Significance
Insects have gustatory receptors with which they taste chemicals and make important choices about foods, mates, and egg deposition sites. Gustatory receptors are novel proteins, and understanding how they recognize diverse chemicals is an unmet challenge. We developed a method to examine taste receptor function using a Drosophila olfactory neuron as a host for expressing taste receptors. We individually tested all sweet receptors of the fly, which resulted in specific sugar responses in the olfactory neuron. We also successfully expressed a bitter receptor and a mosquito taste receptor. Our study describes a systematic view of how the fly’s taste receptors detect sweet substances and holds promise for uncovering functions of taste receptors from insects that transmit diseases or damage crops.
Keywords: heterologous expression, chemosensory, chemoreceptor
Abstract
Sweet taste cells play critical roles in food selection and feeding behaviors. Drosophila sweet neurons express eight gustatory receptors (Grs) belonging to a highly conserved clade in insects. Despite ongoing efforts, little is known about the fundamental principles that underlie how sweet tastants are detected by these receptors. Here, we provide a systematic functional analysis of Drosophila sweet receptors using the ab1C CO2-sensing olfactory neuron as a unique in vivo decoder. We find that each of the eight receptors of this group confers sensitivity to one or more sweet tastants, indicating direct roles in ligand recognition for all sweet receptors. Receptor response profiles are validated by analysis of taste responses in corresponding Gr mutants. The response matrix shows extensive overlap in Gr–ligand interactions and loosely separates sweet receptors into two groups matching their relationships by sequence. We then show that expression of a bitter taste receptor confers sensitivity to selected aversive tastants that match the responses of the neuron that the Gr is derived from. Finally, we characterize an internal fructose-sensing receptor, Gr43a, and its ortholog in the malaria mosquito, AgGr25, in the ab1C expression system. We find that both receptors show robust responses to fructose along with a number of other sweet tastants. Our results provide a molecular basis for tastant detection by the entire repertoire of sweet taste receptors in the fly and lay the foundation for studying Grs in mosquitoes and other insects that transmit deadly diseases.
The detection of high-calorie sweet compounds is a fundamental property of the taste system. In Drosophila, as in mammals, sweet tastants are detected by a distinct subpopulation of sensory cells that drives innate acceptance (1). An investigation of the mechanisms of tastant detection by sweet receptors is therefore necessary for understanding key principles of feeding behaviors.
Insect gustatory receptors (Grs) belong to a novel arthropod superfamily unrelated to mammalian taste receptors (2). The Drosophila melanogaster Gr gene family encodes 68 receptors that are expressed in complex overlapping patterns in chemosensory neurons in both adult and larval stages (3–6). Expression analysis shows that individual Grs are exclusive to either sweet (attractive) or bitter (aversive) taste neurons, delineating separation of sweet and bitter taste receptors within Grs (3–5, 7). Eight Grs have been mapped to sweet taste neurons (5, 8–10), defining a distinct clade of receptors whose members are found across insects and arthropods (11). Of these, Gr5a and Gr64a are broadly required for responses to complementary subsets of sugars in labellar sweet taste neurons (9). Several lines of evidence suggest that one or more of the remaining six receptors are coexpressed with Gr5a in sweet neurons of the fly labellum (8–10, 12). Genetic analysis for some of these other Grs suggests that they are also necessary for responses to sweet tastants, although the breadth of sugar response defects can vary between different Gr mutants (12–14).
Receptor expression in a heterologous context is crucial for deciphering its response properties. Our current understanding of how volatile chemicals are encoded by insect odorant receptor (Or) proteins was made possible by both cell-based and in vivo expression systems that allowed comprehensive functional analysis of Or gene repertoires in D. melanogaster (15, 16) and the malaria mosquito Anopheles gambiae (17, 18). The success of ectopic analysis of Ors is in stark contrast to similar studies for Grs, which have met with limited progress (19–22), despite over a decade of efforts. Although the antennal CO2 receptors Gr21a and Gr63a were successfully expressed in an “empty” olfactory neuron lacking functional Ors (20, 21), attempts to express other Gr genes have been largely futile. Only few instances of functional Gr expression in tissue culture have been reported, which include Gr5a and Gr43a from Drosophila, BmGr8 and BmGr9 from the silkworm Bombyx mori, and PxutGr1 from the swallowtail butterfly Papilio xuthus (19, 22–24). In each case, expression of a single receptor was found to be sufficient to confer tastant responses. To date, however, these instances remain notable exceptions. Because Grs represent a highly divergent chemoreceptor family across insects (25), limitations in examining their functional properties embody a critical gap in the field.
We recently expressed Gr64e in the Gr21a/Gr63a CO2-sensing neuron in the olfactory system and showed that it confers sensitivity to glycerol (12). Here we investigate how tastants are detected by the entire repertoire of sweet taste receptors in Drosophila. We express each receptor individually in the ab1C neuron and examine its responses to a diagnostic panel of sweet compounds derived from the response profile of sweet taste neurons (9). We find that Gr5a and Gr64a confer complementary responses and that every other receptor is activated by one or more sweet tastants. Typically, ectopic responses of other receptors overlap with either Gr5a or Gr64a, but not with both. Ectopic responses are validated by tastant response defects in corresponding Gr mutants. We extend our study by testing a bitter receptor, Gr59c, and observe bitter responses that match previously associated ligands for Gr59c (5). We also test an internal fructose-sensing receptor, Gr43a, and its malaria mosquito ortholog, AgGr25. Both Gr43a and AgGr25 confer robust responses to fructose and some other sweet tastants. Importantly, our results show that AgGr25 can function independently, in the absence of other mosquito proteins. Together, our findings reveal tastant detection properties of Drosophila sweet taste receptors and describe and provide a tool for further analysis of insect taste receptors.
Results
An Ectopic Expression System to Decode Taste Receptors.
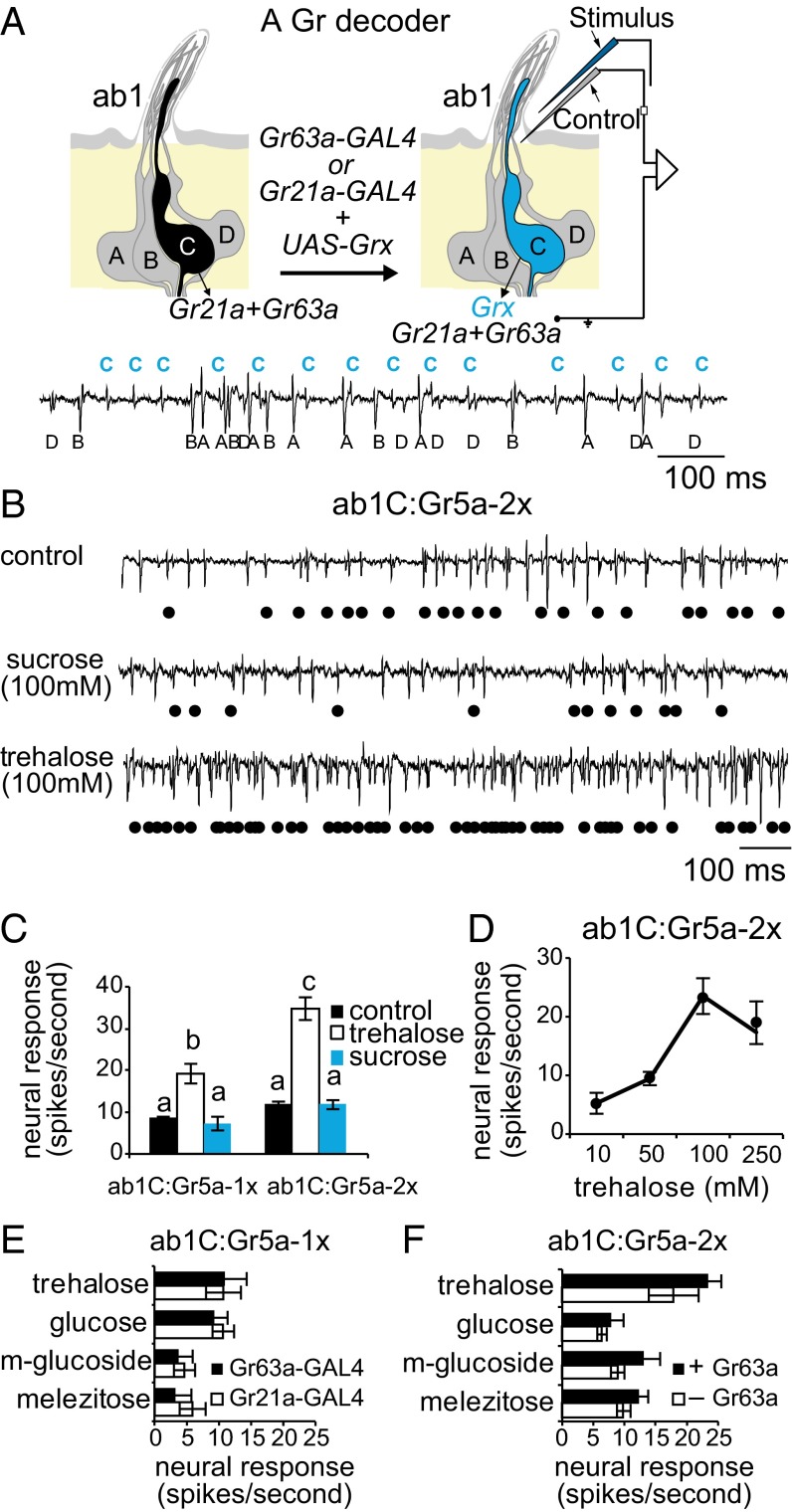
We selected the CO2-sensing ab1C olfactory neuron for ectopic expression of Grs based on our previous study (12). The ab1C neuron, housed with three other neurons in ab1 large basoconic sensilla in the fly antenna, is unique in that it expresses Gr21a and Gr63a but no members of the Or or Ir gene families (20, 21, 26, 27). Exogenous receptors can be expressed in the ab1C neuron via the GAL4/Upstream Activating Sequence (UAS) system by using either Gr21a or Gr63a promoter sequences (Fig. 1A).
Fig. 1.
An in vivo ectopic expression system for analysis of individual Grs. (A) Schematic of ectopic expression in the ab1C neuron with trace from wild-type ab1 sensillum depicting activities of the four ab1 neurons. Glass micropipettes for tastant recordings contain sensillum lymph ringer (SLR) control (gray) or stimulus in SLR (blue). (B) Sample ab1 recordings in flies expressing Gr5a in ab1C neurons (ab1C:Gr5a-2x). Black dots indicate ab1C spikes. (C) Mean responses of ab1C:Gr5a-1x and ab1C:Gr5a-2x neurons. Baseline activity to SLR is not subtracted from stimulus-evoked activity. Sugars were tested at a concentration of 100 mM. Letters indicate statistical significance (P < 0.001; one-way ANOVA with Tukey’s post hoc test; n = 6–12). (D) Dose-dependent response to trehalose (n = 6–12). (E) Mean responses of ab1C:Gr5a neurons generated with Gr21a– or Gr63a–GAL4 as indicated to 100 mM sugars (n = 6). (F) Mean responses of ab1C:Gr5a-2x neurons in wild-type (+Gr63a) or ΔGr63a (–Gr63a) flies to 100 mM sugars (n = 10–14). All genotypes in E and F were compared with each other by using two-way ANOVA with Tukey’s post hoc test. Only genotypes with one copy of UAS–Gr5a (E) are significantly different from genotypes with two copies of UAS–Gr5a (F) (P < 0.05).
One consideration is that tastants would have to be delivered by contact with the lymph of the ab1 sensillum. Previous studies have shown that nonvolatile agonists or antagonists of the Or coreceptor, Orco, are able to act on olfactory neurons simply by their inclusion in the recording micropipette (28, 29). Others have reported injected delivery of inhibitors into the sensillum lymph that mimics the effects of in vitro studies (30–32). We therefore dissolved tastants in electrolyte solution and delivered them by piercing the sensillum. As a control, we used this technique to deliver 10−4 ethyl acetate [solubility in water ∼8% (vol/vol) at 25 °C] and compared ensuing activity with responses obtained via an odor delivery system. In both cases, we observed robust responses to ethyl acetate (Fig. S1). Although neuronal activity was lower when the stimulus was delivered by micropipette, these results show that the dissolved stimulus is sufficient for olfactory neuron activity.
We next systematically characterized the suitability of the ab1C ectopic expression system for decoding taste receptors. We first expressed a trehalose receptor, Gr5a (19, 33), and asked whether its expression was sufficient to confer trehalose response in the ab1C neuron. Each ab1 sensillum was pierced twice for recordings, first to measure baseline activity with electrolyte alone and a second time to measure ligand-evoked activity by using electrolyte solution containing either trehalose or sucrose. Application of sucrose did not alter the firing rate of ab1C neurons expressing Gr5a, designated ab1C:Gr5a (Fig. 1 B and C). By contrast, trehalose evoked a significant increase in ab1C:Gr5a activity. Subsequent recordings with electrolyte solution showed a return of ab1C activity to baseline levels (Fig. S1). Notably, the response was dependent on the gene dosage of Gr5a (Fig. 1C) as well as the concentration of trehalose (Fig. 1D). Regardless of the number of copies of UAS–Gr5a, response to sucrose was indistinguishable from that of electrolyte alone (Fig. 1 B and C), suggesting that Gr5a-mediated response was specific for trehalose.
Flies lacking Gr5a have reduced taste neuron responses not only to trehalose, but also to three other tested sugars: glucose, methyl-α-glucopyranoside (m-glucoside), and melezitose (9). One possible explanation is that Gr5a is directly involved in detection of all four sugar tastants. Alternatively, Gr5a may be involved only indirectly, perhaps as a coreceptor, for detection of glucose, m-glucoside, and melezitose. To distinguish between these possibilities, we examined responses of the ab1C:Gr5a neuron to the four sugars. We also took this opportunity to compare response profiles of ab1C:Gr5a neurons generated independently using either Gr21a–GAL4 or Gr63a–GAL4.
Our recordings showed that the ab1C:Gr5a neuron responded to all Gr5a-dependent sugars (Fig. 1E), indicating a direct role for Gr5a in detecting these sweet tastants. Moreover, similar levels of mean responses to each sugar were obtained irrespective of the GAL4 driver that was used (Fig. 1E). Therefore, in subsequent experiments, Gr21a–GAL4 and Gr63a–GAL4 were treated as equivalent, and receptors were expressed singly in the ab1C neuron via two copies each of GAL4 and UAS transgenes.
It is curious that Gr5a was functional in the ab1C neuron when other attempts at heterologous expression of Gr5a in vivo have failed. We wondered whether the endogenous Gr21a/Gr63a receptor was a factor in its suitability. Although a Gr21a mutant is not available, we examined Gr5a-mediated responses in ab1C neurons of ΔGr63a flies (20). Responses to the four sugars were not significantly altered by the absence of Gr63a (Fig. 1F), indicating that an intact Gr21a/Gr63a receptor is not necessary for functional expression of an exogenous Gr gene, Gr5a. Importantly, no new responses were observed in ab1C:Gr5a neurons of Gr63a mutants (sucrose, 0.17 ± 0.21; maltose, 0.08 ± 0.49; maltotriose, 0.08 ± 0.45; fructose, −0.42 ± 0.52; glycerol, 1.00 ± 0.68; n = 6 for each stimulus), thereby ruling out the possibility that Gr63a may interfere with Gr5a function. Based on these results, subsequent ectopic expression experiments were carried out in Gr63a+ flies.
All Sweet Grs Confer Responses to Sweet Tastants.
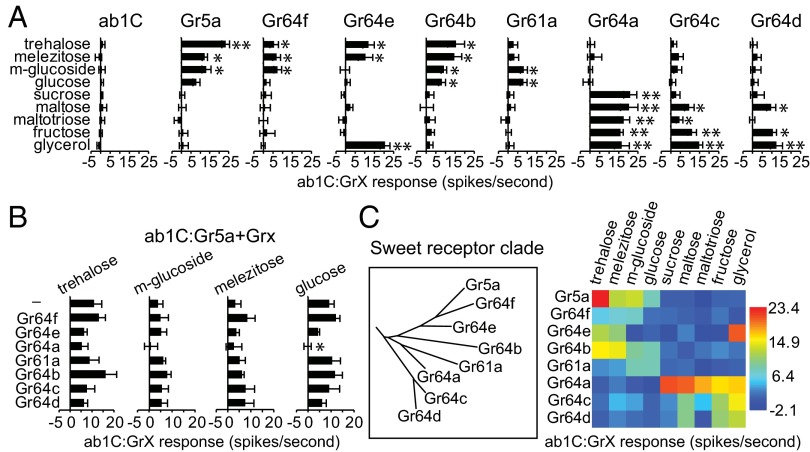
Having established the ab1C neuron as a suitable host for Gr proteins, we performed a systematic analysis of tastant response profiles of the entire sweet Gr clade. Grs were individually expressed in the ab1C neuron, and ab1C:GrX responses were measured against a panel of sweet tastants. Based on our previous observations that only ∼15 compounds from a large stimulus panel strongly activated sweet taste neurons of L-type labellar sensilla, and that all responses were dependent on either Gr5a or Gr64a (9), we selected a diagnostic subset of nine tastants to represent the structural diversity of ligands and the breadth of responses in taste neurons. Specifically, we included four sugars that depend on Gr5a for responses in taste neurons, four others that depend on Gr64a, and a known ligand of Gr64e, glycerol (8, 9, 12). Importantly, the selected tastant panel represents sugars and sugar alcohols that are behaviorally very attractive to flies (34). Sugars were tested at a concentration of 100 mM and glycerol at 10% (vol/vol), concentrations that typically evoke robust responses in taste neurons. One exception was maltotriose, which was tested at a concentration of 250 mM based on initial analysis of dose-dependent responses of ab1C:Gr64a (Fig. S2). Each Gr–tastant combination was tested in at least six sensilla derived from at least two different flies.
ab1C neurons expressing either Gr5a or Gr64a were responsive to complementary subsets of tastants. The ab1C:Gr5a neuron responded to the four Gr5a-dependent sugars (as shown in Fig. 1F), but not to the four Gr64a-dependent sugars or to glycerol (Fig. 2A). Likewise, the ab1C:Gr64a neuron was selectively activated by Gr64a-dependent sugars and glycerol, but not by Gr5a-dependent sugars. Thus, Gr5a and Gr64a, which are broadly required for sugar sensing (9), are directly involved in recognition of nonoverlapping subsets of sweet tastants.
Fig. 2.
Tastant response profiles of sweet taste receptors. (A) Mean electrophysiological responses of ab1C:GrX neurons. All sugars were tested at a concentration of 100 mM, except maltotriose at 250 mM and glycerol at 10% (vol/vol). For each data point, n = 6–14. *P < 0.05; **P < 0.001 [vs. control ab1C flies (w1118)]. (B) Mean electrophysiological responses of Gr5a expressed alone (–) or with the indicated receptor in ab1C neurons. For each data point, n = 6–7. *P < 0.05; **P < 0.001 [vs. Gr5a alone (–)]. (C) Phylogenetic tree of sweet Grs (Left) and heat map of mean neuronal responses of ab1C:GrX neurons to indicated sweet tastants. Data are the same as in A. Heat map was made with PAST (http://folk.uio.no/ohammer/past).
Of the eight Grs that we expressed in the ab1C neuron, every one conferred a significant response to at least one of the tested compounds (Fig. 2A), demonstrating that all receptors of the sweet clade participate in detection of sweet tastants. With the exception of sucrose, each tastant evoked a response from more than one sweet taste receptor. Conversely, each receptor was activated by more than one sweet tastant.
Previous genetic analyses have implicated possible heteromeric complexes in sugar detection. For example, Gr64f is required in combination with Gr5a for response to trehalose (13). We therefore asked whether coexpressed pairs of receptors conferred synergistically higher responses compared with either receptor alone. Specifically, we tested Gr5a in pairwise combinations with the other sweet receptors and measured responses to the Gr5a-dependent sugars. We observed that the presence of another Gr did not result in significant increases in mean sugar responses (Fig. 2B). In fact, coexpression of Gr64a generally depressed responses to Gr5a-dependent sugars, which is consistent with their nonoverlapping functions in taste neurons (9). Thus, at least in the context of the ab1C neuron, expression of an individual sweet taste receptor suffices to confer responses to sweet tastants.
A heat map of ab1C:GrX responses ordered by the sequence relationship between receptor proteins is shown in Fig. 2C. The distribution of responses across the entire set of receptors revealed extensive overlap of Gr–ligand interactions, showing that individual tastants are detected by multiple sweet Grs. Interestingly, even with the limited panel of tastants that we analyzed, two loosely divided subgroups of receptors emerged from the response matrix: one group sharing functional overlap with Gr5a and a second with Gr64a. These results provide the framework to understand how largely distinct subsets of the sweet receptor clade may function with Gr5a or Gr64a to encode sweet tastants.
Ectopic Responses Are Validated by Mutant Analysis.
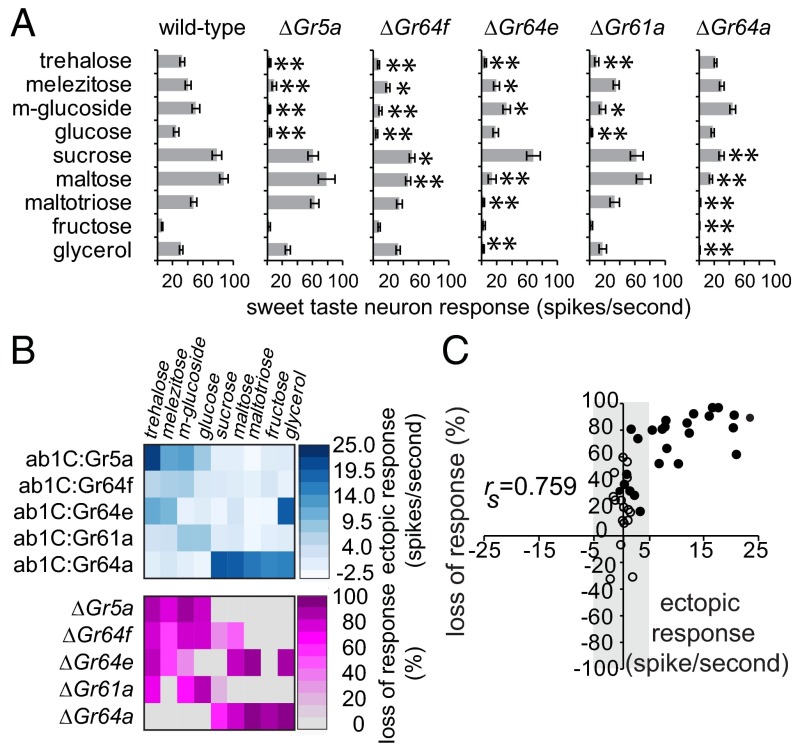
We wanted to validate the observed Gr response profiles in ab1C neurons with physiological analysis of neurons in which these receptors are expressed. We therefore compared the responses of ab1C:GrX neurons to those of sweet taste neurons in the corresponding Gr mutants. Single Gr mutants have been reported for four of these receptors, Gr5a, Gr61a, Gr64a, and Gr64e (8, 9, 12, 33); in addition, a Minos element insertion allele became available for Gr64f (35). Importantly, all five of these receptors are present in sweet taste neurons of L-type sensilla in the labellum as demonstrated by expression or functional analysis (5, 9). We measured responses of sweet taste neurons in L-type sensilla (Fig. S3A) in each of these mutants using our panel of nine sweet tastants, which were tested at concentrations of 100 mM (Fig. 3A and Fig. S3B) and 1 M (Fig. S3C).
Fig. 3.
Sweet taste responses in Gr mutants. (A) Mean responses of sweet taste neurons in L-type sensilla to indicated tastants. Indicated genotypes were: w1118 (wild-type), ΔEP(X)-5 (ΔGr5a), Gr64fMB12243 (ΔGr64f), Gr64eMB03533 (ΔGr64e), Gr61a1 (ΔGr61a), and Gr64a1 (ΔGr64a). All stimuli were tested at a concentration of 100 mM, except glycerol (10%). *P < 0.05; **P < 0.001 (one-way ANOVA with one-tailed Dunnett’s t test vs. wild-type; n = 6–22). (B) Heat maps of ab1C:GrX responses (Upper) and percent reduction in taste neuron responses in corresponding GrX mutants (Lower); the latter only includes data points significantly different from wild-type in C. Percent loss of response was calculated by using [(wild type – mutant)/wild type] x 100. Heat maps were made by using JMP 10 (www.jmp.com). (C) Scatter plot of percent loss of response in Gr mutant and ab1C:GrX response (gain) for each GrX–ligand combination. Filled circles indicate taste neuron responses that are significantly reduced in mutant flies (ΔGrX); open circles indicate those that are not. Shaded area indicates ab1C:GrX responses that are not statistically significant.
We observed complete overlap between Gr5a– and Gr64a–ligand interactions obtained in ab1C:GrX neurons and by loss of sensitivity in Gr5a and Gr64a mutants (Fig. 3 A and B). Although not as complete, there was also substantial overlap between the results of gain-of-function and loss-of-function analyses for Gr61a, Gr64e, and Gr64f (Fig. 3B and Fig. S4A). Of most importance is that every single one of the observed ab1C:GrX responses was validated by a significant reduction in sweet taste neuron response in the corresponding Gr mutant. We visualized the relationship between gain and loss values in a scatter plot (Fig. 3C) and ran a Spearman’s correlation, which showed a strong positive correlation (rs = 0.759, n = 45, P < 0.001). Together, these results authenticate the functional analysis of singly expressed Grs in the ab1C neuron. Moreover, the lack of unsupported interactions in the ab1C neuron makes it unlikely that Gr21a/Gr63a alter the response specificities of ectopically expressed receptors.
The mutant analysis also revealed eight instances in which a Gr (specifically, Gr61a, Gr64e, and Gr64f) was necessary for the full extent of a response in sweet taste neurons but was not sufficient to confer sensitivity to the corresponding tastant in the ab1C:GrX neuron (Fig. 3B). Moreover, the eight responses belonging to this category were not restricted to either Gr5a- or Gr64a-dependent tastants in that Gr61a, Gr64e, and Gr64f mutants all showed significant reductions in response to one or more tastants of both subsets. Our results are consistent with a model in which the tuning breadth of sweet taste neurons is collectively determined by each of the many Grs that are expressed in them and support extensive functional and overlapping interactions among individual receptors of the sweet clade.
Ectopic Analysis Is Validated by a Bitter Receptor.
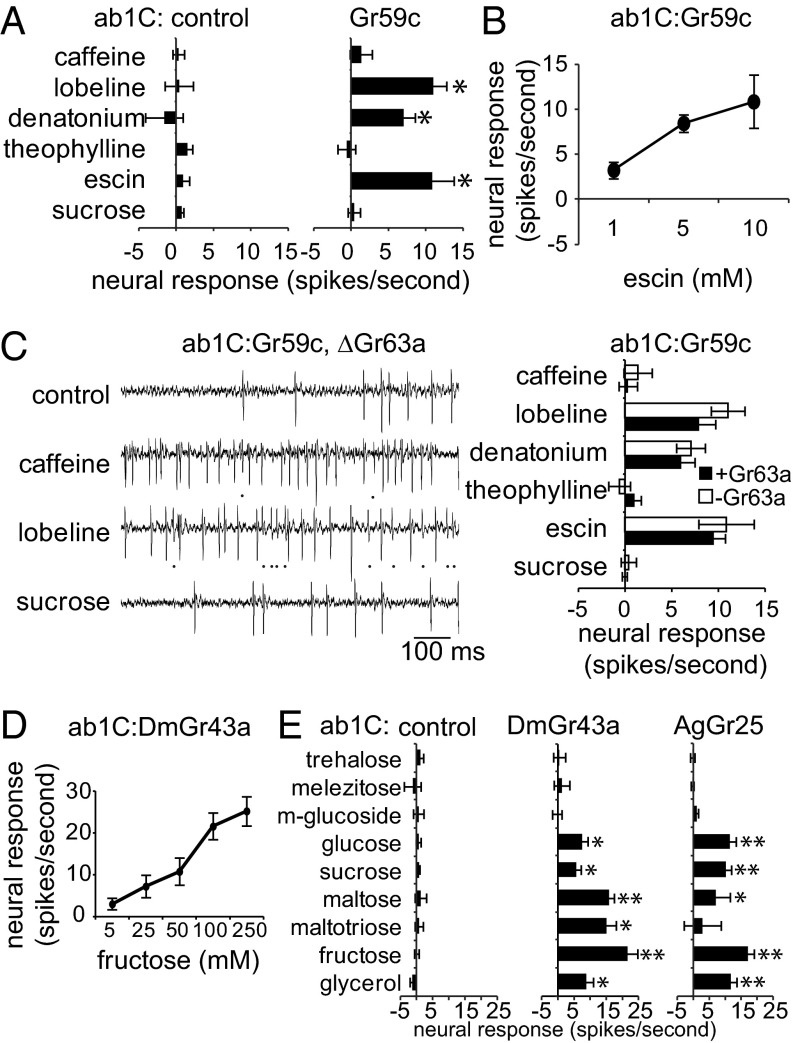
To further investigate the ab1C neuron as a reliable system for reporting GrX activity, we tested a bitter receptor, Gr59c, which has been implicated in detection of specific aversive compounds (5). Gr59c is derived from the I-a class of labellar bitter taste neurons, which respond to lobeline, denatonium, and escin. Misexpression of Gr59c in other classes of labellar bitter neurons increases responses to I-a ligands (5). Consistent with these results, expression of Gr59c in the ab1C neuron conferred responses to lobeline, denatonium, and escin, but not to caffeine, theophylline, or sucrose, which do not activate I-a bitter neurons (Fig. 4 A and B). The Gr59c response profile was not altered by the absence of Gr63a (Fig. 4C). Thus, similar to the sweet receptor Gr5a, the specificity of the Gr59c bitter receptor appears to be preserved in the ab1C neuron.
Fig. 4.
Functional analysis of other Drosophila and mosquito Grs. (A) Mean response of ab1C neurons in control w1118 flies (ab1C) and flies expressing Gr59c (ab1C:Gr59c). *P < 0.05 (vs. control; n = 6–12). Sucrose was tested at a concentration of 100 mM and bitter compounds at 10 mM. (B) Dose-dependent response of ab1C:Gr59c (n = 6–12). (C) Sample recordings and mean responses of ab1C:Gr59c in wild-type (+Gr63a) and ΔGr63a (–Gr63a) flies to indicated stimuli (10 mM). Genotypes are not significantly different (P > 0.05; n = 6). Concentrations were as in A. (D) Dose-dependent response of ab1C:DmGr43a (n = 6–11). (E) Mean responses of ab1C neurons in control w1118 flies (ab1C) and flies expressing Drosophila Gr43a (ab1C:DmGr43a) or its mosquito ortholog (ab1C:AgGr25) to indicated stimuli tested at a concentration of 100 mM, except maltotriose (250 mM) and glycerol (10%). *P < 0.05; **P < 0.001 (vs. ab1C; n = 6–12).
Functional Expression of an Orthologous Pair of Fly and Mosquito Grs.
Recent studies showed that Gr43a functions as an internal fructose-sensing receptor in vivo (36) and confers fructose response when heterologously expressed in COS-7 cells (22). We therefore tested whether expression of Gr43a was sufficient to confer fructose sensitivity in ab1C neurons. Recordings with a range of concentrations revealed a dose-dependent response to fructose in ab1C:Gr43a neurons (Fig. 4C). The concentration range over which Gr43a was active in the ab1C neuron was comparable to that observed by imaging Ca2+ activity in Gr43a-labeled neurons in the legs and the lateral protocerebrum region of the brain (36).
Given the compatibility of the ab1C neuron with Drosophila sweet and bitter taste receptors, we wanted to test whether it could be adopted for functional analysis of taste receptors from other insects such as A. gambiae. Gr genes of D. melanogaster and A. gambiae are highly divergent with few one-to-one orthologs, which include the Gr43a and AgGr25 pair (Fig. S5). We expressed AgGr25 in the ab1C neuron using a UAS–AgGr25 transgene constructed from A. gambiae genomic DNA. We assayed responses of ab1C neurons expressing either DmGr43a or AgGr25 to the nine selected sweet tastants. Both DmGr43a and AgGr25 conferred robust responses to fructose and some other sugars, including glucose (Fig. 4D), which is present in the hemolymph along with fructose and trehalose (37, 38) (Fig. 4E). In fact, there was a large overlap in excitatory responses of DmGr43a and AgGr25, with maltotriose being the only exception that evoked a response exclusively from the fly ortholog. Thus, both DmGr43a and AgGr25 are responsive to sweet tastants. Moreover, the observation that AgGr25 can function in the absence of any other mosquito proteins suggests compatibility between the ab1C neuron and Grs of A. gambiae and potentially other insects as well.
Discussion
We systematically characterized the response properties of individual members of the sweet Gr clade using a unique in vivo ectopic expression system. We also demonstrated the potential utility of this system for functional analysis of other Drosophila Grs as well as those from the malaria mosquito. Our study begins to overcome challenges in studying this highly divergent superfamily of insect Gr proteins and provides a systematic overview of sugar detection by all members of the sweet receptor clade.
We used the Gr21a/Gr63a CO2-sensing olfactory neuron as a host for in vivo expression of individual Grs. The ability of individually expressed Grs to confer tastant responses indicates that they can function in the ab1C neuron in the absence of taste-neuron-specific cofactors or coreceptors. Given the apparent lack of cross-compatibility between receptors and neurons of sweet and bitter categories, it is curious that sweet taste receptors and a bitter receptor could be deorphanized in the morphologically and functionally different CO2-sensing olfactory neuron. Our results are also surprising in view of possible heteromeric configurations for functional taste receptors that are suggested from mutant analyses. However, combinations of two, three, or four sweet taste receptors in the empty neuron yielded none that were capable of conferring responses to sweet tastants (Table S1). Thus, either Gr21a or Gr63a present in the ab1C neuron may be necessary to facilitate functional expression of exogenous taste receptors, although our analysis suggests that the presence of an intact CO2 receptor is not required. However, coexpression of Gr21a and Gr63a with a sweet taste receptor in the ab3A empty neuron system was not sufficient to confer sugar responses (Table S1), suggesting that other properties of the ab1C neuron are likely to factor in as well. Nevertheless, receptor–ligand interactions defined in the ab1C neuron show strong correlation with those identified via endogenous taste neurons, supporting the existence of functional overlap between the two systems.
Our analysis reveals that every sweet Gr can participate directly in detection of sweet tastants. Ors are the closest relatives of Grs and function in heteromeric complexes with Orco, an obligate coreceptor encoded by a highly conserved Or gene. A single Orco-like counterpart has not been identified among Grs, although some evidence suggests that more than one member of the family may adopt such a role, particularly for bitter taste detection (5, 39–41). Orco can also form functional channels by itself (28), a feature that may be shared with some Gr proteins. At least for the sweet Gr clade, it seems unlikely that any member would exclusively serve a universal coreceptor function. Rather, even if these proteins were to function in multimeric complexes, our combined ectopic expression and mutant analyses predict that each would contribute to ligand detection.
We found that recognition of any given sweet tastant is typically distributed across the activities of multiple receptors. Notably, receptors appear to be loosely separated into two groups based on their functional overlap with either Gr5a or Gr64a. The eight Drosophila receptors are thought to originate from a single ancestral gene that gave rise to two lineages—one that includes Gr5a and a second that includes Gr64a—following a duplication event (11). Thus, the two lineages appear to be specialized to some extent for detection of distinct subsets of sweet tastants. It will be interesting to determine whether the two receptors representing each of these lineages in the noninsect arthropod Daphnia pulex (42) display similarly nonoverlapping response profiles. We also note that strong responses to any particular tastant (>14.9 spikes per second in ab1C:GrX neurons, which corresponds to the top third of responses shown in Fig. 2C) were generally evoked from only one or two receptors, suggesting further specialization among them.
In a previous study we found that labellar sweet taste neurons in flies lacking both Gr5a and Gr64a were devoid of responses to all sweet tastants (9). Together with our present findings, one possible model that emerges is that Gr proteins of the sweet clade function with either Gr5a or Gr64a to mediate overlapping, but distinct, responses. Although it is tempting to posit that sweet Grs associate in groups defined by their selectivity for either Gr5a- or Gr64a-dependent sugars, it is important to note that some residual taste responses to Gr64a-dependent sugars are found in ΔGr64a mutants, and likewise for Gr5a (Fig. 3A and Fig. S3C). Thus, a more appropriate scenario might be that interactions are somewhat more promiscuous and allow individual sweet Grs to function with both Gr5a and Gr64a. The idea of such variable coupling between sweet Grs offers an intriguing perspective on the flexibility and adaptability of the insect gustatory system.
Studies have shown that mosquito Ors can function in the Drosophila empty neuron (17, 43). Our results show that a mosquito Gr can function outside its native context in the absence of any other mosquito factors. These results provides a foundation for investigating functional properties of other taste receptors in mosquitoes and for exploring whether this system can be used for studying Gr from other insects.
Experimental Procedures
Insect Stocks and Rearing.
Flies were maintained on standard cornmeal–dextrose medium at 25 °C. Complete genotypes and sources of flies used in every experiment are listed in Table S2. UAS–Gr43a was generated from a PCR-amplified ATG–stop fragment from Canton-S DNA. For UAS–Gr64c and –Gr64d, PCR-amplified ATG–stop fragments from Canton-S genomic DNA were cloned into pJET, sequenced, and transferred to pUAS-T; similarly, UAS–AgGr25 was constructed by using genomic DNA from A. gambiae. A UAS–Gr64b transgene (BL 27324) was mobilized to the third chromosome by using a Delta2–3 transposase stock (BL 3664).
Extracellular Recordings.
Extracellular recordings from the ab1C neuron were performed as described (12). Tastant stimuli were prepared in sensillum lymph ringer (SLR) and stored at −20 °C. Two recording electrodes, one with electrolyte alone (SLR) and a second with stimulus solution (stimulus) were held on the same manipulator. Recordings were first obtained with SLR from three ab1 sensilla for ∼6 s to measure baseline activity of the ab1 neurons. Subsequently, ∼6-s recordings were obtained from the same three sensilla stimulus with the stimulus. Up to three different stimuli were sequentially tested on a single fly; each stimulus was tested on an independent group of three sensilla (i.e., a total of up to 18 recordings—9 SLR and 9 stimulus—per fly). Action potentials of the ab1C neuron were counted in the 2-s period after establishing electrical contact with the sensillum and divided by 2 to obtain a firing rate in spikes per second. Unless otherwise indicated, baseline SLR activity of the ab1C neuron was subtracted from the stimulus-evoked response recorded from the same sensillum.
Tip recordings from labellar taste sensilla in the fly were performed as described (44) by using 30 mM tricholine citrate as electrolyte; tastants were stored at −20 °C, and working aliquots were thawed and kept at 4 °C for no more that 1 wk. Spikes were counted in the 200- to 700-ms window after contact of the stimulus micropipette with the pore of a sensillum.
Tastants.
All compounds were obtained at the highest available purity from Sigma-Aldrich and were as follows: trehalose (T9531), glucose (G7528), or m-glucoside (M9376), melezitose (M5375), sucrose (S7903), maltose (M9171), maltotriose (M8378), fructose (47740), and glycerol (G7893).
Statistical Analyses.
One-way ANOVA and Dunnett’s t tests were used for statistical analysis, unless specified otherwise in the figure legends. All graphs show mean ± SEM.
Supplementary Material
Acknowledgments
We thank A. Ray and S. Turner-Chen for sharing equipment and expertise for olfactory single sensillum recordings; W. Tom for developing the two-electrode recording method; C. Montell for fly stocks; and M. Gordon, S. Charlu, A. Ganguly, G. Pask, and C. Scott for comments on the manuscript. This work was supported by National Science Foundation Integrated Graduate Research and Training Program in Video Bioinformatics Fellowship DGE0903667 (to E.G.F.); National Institutes of Health (NIH) Predoctoral Award F31-AI108266 (to E.G.F.); the University of California; Whitehall Foundation Grant 2010-12-42 (to A.D.); and NIH Grant R01DC013587 (to A.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1311724111/-/DCSupplemental.
References
- 1.Yarmolinsky DA, Zuker CS, Ryba NJ. Common sense about taste: From mammals to insects. Cell. 2009;139(2):234–244. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robertson HM, Warr CG, Carlson JR. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc Natl Acad Sci USA. 2003;100(Suppl 2):14537–14542. doi: 10.1073/pnas.2335847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr Biol. 2004;14(12):1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117(7):981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Weiss LA, Dahanukar A, Kwon JY, Banerjee D, Carlson JR. The molecular and cellular basis of bitter taste in Drosophila. Neuron. 2011;69(2):258–272. doi: 10.1016/j.neuron.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon JY, Dahanukar A, Weiss LA, Carlson JR. Molecular and cellular organization of the taste system in the Drosophila larva. J Neurosci. 2011;31(43):15300–15309. doi: 10.1523/JNEUROSCI.3363-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marella S, et al. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49(2):285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 8.Jiao Y, Moon SJ, Montell C. A Drosophila gustatory receptor required for the responses to sucrose, glucose, and maltose identified by mRNA tagging. Proc Natl Acad Sci USA. 2007;104(35):14110–14115. doi: 10.1073/pnas.0702421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahanukar A, Lei YT, Kwon JY, Carlson JR. Two Gr genes underlie sugar reception in Drosophila. Neuron. 2007;56(3):503–516. doi: 10.1016/j.neuron.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slone J, Daniels J, Amrein H. Sugar receptors in Drosophila. Curr Biol. 2007;17(20):1809–1816. doi: 10.1016/j.cub.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kent LB, Robertson HM. Evolution of the sugar receptors in insects. BMC Evol Biol. 2009;9:41. doi: 10.1186/1471-2148-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wisotsky Z, Medina A, Freeman E, Dahanukar A. Evolutionary differences in food preference rely on Gr64e, a receptor for glycerol. Nat Neurosci. 2011;14(12):1534–1541. doi: 10.1038/nn.2944. [DOI] [PubMed] [Google Scholar]
- 13.Jiao Y, Moon SJ, Wang X, Ren Q, Montell C. Gr64f is required in combination with other gustatory receptors for sugar detection in Drosophila. Curr Biol. 2008;18(22):1797–1801. doi: 10.1016/j.cub.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyamoto T, Chen Y, Slone J, Amrein H. Identification of a Drosophila glucose receptor using Ca2+ imaging of single chemosensory neurons. PLoS ONE. 2013;8(2):e56304. doi: 10.1371/journal.pone.0056304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreher SA, Kwon JY, Carlson JR. The molecular basis of odor coding in the Drosophila larva. Neuron. 2005;46(3):445–456. doi: 10.1016/j.neuron.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125(1):143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 17.Carey AF, Wang G, Su CY, Zwiebel LJ, Carlson JR. Odorant reception in the malaria mosquito Anopheles gambiae. Nature. 2010;464(7285):66–71. doi: 10.1038/nature08834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang G, Carey AF, Carlson JR, Zwiebel LJ. Molecular basis of odor coding in the malaria vector mosquito Anopheles gambiae. Proc Natl Acad Sci USA. 2010;107(9):4418–4423. doi: 10.1073/pnas.0913392107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chyb S, Dahanukar A, Wickens A, Carlson JR. Drosophila Gr5a encodes a taste receptor tuned to trehalose. Proc Natl Acad Sci USA. 2003;100(Suppl 2):14526–14530. doi: 10.1073/pnas.2135339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445(7123):86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- 21.Kwon JY, Dahanukar A, Weiss LA, Carlson JR. The molecular basis of CO2 reception in Drosophila. Proc Natl Acad Sci USA. 2007;104(9):3574–3578. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato K, Tanaka K, Touhara K. Sugar-regulated cation channel formed by an insect gustatory receptor. Proc Natl Acad Sci USA. 2011;108(28):11680–11685. doi: 10.1073/pnas.1019622108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang HJ, et al. Topological and functional characterization of an insect gustatory receptor. PLoS ONE. 2011;6(8):e24111. doi: 10.1371/journal.pone.0024111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozaki K, et al. A gustatory receptor involved in host plant recognition for oviposition of a swallowtail butterfly. Nat Commun. 2011;2:542. doi: 10.1038/ncomms1548. [DOI] [PubMed] [Google Scholar]
- 25.Sánchez-Gracia A, Vieira FG, Rozas J. Molecular evolution of the major chemosensory gene families in insects. Heredity (Edinb) 2009;103(3):208–216. doi: 10.1038/hdy.2009.55. [DOI] [PubMed] [Google Scholar]
- 26.Larsson MC, et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43(5):703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 27.Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136(1):149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones PL, Pask GM, Rinker DC, Zwiebel LJ. Functional agonism of insect odorant receptor ion channels. Proc Natl Acad Sci USA. 2011;108(21):8821–8825. doi: 10.1073/pnas.1102425108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones PL, et al. Allosteric antagonism of insect odorant receptor ion channels. PLoS ONE. 2012;7(1):e30304. doi: 10.1371/journal.pone.0030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olsson SB, Getahun MN, Wicher D, Hansson BS. Piezo controlled microinjection: An in vivo complement for in vitro sensory studies in insects. J Neurosci Methods. 2011;201(2):385–389. doi: 10.1016/j.jneumeth.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 31.Getahun MN, Olsson SB, Lavista-Llanos S, Hansson BS, Wicher D. Insect odorant response sensitivity is tuned by metabotropically autoregulated olfactory receptors. PLoS ONE. 2013;8(3):e58889. doi: 10.1371/journal.pone.0058889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sargsyan V, et al. Phosphorylation via PKC regulates the function of the Drosophila odorant co-receptor. Front Cell Neurosci. 2011;5:5. doi: 10.3389/fncel.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dahanukar A, Foster K, van der Goes van Naters WM, Carlson JR. A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nat Neurosci. 2001;4(12):1182–1186. doi: 10.1038/nn765. [DOI] [PubMed] [Google Scholar]
- 34.Gordesky-Gold B, Rivers N, Ahmed OM, Breslin PA. Drosophila melanogaster prefers compounds perceived sweet by humans. Chem Senses. 2008;33(3):301–309. doi: 10.1093/chemse/bjm088. [DOI] [PubMed] [Google Scholar]
- 35.Bellen HJ, et al. The Drosophila gene disruption project: Progress using transposons with distinctive site specificities. Genetics. 2011;188(3):731–743. doi: 10.1534/genetics.111.126995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyamoto T, Slone J, Song X, Amrein H. A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell. 2012;151(5):1113–1125. doi: 10.1016/j.cell.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee G, Park JH. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics. 2004;167(1):311–323. doi: 10.1534/genetics.167.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson SN. Trehalose - The insect “blood” sugar. Adv Insect Physiol. 2003;31:205–285. [Google Scholar]
- 39.Moon SJ, Köttgen M, Jiao Y, Xu H, Montell C. A taste receptor required for the caffeine response in vivo. Curr Biol. 2006;16(18):1812–1817. doi: 10.1016/j.cub.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 40.Moon SJ, Lee Y, Jiao Y, Montell C. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr Biol. 2009;19(19):1623–1627. doi: 10.1016/j.cub.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee Y, Moon SJ, Montell C. Multiple gustatory receptors required for the caffeine response in Drosophila. Proc Natl Acad Sci USA. 2009;106(11):4495–4500. doi: 10.1073/pnas.0811744106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peñalva-Arana DC, Lynch M, Robertson HM. The chemoreceptor genes of the waterflea Daphnia pulex: Many Grs but no Ors. BMC Evol Biol. 2009;9:79. doi: 10.1186/1471-2148-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hallem EA, Nicole Fox A, Zwiebel LJ, Carlson JR. Olfaction: Mosquito receptor for human-sweat odorant. Nature. 2004;427(6971):212–213. doi: 10.1038/427212a. [DOI] [PubMed] [Google Scholar]
- 44.Benton R, Dahanukar A. Electrophysiological recording from Drosophila taste sensilla. Cold Spring Harb Protoc. 2011;2011(7):839–850. doi: 10.1101/pdb.prot5631. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.