Abstract
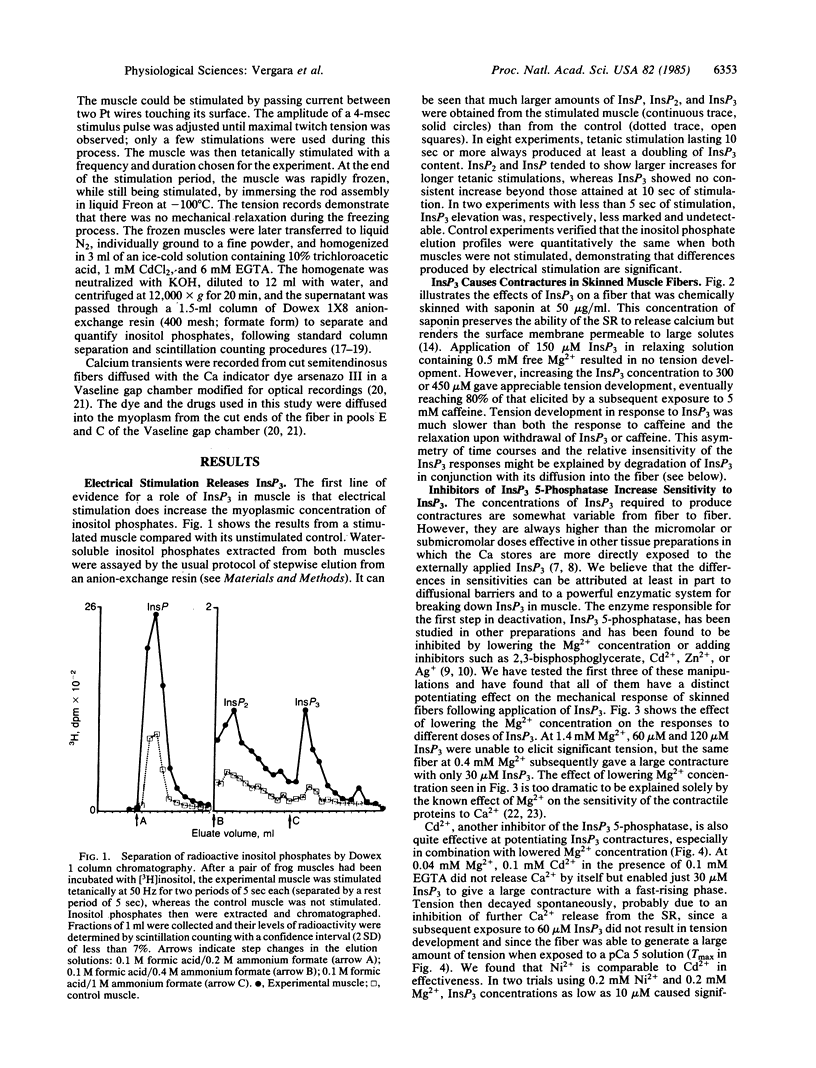
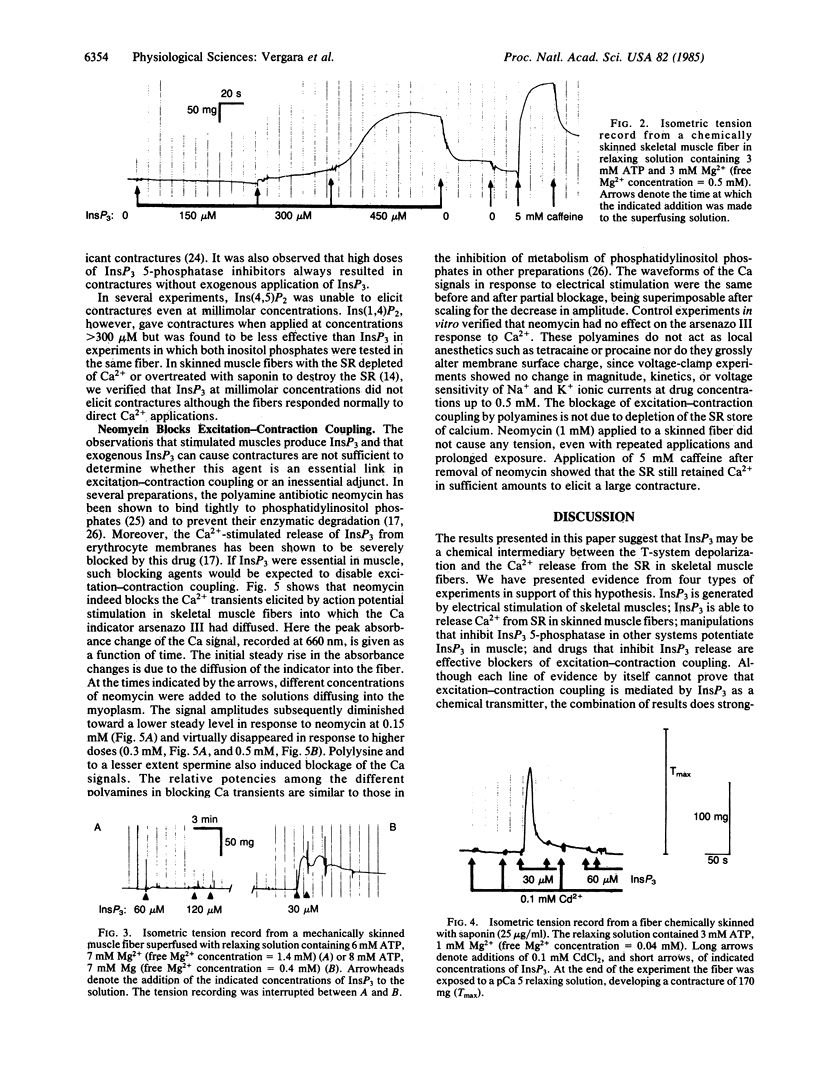
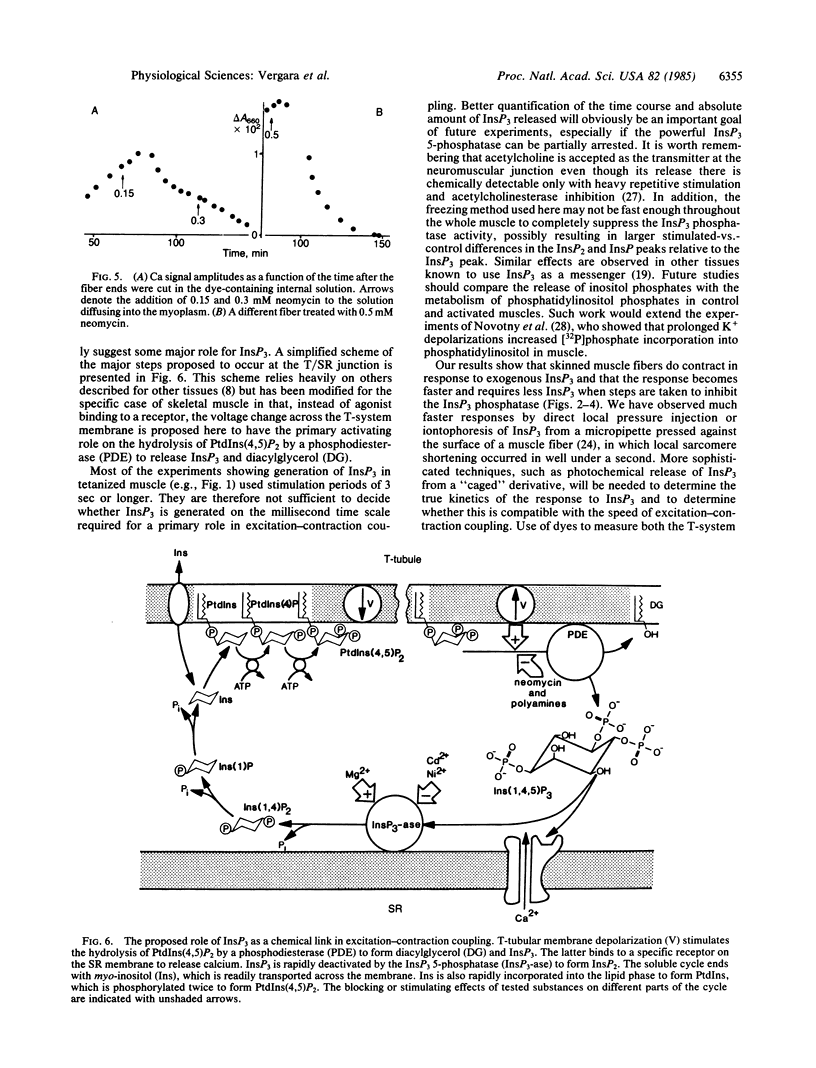
The role of inositol 1,4,5-trisphosphate (InsP3) in excitation-contraction coupling in skeletal muscle was investigated by several methods. The following results were obtained. InsP3 is released by electrical stimulation of muscles. Exogenous InsP3 releases calcium from skinned muscle fibers at relatively high doses under normal conditions but does so at very low concentrations when blockers of the InsP3 5-phosphatase are present. Blockers of InsP3 release are effective blockers of calcium transients elicited by electrical stimulation of muscle fibers. It is proposed that InsP3 acts as a chemical second messenger between transverse (T)-tubular membrane depolarization and calcium release from the sarcoplasmic reticulum in skeletal muscle.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson J. J., Trimm J. L., Weden L., Salama G. Heavy metals induce rapid calcium release from sarcoplasmic reticulum vesicles isolated from skeletal muscle. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1526–1530. doi: 10.1073/pnas.80.6.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W. Differential effects of tetracaine on delayed potassium channels and displacement currents in frog skeletal muscle. J Physiol. 1976 Nov;262(3):613–637. doi: 10.1113/jphysiol.1976.sp011612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Leefmans F. J., Rink T. J., Tsien R. Y. Free calcium ions in neurones of Helix aspersa measured with ion-selective micro-electrodes. J Physiol. 1981 Jun;315:531–548. doi: 10.1113/jphysiol.1981.sp013762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Caputo C., Vergara J., Bezanilla F. Local anaesthetics inhibit tension development and Nile blue fluorescence signals in frog muscle fibres. Nature. 1979 Feb 1;277(5695):400–402. doi: 10.1038/277400a0. [DOI] [PubMed] [Google Scholar]
- Carvalho A. P. Effects of potentiators of muscular contraction on binding of cations by sarcoplasmic reticulum. J Gen Physiol. 1968 Mar;51(3):427–442. doi: 10.1085/jgp.51.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Rakowski R. F., Schneider M. F. A non-linear voltage dependent charge movement in frog skeletal muscle. J Physiol. 1976 Jan;254(2):245–283. doi: 10.1113/jphysiol.1976.sp011232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985 Apr 11;314(6011):534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M., Otsuki I. Control of muscle contraction. Q Rev Biophys. 1969 Nov;2(4):351–384. doi: 10.1017/s0033583500001190. [DOI] [PubMed] [Google Scholar]
- Ellis R. B., Galliard T., Hawthorne J. N. Phosphoinositides. 5. The inositol lipids of ox brain. Biochem J. 1963 Jul;88(1):125–131. doi: 10.1042/bj0880125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M., Iino M. Specific perforation of muscle cell membranes with preserved SR functions by saponin treatment. J Muscle Res Cell Motil. 1980 Mar;1(1):89–100. doi: 10.1007/BF00711927. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C., Nunzi G. Junctional feet and particles in the triads of a fast-twitch muscle fibre. J Muscle Res Cell Motil. 1983 Apr;4(2):233–252. doi: 10.1007/BF00712033. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The effect of sudden changes in ionic concentrations on the membrane potential of single muscle fibres. J Physiol. 1960 Sep;153:370–385. doi: 10.1113/jphysiol.1960.sp006540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F., TAYLOR R. E. Local activation of striated muscle fibres. J Physiol. 1958 Dec 30;144(3):426–441. doi: 10.1113/jphysiol.1958.sp006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Campbell D. T. An improved vaseline gap voltage clamp for skeletal muscle fibers. J Gen Physiol. 1976 Mar;67(3):265–293. doi: 10.1085/jgp.67.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiak M. J., Small D. M., Shipley G. G. Temperature and compositional dependence of the structure of hydrated dimyristoyl lecithin. J Biol Chem. 1979 Jul 10;254(13):6068–6078. [PubMed] [Google Scholar]
- Kerrick W. G., Donaldson S. K. The effects of Mg 2+ on submaximum Ca 2+ -activated tension in skinned fibers of frog skeletal muscle. Biochim Biophys Acta. 1972 Jul 12;275(1):117–122. doi: 10.1016/0005-2728(72)90030-8. [DOI] [PubMed] [Google Scholar]
- Khan M. M., Martell A. E. Thermodynamic quantities associated with the interaction of adenosine triphosphate with metal ions. J Am Chem Soc. 1966 Feb 20;88(4):668–671. doi: 10.1021/ja00956a008. [DOI] [PubMed] [Google Scholar]
- Novotný I., Saleh F., Novotná R. K+ depolarization and phospholipid metabolism in frog sartorius muscle. Gen Physiol Biophys. 1983 Oct;2(5):329–337. [PubMed] [Google Scholar]
- Palade P., Vergara J. Arsenazo III and antipyrylazo III calcium transients in single skeletal muscle fibers. J Gen Physiol. 1982 Apr;79(4):679–707. doi: 10.1085/jgp.79.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter L. T. Synthesis, storage and release of [14C]acetylcholine in isolated rat diaphragm muscles. J Physiol. 1970 Jan;206(1):145–166. doi: 10.1113/jphysiol.1970.sp009003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink T. J., Sanchez A., Hallam T. J. Diacylglycerol and phorbol ester stimulate secretion without raising cytoplasmic free calcium in human platelets. Nature. 1983 Sep 22;305(5932):317–319. doi: 10.1038/305317a0. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M. Mechanism of action of local anaesthetic agents and biotoxins. Br J Anaesth. 1975 Feb;47 Suppl:191–198. [PubMed] [Google Scholar]
- Schacht J. Inhibition by neomycin of polyphosphoinositide turnover in subcellular fractions of guinea-pig cerebral cortex in vitro. J Neurochem. 1976 Nov;27(5):1119–1124. doi: 10.1111/j.1471-4159.1976.tb00318.x. [DOI] [PubMed] [Google Scholar]
- Schacht J. Purification of polyphosphoinositides by chromatography on immobilized neomycin. J Lipid Res. 1978 Nov;19(8):1063–1067. [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Storey D. J., Shears S. B., Kirk C. J., Michell R. H. Stepwise enzymatic dephosphorylation of inositol 1,4,5-trisphosphate to inositol in liver. Nature. 1984 Nov 22;312(5992):374–376. doi: 10.1038/312374a0. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Vergara J., Caputo C. Effects of tetracaine on charge movements and calcium signals in frog skeletal muscle fibers. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1477–1481. doi: 10.1073/pnas.80.5.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicentini L. M., Ambrosini A., Di Virgilio F., Pozzan T., Meldolesi J. Muscarinic receptor-induced phosphoinositide hydrolysis at resting cytosolic Ca2+ concentration in PC12 cells. J Cell Biol. 1985 Apr;100(4):1330–1333. doi: 10.1083/jcb.100.4.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]


