Significance
In vertebrates, the transition of postmeiotic spermatids into spermatozoa (spermiogenesis) is believed to occur indirectly in response to androgens released by the somatic Leydig cells after activation of the luteinizing hormone/choriogonadotropin receptor (LHCGR). In contrast to this indirect model, here we show that distantly related fishes express the homolog of the tetrapod LHCGR (Lhcgrba) also in germ cells, which directly drives spermiogenesis in response to the luteinizing hormone. Our findings reveal a nonsteroidal role of the Lhcgrba pathway in vertebrate germ cells with potential implications for the causes of male infertility.
Keywords: reproduction, testis, fertility
Abstract
In both mammals and teleosts, the differentiation of postmeiotic spermatids to spermatozoa (spermiogenesis) is thought to be indirectly controlled by the luteinizing hormone (LH) acting through the LH/choriogonadotropin receptor (LHCGR) to stimulate androgen secretion in the interstitial Leydig cells. However, a more direct, nonsteroidal role of LH mediating the spermiogenic pathway remains unclear. Using a flatfish with semicystic spermatogenesis, in which spermatids are released into the seminiferous lobule lumen (SLL), where they develop into spermatozoa without direct contact with the supporting Sertoli cells, we show that haploid spermatids express the homolog of the tetrapod LHCGR (Lhcgrba). Both native Lh and intramuscularly injected His-tagged recombinant Lh (rLh) are immunodetected bound to the Lhcgrba of free spermatids in the SLL, showing that circulating gonadotropin can reach the intratubular compartment. In vitro incubation of flatfish spermatids isolated from the SLL with rLh specifically promotes their differentiation into spermatozoa, whereas recombinant follicle-stimulating hormone and steroid hormones are ineffective. Using a repertoire of molecular markers and inhibitors, we find that the Lh-Lhcgrba induction of spermiogenesis is mediated through a cAMP/PKA signaling pathway that initiates the transcription of genes potentially involved in the function of spermatozoa. We further show that Lhcgrba expression in germ cells also occurs in distantly related fishes, suggesting this feature is likely conserved in teleosts regardless of the type of germ cell development. These data reveal a role of LH in vertebrate germ cells, whereby a Lhcgrba-activated signaling cascade in haploid spermatids directs gene expression and the progression of spermiogenesis.
Current models of vertebrate spermatogenesis highlight the role of gonadotropins on the somatic Sertoli and Leydig cells as the major mediators of germ cell development through the secretion of steroid hormones and growth factors (1). The gonadotropins follicle-stimulating hormone (FSH) and luteinizing hormone (LH) exert their actions on spermatogenesis by binding to the FSH receptor (FSHR) in Sertoli cells and the LH/choriogonadotropin receptor (LHCGR) in Leydig cells, respectively (1). In mammals, FSH regulates Sertoli cell functions in the seminiferous tubules, whereas the LHCGR mediates the actions of LH on Leydig cell steroidogenesis and the production of the androgen testosterone (T) to sustain germ cell development and the final differentiation of haploid spermatids to spermatozoa (spermiogenesis) (1). In contrast, in teleost fish, both Fsh and Lh are potent steroidogenic hormones acting through the expression of the Fshr and Lhcgr in Leydig cells (2–4), which results in the production of estrogens, androgens, and progestins that control the different stages of spermatogenesis (5–8).
Studies in mammals indicate that LH is crucial for fertility; in LHCGR and ligand knockout mice, serum T levels are reduced and spermatogenesis is arrested at the round spermatid stage, resulting in azoospermia (9–12). However, the levels of circulating T in LH- and LHCGR-null mice are apparently still sufficient to resume spermatogenesis in wild-type animals (13), and in addition, T replacement therapy in LHCGR mutants only partially restores fertility (14). These data may indicate an additional spermiogenic role of the LH/LHCGR pathway in germ cells that is not mediated by androgens produced by somatic cells. Interestingly, activation of the LHCGR gene promoter is detected in mouse spermatogonia and spermatids (15), and expression of functional LHCGR has been reported in human sperm (16). In addition, a recent study has shown that Lhcgrba-encoding transcripts are expressed in round spermatids of the flatfish Senegalese sole (Solea senegalensis) (17). These findings may suggest the existence of a LH/LHCGR pathway regulating vertebrate germ cell differentiation, but no study has provided direct evidence for such a mechanism. This is in part because in mammals or amphibians, complete spermatogenesis in vitro has been obtained from organ culture systems, but never from isolated germ cells (18, 19).
In teleosts, fertile sperm can be obtained in vitro in the absence of somatic cells (20, 21), and therefore fish can provide excellent experimental models for investigating the endocrine control of spermiogenesis. Here, we selected the Senegalese sole to test the hypothesis that Lh can directly control spermiogenesis through the activation of the Lhcgrba in germ cells. In this flatfish, spermatogenesis occurs in spermatocysts that develop within seminiferous lobules (SLs), but germ cell development is semicystic; that is, round spermatids are released from the supporting Sertoli cells into the SL lumen (SLL), where they elongate and transform into spermatozoa (22). Using this model, we show that the SLL contains Lh and spermatids expressing the Lhcgrba, activation of which by the ligand induces the differentiation of spermatids into spermatozoa through the cAMP/PKA signaling cascade. These findings reveal the presence of a functional LH/LHCGR pathway driving spermiogenesis in the male germ cells of a vertebrate.
Results
Lhcgrba Is Expressed in Teleost Germ Cells.
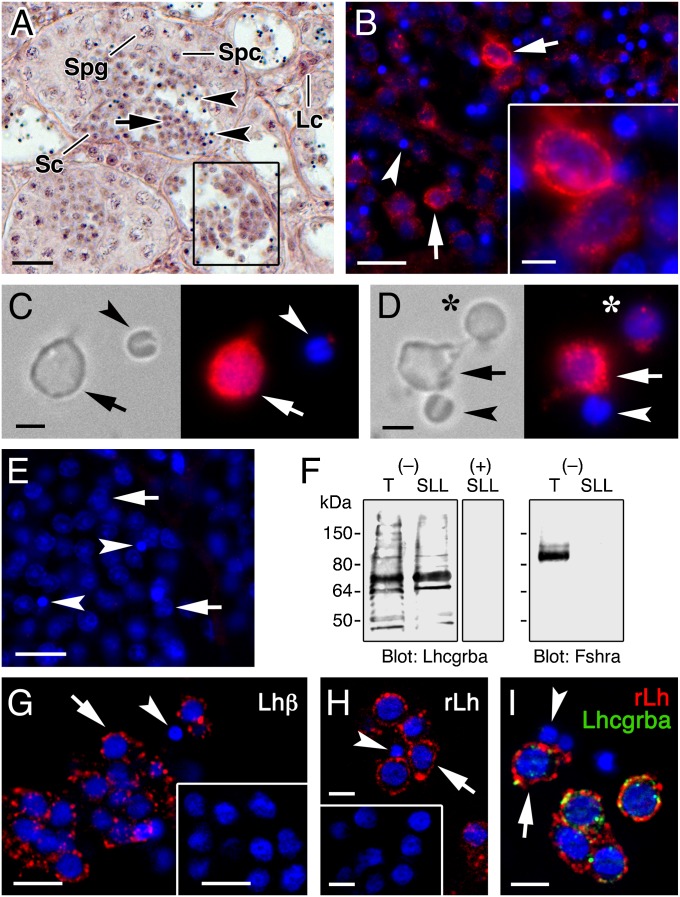
Using a Senegalese sole affinity-purified Lhcgrba antibody, we found that most of the released spermatids in the sole SLL (Fig. 1A) express the Lhcgrba protein in the plasma membrane, whereas spermatozoa do not (Fig. 1B). The identity of the Lhcgrba-positive cells as spermatids was verified by double in situ hybridization and immunostaining of Lhcgrba mRNA and protein with radial spoke head 1 homolog (rsph1) transcripts (Fig. S1), which are potentially involved in the formation of the sperm flagellum (23) and are spermatid-specific. These observations were further corroborated by whole-mount Lhcgrba immunofluorescence and immunoblotting on cells extracted from the SLL, which contains round spermatids, elongating and condensing spermatids, and spermatozoa (Fig. 1 C and D). All cell types except spermatozoa were immunopositive for Lhcgrba (Fig. 1 C and D), whereas Lhcgrba immunoblotting on testis and SLL crude extracts showed a major reactive band of ∼75 kDa, which coincided approximately with the predicted molecular mass of the sole Lhcgrba monomer (75.6 kDa) (Fig. 1F). In contrast, polypeptide bands reactive for the Fshra using a sole specific antibody (4) were detected in testicular extracts but not in spermatids.
Fig. 1.
Senegalese sole spermatids free in the SLL express the Lhcgrba and bind in vivo secreted native and exogenous cognate ligand. (A) Hematoxylin-eosin staining of Senegalese sole semicystic testis. The framed SL shows released spermatids within the SLL. (B) Immunodetection of Lhcgrba (red) in testicular SL sections. (C–D) Whole-mount immunostaining of Lhcgrba (red) in round spermatids and spermatozoa of the SLL. The asterisks indicates a condensing spermatid of smaller size. (E) Section incubated with the Lhcgrba antibody preabsorbed with the immunizing peptide. (F) Lhcgrba and Fshra immunoblots of testis (T) and SLL extracts using intact (−) or preabsorbed (+) antisera. (G) Immunostaining of native Lh (red) bound to spermatids in the SLL, using a piscine Lhβ subunit antibody. The inset shows a control section probed with rLh preadsorbed antisera. (H) Detection of rLh (red) in spermatids of the SLL from a male injected with rLh using a 6xHis tag antibody. Males treated with hormone vehicle were negative (inset). (I) Double immunolabeling of rLh (red) and Lhcgrba (green) in spermatids showing colocalization (yellow) in the cell surface. In B–E and G–I, cell nucleus was counterstained with DAPI (blue). In all panels, round spermatids and spermatozoa are indicated by arrows and arrowheads, respectively. Sc, Sertoli cell; Spg, spermatogonia; Spc, spermatocyte. (Scale bar, A, 20 µm; B, E, G, and G inset, 10 µm; B inset, H, and I, 5 µm; C and D, 2 µm.)
The expression of the Lhcgrba in Senegalese sole spermatids correlated with the immunocytochemical detection of both native Lh (Fig. 1G) and intramuscularly injected His-tagged sole recombinant Lh (rLh) (Fig. 1 H and I) specifically bound to the cell membrane of spermatids. This finding demonstrated that the circulating Lh ligand can reach the SLL, which is consistent with the ability of the SLL fluid extracted from untreated males to activate the Lhcgrba transiently expressed in HEK293T cells (Fig. S2). These data show that the sole SLL contains both Lhcgrba-expressing spermatids and the secreted ligand in vivo.
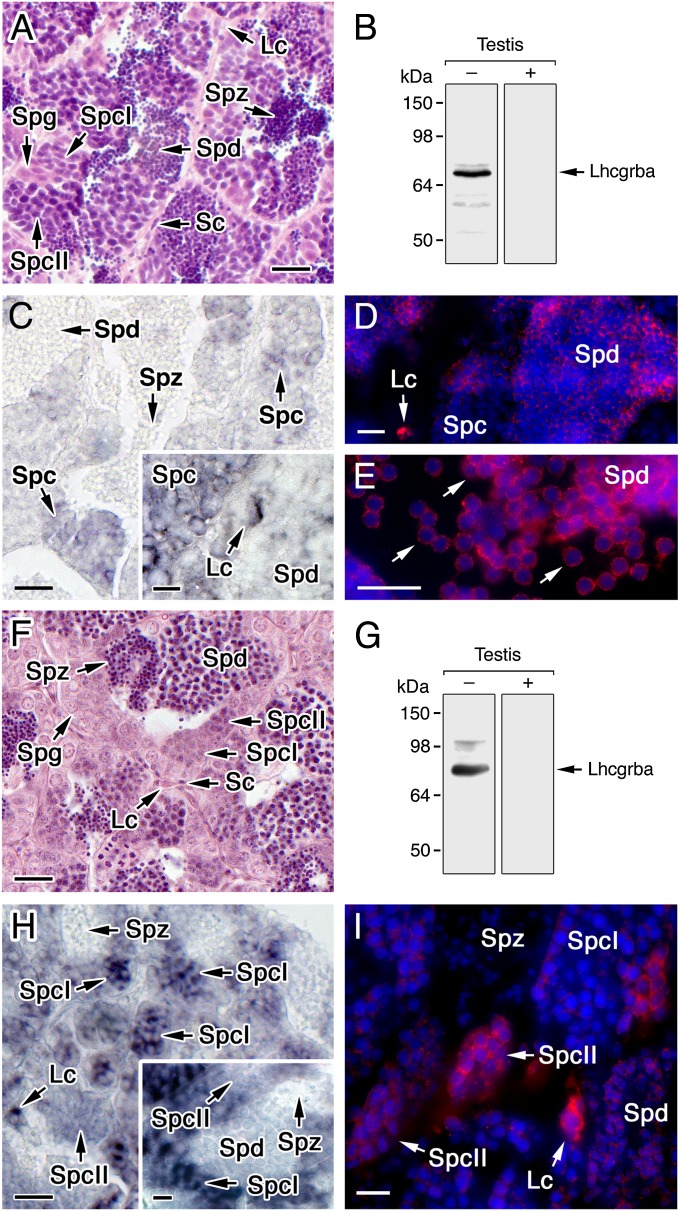
Germ cell Lhcgrba expression in distantly related teleosts, such as the cypriniform zebrafish (Danio rerio) and the perciform gilthead seabream (Sparus aurata), which show cystic spermatogenesis (Fig. 2 A and F), was also investigated. Amino acid alignment of the C-terminal region of sole Lhcgrba with that of the zebrafish and seabream revealed almost complete amino acid identity toward the C terminus of the antigenic peptide used for antibody generation for the three teleosts (Fig. S3). Accordingly, Western blotting of zebrafish and seabream whole testes extracts using the sole Lhcgrba antibody detected one major reactive band at the predicted molecular mass of the corresponding receptor monomers (75.9 and 77.2 kDa, respectively), whereas no reaction was noted with peptide preadsorbed antisera (Fig. 2 B and G). In situ hybridization showed that the lhcgrba was expressed not only in Leydig cells but also in zebrafish spermatocytes (Fig. 2C), as well as in seabream type 1 spermatocytes, and less in type 2 spermatocytes (Fig. 2H). Immunolabeling indicated the presence of Lhcgrba polypeptides in zebrafish round spermatids, in addition to Leydig cells, as well as in elongated spermatids released into the SLL (Fig. 2 D and E). In seabream, strong immunostaining was found in Leydig cells and type 2 spermatocytes, which became weaker in spermatids (Fig. 2I). Taken together, these results suggest that Lhcgrba expression in late spermatocytes and/or spermatids is a conserved feature in teleosts.
Fig. 2.
Teleost germ cells express the Lhcgrba. (A–E) Zebrafish testis. (F–I) Gilthead seabream testis. (A and F) Hematoxylin-eosin staining of testes. (B and G) Lhcgrba immunoblots of whole testis with (+) or without (−) preadsorbed antibody (C and H). In situ hybridization of lhcgrba expression in testes. (D, E, and I) Immunostaining of Lhcgrba (red) superimposed to the DAPI nuclear counterstain (blue), using the sole Lhcgrba antibody. Lc, Leydig cells; Sc, Sertoli cells; Spg, spermatogonia; SpcI, spermatocyte type 1; SpcII, spermatocyte type 2; Spd, spermatid; and Spz, spermatozoa. (Scale bars, A–C and F–H, 20 µm; D, E, and I, 10 µm.)
Lhcgrba Is Transiently Expressed in Spermatids Before Spermatozoa Differentiation.
Flow cytometry enumeration of the whole testis extract of Senegalese sole revealed that the percentage of diploid and haploid cells reached 35–40% and 60–65% of the total cells, respectively (Fig. S4 A and B). Within the haploid population, four different cell subpopulations were noted on the basis of their relative size and SYBR Green I (SGI) fluorescence intensity: round spermatids, differentiating spermatids smaller than the round spermatids, condensing spermatids with lower fluorescence intensity than the differentiating spermatids, and spermatozoa that showed the smallest size and highly condensed chromatin (Fig. S4 A and B). In contrast, flow cytometry of the SLL extracts indicated that diploid cells (doublets of haploid cells) represented less than 1% of the total cells (Fig. S4 A and B). The percentage of spermatozoa in the SLL extract was higher than in the testicular extracts because spermatids attached to the SLs were not recovered. Thus, in the sole SLL, spermatozoa represented the major population of haploid cells, followed by round and differentiating spermatids, and finally condensing spermatids (Fig. S4A, inset).
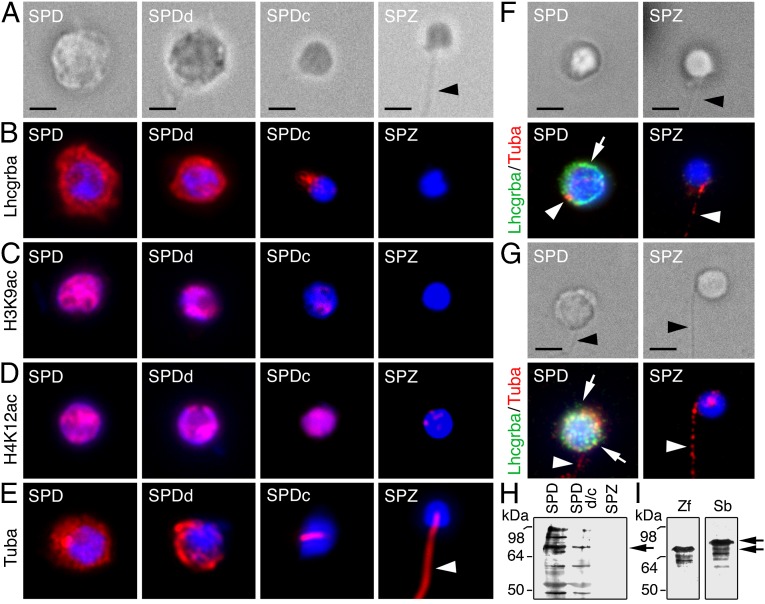
Examination of the SLL germ cell-enriched population after fluorescence-activated cell sorting (FACS) confirmed the presence of four different haploid cell populations (Fig. 3A). Whole-mount immunostaining revealed strong Lhcgrba expression in round and differentiating spermatids, but not in spermatozoa, whereas condensing spermatids showed a faint Lhcgrba staining under the nucleus, suggesting receptor translocation into the residual cytoplasm before complete spermatozoon differentiation (Fig. 3B). Lhcgrba immunoblotting confirmed these observations because immunoreactive bands were detected in round spermatids, which decreased in differentiating and condensing spermatids and completely disappeared in spermatozoa (Fig. 3H).
Fig. 3.
Transient Lhcgrba expression in teleost germ cells precedes spermatozoon differentiation. (A–E) Bright-field (A) and whole-mount immunodetection (red) of Lhcgrba (B), H3K9ac (C), H4K12ac (D), and α-tubulin (Tuba; E) during spermatid differentiation into spermatozoa after FACS of Senegalese sole SLL cell extracts; the signals are superimposed with the DAPI nuclear counterstain (blue). (F and G) Bright-field (Upper) and double immunostaining of Lhcgrba (green; arrows) and Tuba (red; arrowheads) in zebrafish (F) and gilthead seabream (G) FACS-purified haploid cells; nuclei are counterstained with DAPI (blue). (H) Western blot for Lhcgrba on sole sorted SLL cells. (I) Western blots for Lhcgrba in zebrafish (zf) and seabream (sb) sorted haploid cells. SPD, spermatids; SPDd, differentiating spermatids; SPDc, condensed spermatids; SPZ, spermatozoa. Arrowheads point the spermatozoon flagellum. (Scale bar, 2 µm.)
To assess the progression of DNA condensation in the four haploid cell types sorted, we used antibodies against Lys9 acetylated histone 3 (H3K9ac) and Lys12 acetylated histone 4 (H4K12ac), as in vertebrates spermatid DNA condensation is associated with the replacement of histones by transition proteins and protamines (24). An α-tubulin antibody was also used as a marker for the differentiation of the flagellum. The nucleus of Senegalese sole round and differentiating spermatids contained both H3K9ac and H4K12ac; however, although the H3K9ac nuclear localization decreased drastically at the condensing stage, that of H4K12ac did not disappear until full differentiation of spermatozoa (Fig. 3 C and D). Immunostaining for α-tubulin showed that the protein was spread in the cytoplasm in round spermatids, became restricted to half of the cytoplasm in differentiating spermatids and to the nascent flagellar region in the condensing stage, and was distributed along the flagellum of differentiated spermatozoa (Fig. 3E). These results therefore corroborated that the sorted populations from the sole SLL correspond to transitional stages in the differentiation of spermatids into spermatozoa and that the Lhcgrba is only expressed during the initial stages.
To confirm a similar transient Lhcgrba expression in zebrafish and seabream germ cells, we performed double whole-mount immunostaining for Lhcgrba and α-tubulin on FACS-purified testicular haploid cells. In these species, only a single population of haploid cells could be resolved (Fig. S4C), which mostly contained spermatozoa (∼90%) and some round spermatids (Fig. 3 F and G). Nevertheless, as observed in sole, both zebrafish and seabream spermatids, but not spermatozoa, expressed the Lhcgrba, whereas α-tubulin appeared in discrete areas in spermatids, apparently corresponding to the sites of the nascent flagella, and was distributed along the flagellum in spermatozoa (Fig. 3 F and G). Western blot confirmed that zebrafish and seabream sorted spermatids expressed the Lhcgrba (Fig. 3I).
LH Promotes Spermatid Differentiation to Spermatozoa in Vitro.
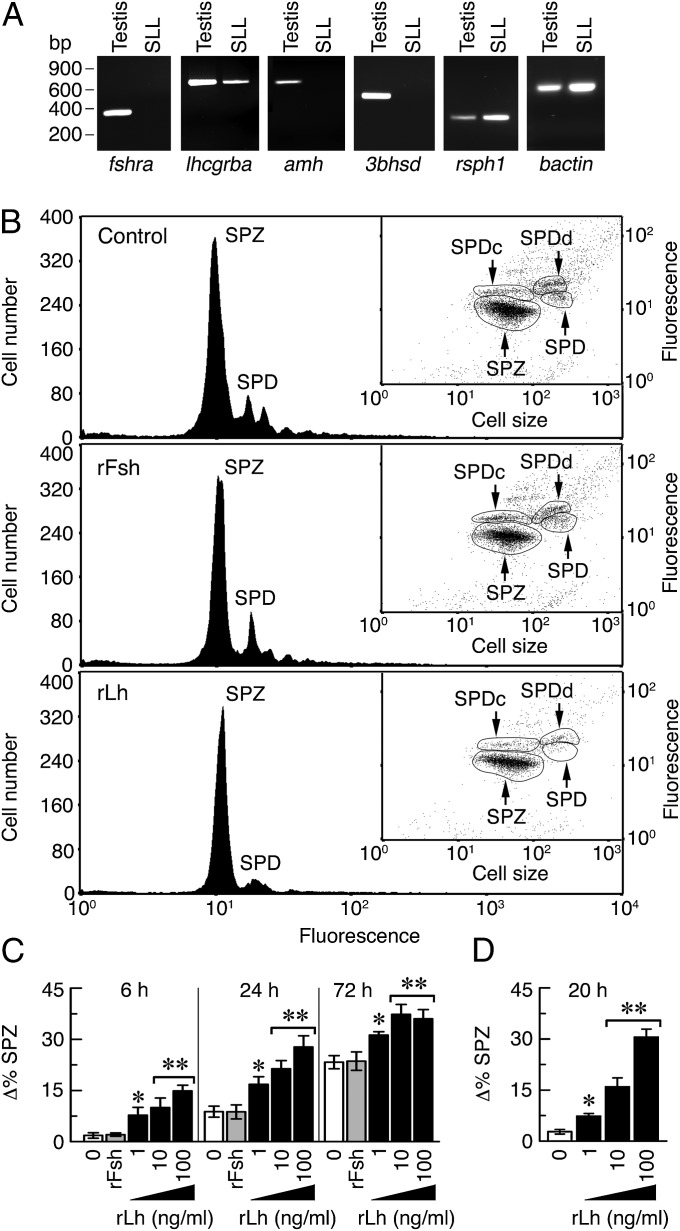
To test whether Lh-activated Lhcgrba can direct the spermatid to spermatozoa transition in Senegalese sole, we incubated SLL cell crude extracts in vitro with rLh. To discard any indirect effect of the hormone on somatic cells that might still be present in the SLL extracts, we first performed RT-PCR analysis of molecular markers for Sertoli cells (fshra and anti-Müllerian hormone, amh), Leydig cells [lhcgrba, fshra, and 3β-hydroxysteroid dehydrogenase (3bhsd)], and spermatids (rsph1) (Fig. 4A). The results showed that although the testis was positive for all markers, the SLL cells only expressed lhcgrba and rsph1, thus confirming the lack of contamination by either Sertoli or Leydig cells.
Fig. 4.
LH directs the transition of Senegalese sole spermatids to spermatozoa in vitro. (A) RT-PCR analysis of fshra, lhcgrba, amh, 3bhsd, rsph1, and bactin expression in testis and SLL crude cell extracts. (B) Representative flow cytometry histograms separated by SGI fluorescence intensity of SLL cells incubated with 100 ng/mL rFsh or rLh, or PBS vehicle (control), for 72 h (see Fig. S4B for time 0). Insets show the corresponding cytogram dot plots in which the haploid cell populations are separated by fluorescence intensity and side scatter, a surrogate of cell size. (C and D) Quantitative flow cytometry analysis of differentiated spermatozoa in the SLL extracts (C) or in FACS-purified spermatids (D) after treatment with rFsh (100 ng/mL) or rLh. Data (mean ± SEM; n = 5) are the increment in the percentage of spermatozoa with respect time 0. *P < 0.05; **P < 0.001, with respect to control cells. SPD, spermatids; SPDd, differentiating spermatids; SPDc, condensed spermatids; SPZ, spermatozoa.
The effect of rLh on spermatid differentiation in vitro was assessed by quantitative flow cytometry of the SLL extracts. As controls, cells were treated with the hormone vehicle or sole homologous recombinant FSH (rFsh). Before hormone treatments, two major fluorescence peaks corresponding to total spermatids (round, differentiating, and condensed) and spermatozoa were identified by flow cytometry (Fig. S4B). After 72 h of culture, the spermatid peak decreased, whereas that of spermatozoa increased equally in the nontreated and rFsh-treated controls (Fig. 4 B and C), suggesting that spermatid differentiation occurred spontaneously in culture and that rFsh had no effect (Fig. 4 B and C). In contrast, rLh induced the differentiation of spermatids into spermatozoa in a time- and dose-dependent manner, with this effect being still significant at 72 h of culture despite the increased levels of spontaneous differentiation in the control groups. Identical results were obtained when culturing FACS-purified spermatids with rLh for 20 h (Fig. 4D). The stimulatory effect of rLh was mimicked by forskolin (FSK) (Fig. S5A), whereas steroids were ineffective (Fig. S5 B–E). These data thus suggest that rLh specifically and directly promotes the differentiation of sole spermatids to spermatozoa.
The cAMP/PKA Signaling Pathway Triggers Spermatid Differentiation.
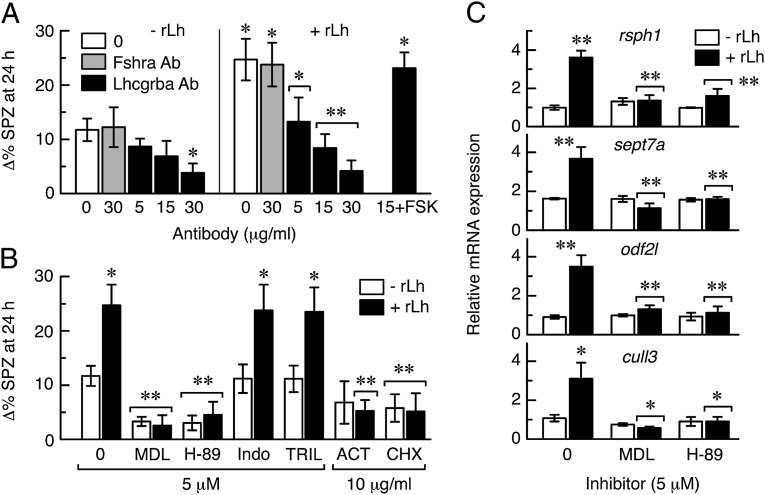
To investigate the signaling cascade involved in rLh-induced spermatid differentiation in sole, we initially verified whether this effect was mediated by its cognate receptor. We examined first whether the Lhcgrba antibody was able to block the sole receptor transiently expressed in HEK293T cells by using luciferase reporter assays (Fig. S6). In these experiments, cAMP-dependent luciferase activity induced by rLh was inhibited in a dose-dependent manner by the addition of the Lhcgrba antibody in the culture medium, whereas the Fshra antibody or IgG had no affect. However, the Lhcgrba antiserum did not reduce the FSK-promoted cAMP surge, suggesting that this antibody specifically blocks the receptor without affecting the adenylyl cyclase enzyme. As observed for cultured cells, treatment of the SLL cell extracts with the Lhcgrba antibody before rLh addition prevented both spontaneous and hormone-induced spermatozoa differentiation, whereas the Fshra antiserum was ineffective (Fig. 5A). Importantly, FSK rescued the inhibition, therefore confirming that the antiserum specifically blocked the Lhcgrba and not the adenylyl cyclase. These data demonstrate that the Lhcgrba mediates the action of rLh on spermatid differentiation into spermatozoa.
Fig. 5.
The Lhcgrba-activated cAMP/PKA signaling pathway drives spermatid differentiation into spermatozoa in vitro. (A) Effect of the Lhcgrba (Lhcgrba Ab) and Fshra (Fshra Ab) antibodies on rLh (10 ng/mL)-stimulated spermatozoa differentiation in Senegalese sole SLL cell extracts. The addition of FSK (10 ng/mL) rescues the Lhcgrba Ab inhibition. (B) Effect of different inhibitors on rLh-induced spermatozoon differentiation. In A and B, data are the increment in the percentage of spermatozoa with respect to time 0. (C) Relative expression of rsph1, sept7a, odf2l, and cull3 quantified by qRT-PCR in SLL extracts treated with rLh in the presence or absence of the MDL and H-89 inhibitors. In all panels, data are the mean ± SEM (n = 3–4). *P < 0.05; **P < 0.001, with respect to cells not treated with rLh or treated with rLh or inhibitor alone (brackets). SPZ, spermatozoa.
The SLL extracts were subsequently incubated with rLh in the presence or absence of inhibitors of the adenylyl cyclase (MDL12330A hydrochloride; MDL), PKA (H-89), 3bHsd (trilostane), and 20β-hydroxysteroid dehydrogenase (indomethacin), as well as DNA transcription (actinomycin D; ACT) and translation (cycloheximide) (Fig. 5B). The MDL and H-89 inhibitors strongly reduced both spontaneous and rLh-induced spermatozoa differentiation, whereas trilostane and indomethacin had no effect, which implies the involvement of a steroid-independent cAMP/PKA pathway during this process. The rLh-stimulated spermatid differentiation was also repressed by both ACT and cycloheximide, suggesting that rLh-promoted de novo protein synthesis is required during the spermatid-to-spermatozoa transition. This hypothesis was further tested by real-time quantitative RT-PCR (qRT-PCR) of rsph1, as well as of septin 7a (sept7a) and outer dense fiber of sperm tails 2-like (odf2l), which are potentially involved in spermatozoa flagellum formation and motility (25, 26), and cullin 3 (cull3), a component of the ubiquitin ligase complex necessary for caspase activation in spermatids (27). The rLh induced an approximately three- to fourfold increase in the expression of the four genes in the SLL cells, which was prevented in the presence of MDL or H-89 (Fig. 5C). Therefore, these data strongly suggest the role of a Lhcgrba/cAMP/PKA signaling pathway in spermatids driving the synthesis de novo of proteins required for spermatozoa differentiation.
Discussion
In this study, we show that Lh activation of its cognate receptor in Senegalese sole haploid spermatids drives their differentiation into spermatozoa in vitro, demonstrating a direct gonadotropic action on the germ cells of a vertebrate. Our data further revealed that Lhcgrba expression occurs in the germ cells of other teleosts with cystic germ cell development, suggesting that this feature may be conserved in evolutionary distant taxa regardless of the type of spermatogenesis. These findings appear to contrast with recent studies in Senegalese sole (28) and zebrafish (3), in which lhcgrba expression in a germ cell-enriched population obtained by laser capture microdissection or FACS from vasa::EGFP transgenic animals, respectively, was not detected by qRT-PCR. The discrepancies may be related to a dilution effect of the non-lhcgrba expressing germ cells on the cells that do express lhcgrba, as in zebrafish, the germ cell-specific vasa promoter is highly activated only in spermatogonia, whereas it is hardly detected in spermatocytes (29).
The sole SLL extracts contained round spermatids, differentiating spermatids, and fully differentiated spermatozoa. Using these somatic cell-free extracts, we demonstrated that spermatids express functional Lhcgrba, as treatment with homologous rLh, which is specific for Lhcgrba (4), promoted the in vitro differentiation into spermatozoa of almost the complete population of spermatids after 3 d of incubation. The inhibition of this process by the Lhcgrba antibodies, and the failure of rFsh to induce any effect, provided further support for this conclusion. The differentiation of sole spermatids in the SLL in vitro was faster than that observed in other teleosts (5, 20, 21), suggesting that these cells probably started the differentiation process in the testis before their collection. In medaka (Oryzias latipes) and zebrafish, in vitro differentiation from isolated spermatocytes to functional spermatozoa requires the presence of fish or fetal bovine sera (21) or gonadotropins (30). Because FBS contains traces of FSH and LH (31), it is possible that in vitro spermiogenesis in fish involves the presence of Lh, thus potentially supporting our observations. However, spermatozoa isolated from the sole SLL, as well as differentiated spermatozoa in vitro, were not motile, suggesting that additional factors produced in the testis or present in standard sera not used in this study may be required for the acquisition of full motility of spermatozoa.
In mammals, transformation of round spermatids into spermatozoa requires the posttranslational modification of existing proteins and the transcriptional activation of postmeiotic genes encoding factors needed for spermatid maturation, as well as of spermatozoa-specific proteins involved in flagellum or acrosome formation (32, 33). This cascade of events is dependent on the cAMP-responsive element modulator (CREM) transcription factor, the activity of which is partly dependent on ACT (activator of CREM in testis) (32–34). PKA phosphorylation also plays an important role for the translocation of transition protein 2 into the nucleus during spermatid elongation (35). Both CREM and ACT, as well as PKA, are thus essential for the formation of fertile spermatozoa, but the primary signal triggering a cAMP surge in round spermatids is yet unclear. Our data in sole indicate a role of the Lhcgrba-activated cAMP/PKA signaling pathway in spermatids, which drives the transcription of genes potentially involved in flagella formation (odfl2, rsph1, and sept7a) and protein ubiquitination (cullin3) during spermiogenesis. Whether a CREM-like transcription factor is also involved remains unknown. Nevertheless, the same LHCGR/cAMP/PKA pathway is the predominant steroidogenic mechanism in Leydig cells (3, 36) and has also been described in human sperm (16). In our study, steroid hormones had no effect on the differentiation of spermatids to spermatozoa in vitro, but a role of steroids during earlier stages of spermatid formation/differentiation in the SL cannot be ruled out.
Previous studies in perciform and siluriform teleosts have shown that the blood–testis barrier forms before spermiogenesis during the transition from spermatocytes to early spermatids, when tight junctions are established between Sertoli cells (37). This barrier would thus make round spermatids that are free in the tubular lumen unavailable for high-molecular mass molecules such as circulating gonadotropins. Our data reveal, however, that both native Lh and intramuscularly injected rLh can reach the spermatids of the SLL, implying that in Senegalese sole the Sertoli cell barrier may form after the release of spermatids or that this barrier is less restrictive than in other teleosts. These observations could explain the relatively high basal differentiation of isolated sole spermatids in vitro without hormone. However, we noted constitutive activity within the Lhcgrba in the absence of hormone ligand in HEK293T cells (Fig. S2), which could also be the underlying mechanism in free spermatids. In addition, because sole Fshra can also bind Lh (4), several other mechanisms can be speculated, such as Fshra-mediated Lh transcytosis through Sertoli cells (38). The cellular pathways for the translocation of Lh into the intratubular compartment and activation of spermatids in vivo thus remain intriguing areas for future investigation.
In conclusion, we provide strong evidence of the involvement of a nonsteroidal Lh/Lhcgrba pathway directing spermiogenesis in teleost germ cells, which coexists with the established Lhcgrba-mediated androgenic pathway in Leydig cells. These findings will provide additional insights into the gonadotropic regulation of spermatogenesis in vertebrates and the causes of male infertility.
Materials and Methods
A detailed description of materials and methods is provided in the SI Materials and Methods. It describes the procedures used for the preparation of testis and SLL cell extracts, flow cytometry, and in vitro incubation of SLL cells and FACS-purified spermatids. It also details the methods for gene expression analyses, immunofluorescence microscopy, immunoblotting, and statistics.
Supplementary Material
Acknowledgments
We thank Dr. Roderick N. Finn for critically reading the manuscript, Dr. Hugo Sarmento for his assistance with flow cytometry analyses, and Òscar Fornas from the Universitat Pompeu Fabra (UPF)/Centre de Regulació Genómica (CRG) Flow Cytometry Unit service. This work was funded by the Spanish Fundación Ramón Areces (to J.C.). F.C. was supported by Juan de la Cierva Programme (Spanish Ministry of Science and Innovation).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. Y.N. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1317838111/-/DCSupplemental.
References
- 1.Holdcraft RW, Braun RE. Hormonal regulation of spermatogenesis. Int J Androl. 2004;27(6):335–342. doi: 10.1111/j.1365-2605.2004.00502.x. [DOI] [PubMed] [Google Scholar]
- 2.García-López A, et al. Leydig cells express follicle-stimulating hormone receptors in African catfish. Endocrinology. 2009;150(1):357–365. doi: 10.1210/en.2008-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.García-López A, et al. Studies in zebrafish reveal unusual cellular expression patterns of gonadotropin receptor messenger ribonucleic acids in the testis and unexpected functional differentiation of the gonadotropins. Endocrinology. 2010;151(5):2349–2360. doi: 10.1210/en.2009-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chauvigné F, et al. Follicle-stimulating hormone and luteinizing hormone mediate the androgenic pathway in Leydig cells of an evolutionary advanced teleost. Biol Reprod. 2012;87(2):35. doi: 10.1095/biolreprod.112.100784. [DOI] [PubMed] [Google Scholar]
- 5.Miura T, Yamauchi K, Takahashi H, Nagahama Y. Hormonal induction of all stages of spermatogenesis in vitro in the male Japanese eel (Anguilla japonica) Proc Natl Acad Sci USA. 1991;88(13):5774–5778. doi: 10.1073/pnas.88.13.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miura T, et al. Estradiol-17β stimulates the renewal of spermatogonial stem cells in males. Biochem Biophys Res Commun. 1999;264(1):230–234. doi: 10.1006/bbrc.1999.1494. [DOI] [PubMed] [Google Scholar]
- 7.Miura T, Higuchi M, Ozaki Y, Ohta T, Miura C. Progestin is an essential factor for the initiation of the meiosis in spermatogenetic cells of the eel. Proc Natl Acad Sci USA. 2006;103(19):7333–7338. doi: 10.1073/pnas.0508419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen SX, et al. A progestin (17α,20β-dihydroxy-4-pregnen-3-one) stimulates early stages of spermatogenesis in zebrafish. Gen Comp Endocrinol. 2013;185:1–9. doi: 10.1016/j.ygcen.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Lei ZM, et al. Targeted disruption of luteinizing hormone/human chorionic gonadotropin receptor gene. Mol Endocrinol. 2001;15(1):184–200. doi: 10.1210/mend.15.1.0586. [DOI] [PubMed] [Google Scholar]
- 10.Zhang FP, Poutanen M, Wilbertz J, Huhtaniemi I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol Endocrinol. 2001;15(1):172–183. doi: 10.1210/mend.15.1.0582. [DOI] [PubMed] [Google Scholar]
- 11.Zhang FP, Pakarainen T, Zhu F, Poutanen M, Huhtaniemi I. Molecular characterization of postnatal development of testicular steroidogenesis in luteinizing hormone receptor knockout mice. Endocrinology. 2004;145(3):1453–1463. doi: 10.1210/en.2003-1049. [DOI] [PubMed] [Google Scholar]
- 12.Ma X, Dong Y, Matzuk MM, Kumar TR. Targeted disruption of luteinizing hormone beta-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc Natl Acad Sci USA. 2004;101(49):17294–17299. doi: 10.1073/pnas.0404743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang FP, Pakarainen T, Poutanen M, Toppari J, Huhtaniemi I. The low gonadotropin-independent constitutive production of testicular testosterone is sufficient to maintain spermatogenesis. Proc Natl Acad Sci USA. 2003;100(23):13692–13697. doi: 10.1073/pnas.2232815100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pakarainen T, Zhang FP, Mäkelä S, Poutanen M, Huhtaniemi I. Testosterone replacement therapy induces spermatogenesis and partially restores fertility in luteinizing hormone receptor knockout mice. Endocrinology. 2005;146(2):596–606. doi: 10.1210/en.2004-0913. [DOI] [PubMed] [Google Scholar]
- 15.Hämäläinen T, Poutanen M, Huhtaniemi I. Promoter function of different lengths of the murine luteinizing hormone receptor gene 5′-flanking region in transfected gonadal cells and in transgenic mice. Endocrinology. 2001;142(6):2427–2434. doi: 10.1210/endo.142.6.7994. [DOI] [PubMed] [Google Scholar]
- 16.Eblen A, Bao S, Lei ZM, Nakajima ST, Rao CV. The presence of functional luteinizing hormone/chorionic gonadotropin receptors in human sperm. J Clin Endocrinol Metab. 2001;86(6):2643–2648. doi: 10.1210/jcem.86.6.7533. [DOI] [PubMed] [Google Scholar]
- 17.Chauvigné F, et al. Functional and evolutionary analysis of flatfish gonadotropin receptors reveals cladal- and lineage-level divergence of the teleost glycoprotein receptor family. Biol Reprod. 2010;82(6):1088–1102. doi: 10.1095/biolreprod.109.082289. [DOI] [PubMed] [Google Scholar]
- 18.Abe SI. Cell culture of spermatogenic cells from amphibians. Dev Growth Differ. 1988;30:209–218. doi: 10.1111/j.1440-169X.1988.00209.x. [DOI] [PubMed] [Google Scholar]
- 19.Song HW, Wilkinson MF. In vitro spermatogenesis: A long journey to get tails. Spermatogenesis. 2012;2(4):238–244. doi: 10.4161/spmg.22069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saiki A, et al. Establishment of in vitro spermatogenesis from spermatocytes in the medaka, Oryzias latipes. Dev Growth Differ. 1997;39(3):337–344. doi: 10.1046/j.1440-169x.1997.t01-2-00009.x. [DOI] [PubMed] [Google Scholar]
- 21.Hong Y, et al. Establishment of a normal medakafish spermatogonial cell line capable of sperm production in vitro. Proc Natl Acad Sci USA. 2004;101(21):8011–8016. doi: 10.1073/pnas.0308668101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.García-López A, Martínez-Rodríguez G, Sarasquete C. Male reproductive system in Senegalese sole Solea senegalensis (Kaup): Anatomy, histology and histochemistry. Histol Histopathol. 2005;20(4):1179–1189. doi: 10.14670/HH-20.1179. [DOI] [PubMed] [Google Scholar]
- 23.Gingras D, et al. Molecular cloning and characterization of a radial spoke head protein of sea urchin sperm axonemes: Involvement of the protein in the regulation of sperm motility. Mol Biol Cell. 1998;9(2):513–522. doi: 10.1091/mbc.9.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hazzouri M, et al. Regulated hyperacetylation of core histones during mouse spermatogenesis: Involvement of histone deacetylases. Eur J Cell Biol. 2000;79(12):950–960. doi: 10.1078/0171-9335-00123. [DOI] [PubMed] [Google Scholar]
- 25.Schlecht U, et al. Expression profiling of mammalian male meiosis and gametogenesis identifies novel candidate genes for roles in the regulation of fertility. Mol Biol Cell. 2004;15(3):1031–1043. doi: 10.1091/mbc.E03-10-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin YH, Kuo YC, Chiang HS, Kuo PL. The role of the septin family in spermiogenesis. Spermatogenesis. 2011;1(4):298–302. doi: 10.4161/spmg.1.4.18326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arama E, Bader M, Rieckhof GE, Steller H. A ubiquitin ligase complex regulates caspase activation during sperm differentiation in Drosophila. PLoS Biol. 2007;5(10):e251. doi: 10.1371/journal.pbio.0050251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marín-Juez R, Viñas J, Mechaly AS, Planas JV, Piferrer F. Stage-specific gene expression during spermatogenesis in the Senegalese sole (Solea senegalensis), a fish with semi-cystic type of spermatogenesis, as assessed by laser capture microdissection and absolute quantitative PCR. Gen Comp Endocrinol. 2013;188:242–250. doi: 10.1016/j.ygcen.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 29.Nóbrega RH, et al. Spermatogonial stem cell niche and spermatogonial stem cell transplantation in zebrafish. PLoS ONE. 2010;5(9):e12808. doi: 10.1371/journal.pone.0012808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakai N. Transmeiotic differentiation of zebrafish germ cells into functional sperm in culture. Development. 2002;129(14):3359–3365. doi: 10.1242/dev.129.14.3359. [DOI] [PubMed] [Google Scholar]
- 31.Price PJ, Gregory EA. Relationship between in vitro growth promotion and biophysical and biochemical properties of the serum supplement. In Vitro. 1982;18(6):576–584. doi: 10.1007/BF02810081. [DOI] [PubMed] [Google Scholar]
- 32.Blendy JA, Kaestner KH, Weinbauer GF, Nieschlag E, Schütz G. Severe impairment of spermatogenesis in mice lacking the CREM gene. Nature. 1996;380(6570):162–165. doi: 10.1038/380162a0. [DOI] [PubMed] [Google Scholar]
- 33.Nantel F, et al. Spermiogenesis deficiency and germ-cell apoptosis in CREM-mutant mice. Nature. 1996;380(6570):159–162. doi: 10.1038/380159a0. [DOI] [PubMed] [Google Scholar]
- 34.Kotaja N, et al. Abnormal sperm in mice with targeted deletion of the act (activator of cAMP-responsive element modulator in testis) gene. Proc Natl Acad Sci USA. 2004;101(29):10620–10625. doi: 10.1073/pnas.0401947101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meetei AR, Ullas KS, Vasupradha V, Rao MR. Involvement of protein kinase A in the phosphorylation of spermatidal protein TP2 and its effect on DNA condensation. Biochemistry. 2002;41(1):185–195. doi: 10.1021/bi0117652. [DOI] [PubMed] [Google Scholar]
- 36.Stocco DM, Wang X, Jo Y, Manna PR. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: More complicated than we thought. Mol Endocrinol. 2005;19(11):2647–2659. doi: 10.1210/me.2004-0532. [DOI] [PubMed] [Google Scholar]
- 37.Schulz RW, et al. Sertoli cell proliferation in the adult testis—evidence from two fish species belonging to different orders. Biol Reprod. 2005;73(5):891–898. doi: 10.1095/biolreprod.105.039891. [DOI] [PubMed] [Google Scholar]
- 38.Ghinea N, Mai TV, Groyer-Picard MT, Milgrom E. How protein hormones reach their target cells. Receptor-mediated transcytosis of hCG through endothelial cells. J Cell Biol. 1994;125(1):87–97. doi: 10.1083/jcb.125.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.