One of the proteins encoded by the α‐herpesvirus HSV‐1 is ICP27, which disrupts host mRNA processing 120, 121 while allowing the export of intronless viral transcripts 122, 123, 124. ICP27 binds directly to the RNA export factor ALY/REF and NXF1, which recruits viral mRNAs to export receptors for preferential transport into the cytoplasm 122, 123, 124. Interestingly, other related herpesviruses do not inhibit host mRNA processing during infection, but do encode an ICP27‐like protein that favors viral mRNA export. These viruses include human cytomegalovirus (hCMV), Kaposi's sarcoma‐associated herpesvirus (KSHV), Epstein–Barr virus (EBV) and varicella‐zoster virus (VZV) 125, 126, 127, 128, 129.
Nucleocytoplasmic Transport: A New Frontier in Antiviral Therapy
These studies show that viruses dedicate many resources to the disruption of nucleocytoplasmic trafficking and the host can antagonize these effects. These findings attest to the importance of these pathways in proviral and antiviral mechanisms. As such, nuclear import and export pathways are enticing targets for the development of novel antiviral therapeutics. Recently, screens for small‐molecule inhibitors of the NS1 protein of IAV revealed both unique virus/host interactions and potentially useful antiviral strategies 130, 131. In one study, a high‐throughput screen was performed to identify small molecules that could antagonize NS1‐mediated inhibition of host gene expression. One of the compounds identified interfered with infection of several viruses by inducing the expression of REDD1 (also known as DDIT4), an inhibitor of the mTORC1 pathway that is required for IAV infection 130, 132. Overexpression of REDD1 resisted IAV infection while REDD1 knockout cells were more permissive to viral replication, demonstrating a role for REDD1 as a host defense factor. Interestingly, a second compound identified in the same screen uncovered a new link between pyrimidine biosynthesis pathways and mRNA nuclear export. This compound, a quinoline carboxylic acid, directly inhibited the host enzyme dihydroorotate dehydrogenase (DHODH), which is required for de novo pyrimidine biosynthesis but not for synthesis of pyrimidines via the salvage pathway. Thus, pyrimidine synthesis is not totally shut down and cytotoxicity does not occur upon compound treatment. The inhibition of DHODH led to an increase in NXF1 expression and subsequent relief of mRNA export block mediated by both IAV NS1 and VSV M proteins 102. The compound inhibited viral replication owing to the reduction of pyrimidine pools that viruses need to execute robust viral transcription and the release of host mRNA export block, which resulted in expression of antiviral factors 102, 133. Simultaneously, host cells maintained homeostasis by utilizing the salvage pathway or pyrimidines derived from the partially inhibited de novo biosynthesis pathway 102, 133.
Another strategy to inhibit virus replication is to target interactions between viral proteins and their nuclear transport receptors to prevent their import into the nucleus to promote viral replication. This was recently shown to be the case for the NS5 protein of dengue virus (DENV2) in a study in which the small‐molecule Ivermectin was able to inhibit NS5 interaction with Kapα/β, resulting in inhibition of nuclear import and infection 134.
Together, these studies show that the use of high‐throughput screening techniques to discover small molecules that alter host or viral gene expression has broad implications for the development of antiviral agents. Furthermore, the study of these compounds may reveal new interactions and regulatory functions of nuclear transport pathways, much like the use of viruses as tools of discovery.
Acknowledgments
We thank Angela Diehl for outstanding design of the figures. This work was supported by NIH R01AI079110, R01AI089539 and CPRIT RP121003‐RP120718‐P2; M. L. Y and M. A. M were supported by NIH T32‐CA124334.
References
- 1. Wente SR, Rout MP. The nuclear pore complex and nuclear transport. Cold Spring Harb Perspect Biol 2010;2:a000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cronshaw JM, Krutchinsky AN, Zhang W, Chait BT, Matunis MJ. Proteomic analysis of the mammalian nuclear pore complex. J Cell Biol 2002;158:915–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rout MP, Aitchison JD, Suprapto A, Hjertaas K, Zhao Y, Chait BT. The yeast nuclear pore complex: composition, architecture, and transport mechanism. J Cell Biol 2000;148:635–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Raices M, D'Angelo MA. Nuclear pore complex composition: a new regulator of tissue‐specific and developmental functions. Nat Rev Mol Cell Biol 2012;13:687–699. [DOI] [PubMed] [Google Scholar]
- 5. Suntharalingam M, Wente SR. Peering through the pore: nuclear pore complex structure, assembly, and function. Dev Cell 2003;4:775–789. [DOI] [PubMed] [Google Scholar]
- 6. Rout MP, Aitchison JD, Magnasco MO, Chait BT. Virtual gating and nuclear transport: the hole picture. Trends Cell Biol 2003;13:622–628. [DOI] [PubMed] [Google Scholar]
- 7. Cullen BR. Nuclear RNA export. J Cell Sci 2003;116(Pt 4):587–597. [DOI] [PubMed] [Google Scholar]
- 8. Kau TR, Way JC, Silver PA. Nuclear transport and cancer: from mechanism to intervention. Nat Rev Cancer 2004;4:106–117. [DOI] [PubMed] [Google Scholar]
- 9. Vasu SK, Forbes DJ. Nuclear pores and nuclear assembly. Curr Opin Cell Biol 2001;13:363–375. [DOI] [PubMed] [Google Scholar]
- 10. Weis K. Regulating access to the genome: nucleocytoplasmic transport throughout the cell cycle. Cell 2003;112:441–451. [DOI] [PubMed] [Google Scholar]
- 11. Rodriguez‐Navarro S, Hurt E. Linking gene regulation to mRNA production and export. Curr Opin Cell Biol 2011;23:302–309. [DOI] [PubMed] [Google Scholar]
- 12. Lee BJ, Cansizoglu AE, Suel KE, Louis TH, Zhang Z, Chook YM. Rules for nuclear localization sequence recognition by karyopherin beta 2. Cell 2006;126:543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pemberton LF, Paschal BM. Mechanisms of receptor‐mediated nuclear import and nuclear export. Traffic 2005;6:187–198. [DOI] [PubMed] [Google Scholar]
- 14. Gustin KE, Sarnow P. Effects of poliovirus infection on nucleo‐cytoplasmic trafficking and nuclear pore complex composition. EMBO J 2001;20(1–2):240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gustin KE, Sarnow P. Inhibition of nuclear import and alteration of nuclear pore complex composition by rhinovirus. J Virol 2002;76:8787–8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Castello A, Izquierdo JM, Welnowska E, Carrasco L. RNA nuclear export is blocked by poliovirus 2A protease and is concomitant with nucleoporin cleavage. J Cell Sci 2009;122(Pt 20):3799–3809. [DOI] [PubMed] [Google Scholar]
- 17. Belov GA, Lidsky PV, Mikitas OV, Egger D, Lukyanov KA, Bienz K, Agol VI. Bidirectional increase in permeability of nuclear envelope upon poliovirus infection and accompanying alterations of nuclear pores. J Virol 2004;78:10166–10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park N, Katikaneni P, Skern T, Gustin KE. Differential targeting of nuclear pore complex proteins in poliovirus‐infected cells. J Virol 2008;82:1647–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park N, Skern T, Gustin KE. Specific cleavage of the nuclear pore complex protein Nup62 by a viral protease. J Biol Chem 2010;285:28796–28805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Watters K, Palmenberg AC. Differential processing of nuclear pore complex proteins by rhinovirus 2A proteases from different species and serotypes. J Virol 2011;85:10874–10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ghildyal R, Jordan B, Li D, Dagher H, Bardin PG, Gern JE, Jans DA. Rhinovirus 3C protease can localize in the nucleus and alter active and passive nucleocytoplasmic transport. J Virol 2009;83:7349–7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shuai K, Liu B. Regulation of JAK‐STAT signalling in the immune system. Nat Rev Immunol 2003;3:900–911. [DOI] [PubMed] [Google Scholar]
- 23. Kopecky‐Bromberg SA, Martinez‐Sobrido L, Frieman M, Baric RA, Palese P. Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J Virol 2007;81:548–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frieman M, Yount B, Heise M, Kopecky‐Bromberg SA, Palese P, Baric RS. Severe acute respiratory syndrome coronavirus ORF6 antagonizes STAT1 function by sequestering nuclear import factors on the rough endoplasmic reticulum/Golgi membrane. J Virol 2007;81:9812–9824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hussain S, Perlman S, Gallagher TM. Severe acute respiratory syndrome coronavirus protein 6 accelerates murine hepatitis virus infections by more than one mechanism. J Virol 2008;82:7212–7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mateo M, Reid SP, Leung LW, Basler CF, Volchkov VE. Ebolavirus VP24 binding to karyopherins is required for inhibition of interferon signaling. J Virol 2010;84:1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reid SP, Leung LW, Hartman AL, Martinez O, Shaw ML, Carbonnelle C, Volchkov VE, Nichol ST, Basler CF. Ebola virus VP24 binds karyopherin alpha1 and blocks STAT1 nuclear accumulation. J Virol 2006;80:5156–5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reid SP, Valmas C, Martinez O, Sanchez FM, Basler CF. Ebola virus VP24 proteins inhibit the interaction of NPI‐1 subfamily karyopherin alpha proteins with activated STAT1. J Virol 2007;81:13469–13477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shabman RS, Gulcicek EE, Stone KL, Basler CF. The Ebola virus VP24 protein prevents hnRNP C1/C2 binding to karyopherin alpha1 and partially alters its nuclear import. J Infect Dis 2011;204(Suppl. 3):S904–S910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ertel KJ, Brunner JE, Semler BL. Mechanistic consequences of hnRNP C binding to both RNA termini of poliovirus negative‐strand RNA intermediates. J Virol 2010;84:4229–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takayama I, Sato H, Watanabe A, Omi‐Furutani M, Sugai A, Kanki K, Yoneda M, Kai C. The nucleocapsid protein of measles virus blocks host interferon response. Virology 2012;424:45–55. [DOI] [PubMed] [Google Scholar]
- 32. Holloway G, Truong TT, Coulson BS. Rotavirus antagonizes cellular antiviral responses by inhibiting the nuclear accumulation of STAT1, STAT2, and NF‐kappaB. J Virol 2009;83:4942–4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Porter FW, Bochkov YA, Albee AJ, Wiese C, Palmenberg AC. A picornavirus protein interacts with Ran‐GTPase and disrupts nucleocytoplasmic transport. Proc Natl Acad Sci U S A 2006;103:12417–12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Porter FW, Palmenberg AC. Leader‐induced phosphorylation of nucleoporins correlates with nuclear trafficking inhibition by cardioviruses. J Virol 2009;83:1941–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bardina MV, Lidsky PV, Sheval EV, Fominykh KV, van Kuppeveld FJ, Polyakov VY, Agol VI. Mengovirus‐induced rearrangement of the nuclear pore complex: hijacking cellular phosphorylation machinery. J Virol 2009;83:3150–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lidsky PV, Hato S, Bardina MV, Aminev AG, Palmenberg AC, Sheval EV, Polyakov VY, van Kuppeveld FJ, Agol VI. Nucleocytoplasmic traffic disorder induced by cardioviruses. J Virol 2006;80:2705–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Delhaye S, van Pesch V, Michiels T. The leader protein of Theiler's virus interferes with nucleocytoplasmic trafficking of cellular proteins. J Virol 2004;78:4357–4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ricour C, Borghese F, Sorgeloos F, Hato SV, van Kuppeveld FJ, Michiels T. Random mutagenesis defines a domain of Theiler's virus leader protein that is essential for antagonism of nucleocytoplasmic trafficking and cytokine gene expression. J Virol 2009;83:11223–11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Atasheva S, Garmashova N, Frolov I, Frolova E. Venezuelan equine encephalitis virus capsid protein inhibits nuclear import in Mammalian but not in mosquito cells. J Virol 2008;82:4028–4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Atasheva S, Fish A, Fornerod M, Frolova EI. Venezuelan equine Encephalitis virus capsid protein forms a tetrameric complex with CRM1 and importin alpha/beta that obstructs nuclear pore complex function. J Virol 2010;84:4158–4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nelson LM, Rose RC, Moroianu J. The L1 major capsid protein of human papillomavirus type 11 interacts with Kap beta2 and Kap beta3 nuclear import receptors. Virology 2003;306:162–169. [DOI] [PubMed] [Google Scholar]
- 42. Darshan MS, Lucchi J, Harding E, Moroianu J. The l2 minor capsid protein of human papillomavirus type 16 interacts with a network of nuclear import receptors. J Virol 2004;78:12179–12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Malik P, Tabarraei A, Kehlenbach RH, Korfali N, Iwasawa R, Graham SV, Schirmer EC. Herpes simplex virus ICP27 protein directly interacts with the nuclear pore complex through Nup62, inhibiting host nucleocytoplasmic transport pathways. J Biol Chem 2012;287:12277–12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen IH, Sciabica KS, Sandri‐Goldin RM. ICP27 interacts with the RNA export factor Aly/REF to direct herpes simplex virus type 1 intronless mRNAs to the TAP export pathway. J Virol 2002;76:12877–12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Enninga J, Levy DE, Blobel G, Fontoura BM. Role of nucleoporin induction in releasing an mRNA nuclear export block. Science 2002;295:1523–1525. [DOI] [PubMed] [Google Scholar]
- 46. Ball LA. Virus replication strategies. Fields Virol 2007;5:120–140. [Google Scholar]
- 47. Fornerod M, Ohno M, Yoshida M, Mattaj IW. CRM1 is an export receptor for leucine‐rich nuclear export signals. Cell 1997;90:1051–1060. [DOI] [PubMed] [Google Scholar]
- 48. Stade K, Ford CS, Guthrie C, Weis K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell 1997;90:1041–1050. [DOI] [PubMed] [Google Scholar]
- 49. Gruter P, Tabernero C, von Kobbe C, Schmitt C, Saavedra C, Bachi A, Wilm M, Felber BK, Izaurralde E. TAP, the human homolog of Mex67p, mediates CTE‐dependent RNA export from the nucleus. Mol Cell 1998;1:649–659. [DOI] [PubMed] [Google Scholar]
- 50. Shatkin AJ, Manley JL. The ends of the affair: capping and polyadenylation. Nat Struct Biol 2000;7:838–842. [DOI] [PubMed] [Google Scholar]
- 51. Cheng H, Dufu K, Lee CS, Hsu JL, Dias A, Reed R. Human mRNA export machinery recruited to the 5' end of mRNA. Cell 2006;127:1389–1400. [DOI] [PubMed] [Google Scholar]
- 52. Strasser K, Masuda S, Mason P, Pfannstiel J, Oppizzi M, Rodriguez‐Navarro S, Rondon AG, Aguilera A, Struhl K, Reed R, Hurt E. TREX is a conserved complex coupling transcription with messenger RNA export. Nature 2002;417:304–308. [DOI] [PubMed] [Google Scholar]
- 53. Masuda S, Das R, Cheng H, Hurt E, Dorman N, Reed R. Recruitment of the human TREX complex to mRNA during splicing. Genes Dev 2005;19:1512–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhou Z, Luo MJ, Straesser K, Katahira J, Hurt E, Reed R. The protein Aly links pre‐messenger‐RNA splicing to nuclear export in metazoans. Nature 2000;407:401–405. [DOI] [PubMed] [Google Scholar]
- 55. Chavez S, Beilharz T, Rondon AG, Erdjument‐Bromage H, Tempst P, Svejstrup JQ, Lithgow T, Aguilera A. A protein complex containing Tho2, Hpr1, Mft1 and a novel protein, Thp2, connects transcription elongation with mitotic recombination in Saccharomyces cerevisiae . EMBO J 2000;19:5824–5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rehwinkel J, Herold A, Gari K, Kocher T, Rode M, Ciccarelli FL, Wilm M, Izaurralde E. Genome‐wide analysis of mRNAs regulated by the THO complex in Drosophila melanogaster . Nat Struct Mol Biol 2004;11:558–566. [DOI] [PubMed] [Google Scholar]
- 57. Piruat JI, Aguilera A. A novel yeast gene, THO2, is involved in RNA pol II transcription and provides new evidence for transcriptional elongation‐associated recombination. EMBO J 1998;17:4859–4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Luo ML, Zhou Z, Magni K, Christoforides C, Rappsilber J, Mann M, Reed R. Pre‐mRNA splicing and mRNA export linked by direct interactions between UAP56 and Aly. Nature 2001;413:644–647. [DOI] [PubMed] [Google Scholar]
- 59. Dufu K, Livingstone MJ, Seebacher J, Gygi SP, Wilson SA, Reed R. ATP is required for interactions between UAP56 and two conserved mRNA export proteins, Aly and CIP29, to assemble the TREX complex. Genes Dev 2010;24:2043–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Taniguchi I, Ohno M. ATP‐dependent recruitment of export factor Aly/REF onto intronless mRNAs by RNA helicase UAP56. Mol Cell Biol 2008;28:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stutz F, Bachi A, Doerks T, Braun IC, Seraphin B, Wilm M, Bork P, Izaurralde E. REF, an evolutionary conserved family of hnRNP‐like proteins, interacts with TAP/Mex67p and participates in mRNA nuclear export. RNA 2000;6:638–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Strasser K, Hurt E. Yra1p, a conserved nuclear RNA‐binding protein, interacts directly with Mex67p and is required for mRNA export. EMBO J 2000;19:410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bachi A, Braun IC, Rodrigues JP, Pante N, Ribbeck K, von Kobbe C, Kutay U, Wilm M, Gorlich D, Carmo‐Fonseca M, Izaurralde E. The C‐terminal domain of TAP interacts with the nuclear pore complex and promotes export of specific CTE‐bearing RNA substrates. RNA 2000;6:136–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Carmody SR, Wente SR. mRNA nuclear export at a glance. J Cell Sci 2009;122(Pt 12):1933–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bailer SM, Siniossoglou S, Podtelejnikov A, Hellwig A, Mann M, Hurt E. Nup116p and nup100p are interchangeable through a conserved motif which constitutes a docking site for the mRNA transport factor gle2p. EMBO J 1998;17:1107–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bharathi A, Ghosh A, Whalen WA, Yoon JH, Pu R, Dasso M, Dhar R. The human RAE1 gene is a functional homologue of Schizosaccharomyces pombe rae1 gene involved in nuclear export of Poly(A) + RNA. Gene 1997;198(1–2):251–258. [DOI] [PubMed] [Google Scholar]
- 67. Blevins MB, Smith AM, Phillips EM, Powers MA. Complex formation among the RNA export proteins Nup98, Rae1/Gle2, and TAP. J Biol Chem 2003;278:20979–20988. [DOI] [PubMed] [Google Scholar]
- 68. Brown JA, Bharathi A, Ghosh A, Whalen W, Fitzgerald E, Dhar R. A mutation in the Schizosaccharomyces pombe rae1 gene causes defects in poly(A) + RNA export and in the cytoskeleton. J Biol Chem 1995;270:7411–7419. [DOI] [PubMed] [Google Scholar]
- 69. Faria PA, Chakraborty P, Levay A, Barber GN, Ezelle HJ, Enninga J, Arana C, van Deursen J, Fontoura BM. VSV disrupts the Rae1/mrnp41 mRNA nuclear export pathway. Mol Cell 2005;17:93–102. [DOI] [PubMed] [Google Scholar]
- 70. Kraemer D, Blobel G. mRNA binding protein mrnp 41 localizes to both nucleus and cytoplasm. Proc Natl Acad Sci U S A 1997;94:9119–9124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pritchard CE, Fornerod M, Kasper LH, van Deursen JM. RAE1 is a shuttling mRNA export factor that binds to a GLEBS‐like NUP98 motif at the nuclear pore complex through multiple domains. J Cell Biol 1999;145:237–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ren Y, Seo HS, Blobel G, Hoelz A. Structural and functional analysis of the interaction between the nucleoporin Nup98 and the mRNA export factor Rae1. Proc Natl Acad Sci U S A 2010;107:10406–10411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Whalen WA, Bharathi A, Danielewicz D, Dhar R. Advancement through mitosis requires rae1 gene function in fission yeast. Yeast 1997;13:1167–1179. [DOI] [PubMed] [Google Scholar]
- 74. Kuss SK, Mata MA, Zhang L, Fontoura BM. Nuclear imprisonment: viral strategies to arrest host mRNA nuclear export. Viruses 2013;5:1824–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Alcazar‐Roman AR, Tran EJ, Guo S, Wente SR. Inositol hexakisphosphate and Gle1 activate the DEAD‐box protein Dbp5 for nuclear mRNA export. Nat Cell Biol 2006;8:711–716. [DOI] [PubMed] [Google Scholar]
- 76. Weirich CS, Erzberger JP, Flick JS, Berger JM, Thorner J, Weis K. Activation of the DExD/H‐box protein Dbp5 by the nuclear‐pore protein Gle1 and its coactivator InsP6 is required for mRNA export. Nat Cell Biol 2006;8:668–676. [DOI] [PubMed] [Google Scholar]
- 77. Tran EJ, Zhou Y, Corbett AH, Wente SR. The DEAD‐box protein Dbp5 controls mRNA export by triggering specific RNA:protein remodeling events. Mol Cell 2007;28:850–859. [DOI] [PubMed] [Google Scholar]
- 78. Montpetit B, Thomsen ND, Helmke KJ, Seeliger MA, Berger JM, Weis K. A conserved mechanism of DEAD‐box ATPase activation by nucleoporins and InsP6 in mRNA export. Nature 2011;472:238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tsai NP, Lin YL, Tsui YC, Wei LN. Dual action of epidermal growth factor: extracellular signal‐stimulated nuclear‐cytoplasmic export and coordinated translation of selected messenger RNA. J Cell Biol 2010;188:325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yang J, Bogerd HP, Wang PJ, Page DC, Cullen BR. Two closely related human nuclear export factors utilize entirely distinct export pathways. Mol Cell 2001;8:397–406. [DOI] [PubMed] [Google Scholar]
- 81. Culjkovic B, Topisirovic I, Skrabanek L, Ruiz‐Gutierrez M, Borden KL. eIF4E is a central node of an RNA regulon that governs cellular proliferation. J Cell Biol 2006;175:415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Natalizio BJ, Wente SR. Postage for the messenger: designating routes for nuclear mRNA export. Trends Cell Biol 2013;23:365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Malim MH, Bohnlein S, Hauber J, Cullen BR. Functional dissection of the HIV‐1 Rev trans‐activator – derivation of a trans‐dominant repressor of Rev function. Cell 1989;58:205–214. [DOI] [PubMed] [Google Scholar]
- 84. Malim MH, Hauber J, Le SY, Maizel JV, Cullen BR. The HIV‐1 rev trans‐activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature 1989;338:254–257. [DOI] [PubMed] [Google Scholar]
- 85. Malim MH, Tiley LS, McCarn DF, Rusche JR, Hauber J, Cullen BR. HIV‐1 structural gene expression requires binding of the Rev trans‐activator to its RNA target sequence. Cell 1990;60:675–683. [DOI] [PubMed] [Google Scholar]
- 86. Bray M, Prasad S, Dubay JW, Hunter E, Jeang KT, Rekosh D, Hammarskjold ML. A small element from the Mason‐Pfizer monkey virus genome makes human immunodeficiency virus type 1 expression and replication Rev‐independent. Proc Natl Acad Sci U S A 1994;91:1256–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Buser C, Walther P, Mertens T, Michel D. Cytomegalovirus primary envelopment occurs at large infoldings of the inner nuclear membrane. J Virol 2007;81:3042–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Speese SD, Ashley J, Jokhi V, Nunnari J, Barria R, Li Y, Ataman B, Koon A, Chang YT, Li Q, Moore MJ, Budnik V. Nuclear envelope budding enables large ribonucleoprotein particle export during synaptic Wnt signaling. Cell 2012;149:832–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Darlington RW, Moss LH III. Herpesvirus envelopment. J Virol 1968;2:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Burke B, Stewart CL. The nuclear lamins: flexibility in function. Nat Rev Mol Cell Biol 2013;14:13–24. [DOI] [PubMed] [Google Scholar]
- 91. Garcia‐Sastre A. Induction and evasion of type I interferon responses by influenza viruses. Virus Res 2011;162(1–2):12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hale BG, Randall RE, Ortin J, Jackson D. The multifunctional NS1 protein of influenza A viruses. J Gen Virol 2008;89(Pt 10):2359–2376. [DOI] [PubMed] [Google Scholar]
- 93. Luna R, Gaillard H, Gonzalez‐Aguilera C, Aguilera A. Biogenesis of mRNPs: integrating different processes in the eukaryotic nucleus. Chromosoma 2008;117:319–331. [DOI] [PubMed] [Google Scholar]
- 94. Qiu Y, Nemeroff M, Krug RM. The influenza virus NS1 protein binds to a specific region in human U6 snRNA and inhibits U6‐U2 and U6‐U4 snRNA interactions during splicing. RNA 1995;1:304–316. [PMC free article] [PubMed] [Google Scholar]
- 95. Wolff T, O'Neill RE, Palese P. NS1‐binding protein (NS1‐BP): a novel human protein that interacts with the influenza A virus nonstructural NS1 protein is relocalized in the nuclei of infected cells. J Virol 1998;72:7170–7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Nemeroff ME, Barabino SM, Li Y, Keller W, Krug RM. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3'end formation of cellular pre‐mRNAs. Mol Cell 1998;1:991–1000. [DOI] [PubMed] [Google Scholar]
- 97. Chen Z, Li Y, Krug RM. Influenza A virus NS1 protein targets poly(A)‐binding protein II of the cellular 3'‐end processing machinery. EMBO J 1999;18:2273–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Poon LL, Pritlove DC, Fodor E, Brownlee GG. Direct evidence that the poly(A) tail of influenza A virus mRNA is synthesized by reiterative copying of a U track in the virion RNA template. J Virol 1999;73:3473–3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Robertson JS, Schubert M, Lazzarini RA. Polyadenylation sites for influenza virus mRNA. J Virol 1981;38:157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Tsai PL, Chiou NT, Kuss S, Garcia‐Sastre A, Lynch KW, Fontoura BM. Cellular RNA binding proteins NS1‐BP and hnRNP K regulate influenza A virus RNA splicing. PLoS Pathog 2013;9:e1003460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Satterly N, Tsai PL, van Deursen J, Nussenzveig DR, Wang Y, Faria PA, Levay A, Levy DE, Fontoura BM. Influenza virus targets the mRNA export machinery and the nuclear pore complex. Proc Natl Acad Sci U S A 2007;104:1853–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhang L, Das P, Schmolke M, Manicassamy B, Wang Y, Deng X, Cai L, Tu BP, Forst CV, Roth MG, Levy DE, Garcia‐Sastre A, de Brabander J, Phillips MA, Fontoura BM. Inhibition of pyrimidine synthesis reverses viral virulence factor‐mediated block of mRNA nuclear export. J Cell Biol 2012;196:315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Petersen JM, Her LS, Dahlberg JE. Multiple vesiculoviral matrix proteins inhibit both nuclear export and import. Proc Natl Acad Sci U S A 2001;98:8590–8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Petersen JM, Her LS, Varvel V, Lund E, Dahlberg JE. The matrix protein of vesicular stomatitis virus inhibits nucleocytoplasmic transport when it is in the nucleus and associated with nuclear pore complexes. Mol Cell Biol 2000;20:8590–8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. von Kobbe C, van Deursen JM, Rodrigues JP, Sitterlin D, Bachi A, Wu X, Wilm M, Carmo‐Fonseca M, Izaurralde E. Vesicular stomatitis virus matrix protein inhibits host cell gene expression by targeting the nucleoporin Nup98. Mol Cell 2000;6:1243–1252. [DOI] [PubMed] [Google Scholar]
- 106. Her LS, Lund E, Dahlberg JE. Inhibition of Ran guanosine triphosphatase‐dependent nuclear transport by the matrix protein of vesicular stomatitis virus. Science 1997;276:1845–1848. [DOI] [PubMed] [Google Scholar]
- 107. Glodowski DR, Petersen JM, Dahlberg JE. Complex nuclear localization signals in the matrix protein of vesicular stomatitis virus. J Biol Chem 2002;277:46864–46870. [DOI] [PubMed] [Google Scholar]
- 108. Rajani KR, Pettit Kneller EL, McKenzie MO, Horita DA, Chou JW, Lyles DS. Complexes of vesicular stomatitis virus matrix protein with host Rae1 and Nup98 involved in inhibition of host transcription. PLoS Pathog 2012;8:e1002929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Capelson M, Liang Y, Schulte R, Mair W, Wagner U, Hetzer MW. Chromatin‐bound nuclear pore components regulate gene expression in higher eukaryotes. Cell 2010;140:372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kalverda B, Pickersgill H, Shloma VV, Fornerod M. Nucleoporins directly stimulate expression of developmental and cell‐cycle genes inside the nucleoplasm. Cell 2010;140:360–371. [DOI] [PubMed] [Google Scholar]
- 111. Blower MD, Nachury M, Heald R, Weis K. A Rae1‐containing ribonucleoprotein complex is required for mitotic spindle assembly. Cell 2005;121:223–234. [DOI] [PubMed] [Google Scholar]
- 112. Cross MK, Powers MA. Nup98 regulates bipolar spindle assembly through association with microtubules and opposition of MCAK. Mol Biol Cell 2011;22:661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Chakraborty P, Seemann J, Mishra RK, Wei JH, Weil L, Nussenzveig DR, Heiber J, Barber GN, Dasso M, Fontoura BM. Vesicular stomatitis virus inhibits mitotic progression and triggers cell death. EMBO Rep 2009;10:1154–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Barber GN. Vesicular stomatitis virus as an oncolytic vector. Viral Immunol 2004;17:516–527. [DOI] [PubMed] [Google Scholar]
- 115. Barber GN. VSV‐tumor selective replication and protein translation. Oncogene 2005;24:7710–7719. [DOI] [PubMed] [Google Scholar]
- 116. Hastie E, Grdzelishvili VZ. Vesicular stomatitis virus as a flexible platform for oncolytic virotherapy against cancer. J Gen Virol 2012;93(Pt 12):2529–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Querido E, Blanchette P, Yan Q, Kamura T, Morrison M, Boivin D, Kaelin WG, Conaway RC, Conaway JW, Branton PE. Degradation of p53 by adenovirus E4orf6 and E1B55K proteins occurs via a novel mechanism involving a Cullin‐containing complex. Genes Dev 2001;15:3104–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Woo JL, Berk AJ. Adenovirus ubiquitin‐protein ligase stimulates viral late mRNA nuclear export. J Virol 2007;81:575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Blanchette P, Kindsmuller K, Groitl P, Dallaire F, Speiseder T, Branton PE, Dobner T. Control of mRNA export by adenovirus E4orf6 and E1B55K proteins during productive infection requires E4orf6 ubiquitin ligase activity. J Virol 2008;82:2642–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bryant HE, Wadd SE, Lamond AI, Silverstein SJ, Clements JB. Herpes simplex virus IE63 (ICP27) protein interacts with spliceosome‐associated protein 145 and inhibits splicing prior to the first catalytic step. J Virol 2001;75:4376–4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lindberg A, Kreivi JP. Splicing inhibition at the level of spliceosome assembly in the presence of herpes simplex virus protein ICP27. Virology 2002;294:189–198. [DOI] [PubMed] [Google Scholar]
- 122. Johnson LA, Li L, Sandri‐Goldin RM. The cellular RNA export receptor TAP/NXF1 is required for ICP27‐mediated export of herpes simplex virus 1 RNA, but the TREX complex adaptor protein Aly/REF appears to be dispensable. J Virol 2009;83:6335–6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Johnson LA, Sandri‐Goldin RM. Efficient nuclear export of herpes simplex virus 1 transcripts requires both RNA binding by ICP27 and ICP27 interaction with TAP/NXF1. J Virol 2009;83:1184–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Koffa MD, Clements JB, Izaurralde E, Wadd S, Wilson SA, Mattaj IW, Kuersten S. Herpes simplex virus ICP27 protein provides viral mRNAs with access to the cellular mRNA export pathway. EMBO J 2001;20:5769–5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Boyne JR, Colgan KJ, Whitehouse A. Recruitment of the complete hTREX complex is required for Kaposi's sarcoma‐associated herpesvirus intronless mRNA nuclear export and virus replication. PLoS Pathog 2008;4:e1000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Williams BJ, Boyne JR, Goodwin DJ, Roaden L, Hautbergue GM, Wilson SA, Whitehouse A. The prototype gamma‐2 herpesvirus nucleocytoplasmic shuttling protein, ORF 57, transports viral RNA through the cellular mRNA export pathway. Biochem J 2005;387(Pt 2):295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hiriart E, Bardouillet L, Manet E, Gruffat H, Penin F, Montserret R, Farjot G, Sergeant A. A region of the Epstein‐Barr virus (EBV) mRNA export factor EB2 containing an arginine‐rich motif mediates direct binding to RNA. J Biol Chem 2003;278:37790–37798. [DOI] [PubMed] [Google Scholar]
- 128. Ote I, Lebrun M, Vandevenne P, Bontems S, Medina‐Palazon C, Manet E, Piette J, Sadzot‐Delvaux C. Varicella‐zoster virus IE4 protein interacts with SR proteins and exports mRNAs through the TAP/NXF1 pathway. PLoS One 2009;4:e7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lischka P, Toth Z, Thomas M, Mueller R, Stamminger T. The UL69 transactivator protein of human cytomegalovirus interacts with DEXD/H‐Box RNA helicase UAP56 to promote cytoplasmic accumulation of unspliced RNA. Mol Cell Biol 2006;26:1631–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Mata MA, Satterly N, Versteeg GA, Frantz D, Wei S, Williams N, Schmolke M, Pena‐Llopis S, Brugarolas J, Forst CV, White MA, Garcia‐Sastre A, Roth MG, Fontoura BM. Chemical inhibition of RNA viruses reveals REDD1 as a host defense factor. Nat Chem Biol 2011;7:712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Basu D, Walkiewicz MP, Frieman M, Baric RS, Auble DT, Engel DA. Novel influenza virus NS1 antagonists block replication and restore innate immune function. J Virol 2009;83:1881–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Konig R, Stertz S, Zhou Y, Inoue A, Hoffmann HH, Bhattacharyya S, Alamares JG, Tscherne DM, Ortigoza MB, Liang Y, Gao Q, Andrews SE, Bandyopadhyay S, De Jesus P, Tu BP, et al. Human host factors required for influenza virus replication. Nature 2010;463:813–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Hoffmann HH, Kunz A, Simon VA, Palese P, Shaw ML. Broad‐spectrum antiviral that interferes with de novo pyrimidine biosynthesis. Proc Natl Acad Sci U S A 2011;108:5777–5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Tay MY, Fraser JE, Chan WK, Moreland NJ, Rathore AP, Wang C, Vasudevan SG, Jans DA. Nuclear localization of dengue virus (DENV) 1–4 non‐structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antiviral Res 2013;99:301–306. [DOI] [PubMed] [Google Scholar]


