Abstract
SUMMARY
The agents of human treponematoses include four closely related members of the genus Treponema: three subspecies of Treponema pallidum plus Treponema carateum. T. pallidum subsp. pallidum causes venereal syphilis, while T. pallidum subsp. pertenue, T. pallidum subsp. endemicum, and T. carateum are the agents of the endemic treponematoses yaws, bejel (or endemic syphilis), and pinta, respectively. All human treponematoses share remarkable similarities in pathogenesis and clinical manifestations, consistent with the high genetic and antigenic relatedness of their etiological agents. Distinctive features have been identified in terms of age of acquisition, most common mode of transmission, and capacity for invasion of the central nervous system and fetus, although the accuracy of these purported differences is debated among investigators and no biological basis for these differences has been identified to date. In 2012, the World Health Organization (WHO) officially set a goal for yaws eradication by 2020. This challenging but potentially feasible endeavor is favored by the adoption of oral azithromycin for mass treatment and the currently focused distribution of yaws and endemic treponematoses and has revived global interest in these fascinating diseases and their causative agents.
INTRODUCTION
The human treponematoses comprise venereal syphilis and the endemic treponematoses called yaws, bejel, and pinta. The etiological agents of these diseases are Gram-negative bacteria that belong to the order Spirochaetales, family Spirochaetaceae, and genus Treponema. The syphilis, yaws, and bejel spirochetes were originally classified as separate species but are now considered to be subspecies of Treponema pallidum (T. pallidum subsp. pallidum, T. pallidum subsp. pertenue, and T. pallidum subsp. endemicum, respectively) based upon DNA hybridization evidence of their remarkably high genetic relatedness (1, 2). The lack of availability of an isolate of the agent of pinta has precluded genetic analyses of this organism, and it retains its separate name, T. carateum.
All human treponematoses share impressive similarities in pathogenesis and natural history (Table 1). All are transmitted by direct contact with infectious lesions and are chronic infections that are manifest in multiple stages involving the skin. All except pinta can progress to cause serious and destructive lesions of skin, bone, and cartilage. As in venereal syphilis, the clinical manifestations of the endemic treponematoses are commonly divided into an early stage (encompassing primary and secondary manifestations) and a late stage. Early-stage lesions are highly infectious and can persist for weeks to months, or even years, following appearance. Once the early manifestations spontaneously regress due to the host's immune response against the pathogen, the patient enters a state of latency that in many cases lasts for a lifetime. In a relatively small percentage of cases, however, the infection may progress from latency to tertiary disease, characterized by destruction of tissues.
TABLE 1.
Classical features of human treponematoses
| Feature | Yaws | Bejel (endemic syphilis) | Pinta | Venereal syphilis |
|---|---|---|---|---|
| Causative agent | T. pallidum subsp. pertenue | T. pallidum subsp. endemicum | T. carateum | T. pallidum subsp. pallidum |
| Geographical distribution | Western/Central Africa, Southeast Asia, Pacific Islands | Sahelian Africa, Saudi Arabia | Central and South America | Global |
| Climatic conditions | Tropical (hot and humid) | Hot and dry (semiarid/arid) | Warm (semiarid) | All |
| Age group (peak incidence of lesions) | Children (<15 yr) | Children (2–15 yr) | Children and adults | Adults |
| Common mode of transmission | Skin-to-skin contact | Mucous membrane and skin-to-skin contact (sharing of eating utensils and drinking vessels) | Skin-to-skin contact | Sexual and congenital; occasionally nonsexual contact |
| Most common location of primary lesion | Lower extremities | Oral mucosa (rarely seen) | Extremities | Genitalia, anal, and oral mucosae |
| Most affected organs | Skin and bone | Oral and nasal mucosae, intertriginous areas and bone | Skin | Skin; systemic involvement, including central nervous system and fetus |
| Late complications in the absence of treatment | Destructive osteitis, saddle nose, destruction of the palate and nasal septum, painful lesions on soles | Destructive osteitis of the nose, palate, and nasal septum | Depigmented lesions over hands, wrists, elbows, feet, and ankles | Neurological (optic atrophy, paresis, tabes dorsalis), cardiovascular (aortitis, aortic aneurysm), gummatous (skin, lung, liver, brain, other organs) |
| Vertical transmission | Not commonly recognized but asserted (43) | Not commonly recognized but asserted (332) | Not recognized | Frequent |
| Central nervous system involvement | Believed to be rare | Believed to be extremely rare or absent | Not recognized | Frequent |
| Animal models | Rabbit ≅ hamster | Rabbit ≅ hamster | Primate only | Rabbit > primate > guinea pig > hamster |
| Genome size (kbp) | 1,139.3–1,139.7 | 1,137.7a | NDb | 1,138.0–1,140.0 |
| Genomic identity with T. pallidum subsp. pallidum (%) | 99.8 | 99.7a | ND | NAc |
Štaudová et al., unpublished data.
ND, no data.
NA, not applicable.
Despite many similarities, some differences among the human treponematoses have been described. The endemic treponematoses are transmitted primarily in childhood, often in the poorest rural communities of the tropical belt (Fig. 1). In contrast, venereal syphilis is globally distributed and is transmitted primarily by sexual activity of adolescents and adults. In pregnant women with syphilis, T. pallidum subsp. pallidum readily crosses the placenta to infect the fetus, causing spontaneous abortion, stillbirth, or congenital infection of the newborn, while congenital infection has been stated not to occur in endemic treponematoses. Furthermore, despite recognition of cardiovascular, neurological, and ophthalmological manifestations during syphilis infection, these manifestations are rarely or not reported for endemic treponematoses (Table 1). One of the goals of this work is to critically review the data relevant to the often-described differences among these diseases and the possible biological, social, and environmental factors behind these differences.
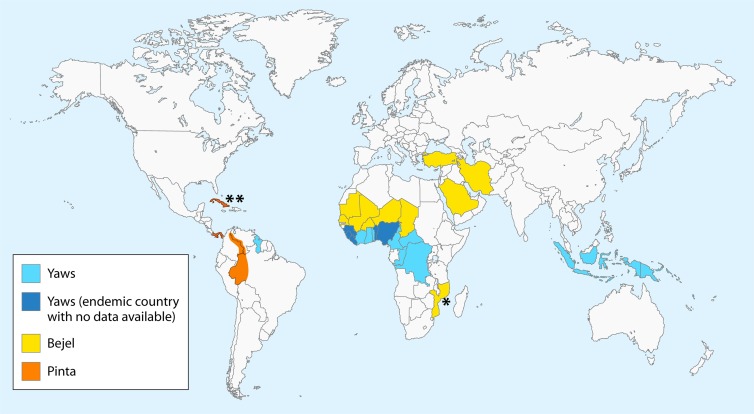
FIG 1.
Current geographical distribution of endemic treponematoses. The map was designed according to epidemiological data on yaws available at http://www.who.int/yaws/epidemiology/en/ and the gathered bibliography, including work by Harding (333), Bendel (334), and da Cruz-Ferreira and Sterneberg (335). *, based on a 2006 case report article describing two cases of bejel in children in Mozambique (267). **, based on an early pinta lesion identified in a female resident of Cuba visiting Austria (274). Officially, the last case of pinta in Cuba was reported in 1975.
Our overall understanding of the pathogenesis of human treponematoses is limited (i) by the inability to grow these spirochetes in vitro, requiring expensive and difficult continual propagation in laboratory animals, (ii) by their remarkably fragile cellular ultrastructure, permitting only limited mechanical manipulation of the pathogen to preserve its integrity and viability, and (iii) by the bacterium's limited viability outside a host. The rabbit is widely used for propagation of isolates of the T. pallidum subspecies.
ANIMAL MODELS FOR THE STUDY OF THE HUMAN TREPONEMATOSES
Rabbits are highly susceptible to T. pallidum infection (3, 4), and it has been shown that, upon intradermal (i.d.) infection with T. pallidum subsp. pallidum, New Zealand White (NZW) rabbits develop lesions that are clinically and histologically very similar to human primary lesions. Secondary syphilis lesions can also appear after healing of primary lesion in the rabbit model (5, 6). Infected rabbits mount cytokine responses against the pathogen that mirror those of humans during natural infection (6–9). Development of alternative experimental models of treponemal infection has been attempted to overcome the unavailability of inbred rabbit strains and to facilitate immunological studies, but with relatively low success. Hamsters were shown to be very good models for yaws and bejel but were less susceptible to infection with T. pallidum subsp. pallidum (10) (Table 1). Guinea pigs develop primary ulcers only with inocula far larger than necessary in rabbits or humans and with atypical histopathology and cytokine expression profile compared to those of rabbit and human lesions (11, 12). Mice can be infected with T. pallidum subsp. pallidum, but they fail to develop disease manifestations and are thus of scarce utility for the study of the disease (13). Nonhuman primates are very expensive and limited in availability, but they have been used for the study of syphilis and are the only known experimental host for T. carateum, the agent of pinta (5, 14, 15). The histopathologies of early and late pinta lesions in chimpanzees was shown to be very similar to those of the corresponding human lesions, even though T. carateum localized mostly in the upper dermis of infected chimpanzees, while in humans the pathogen is generally seen in the epidermis (16).
CLINICAL MANIFESTATIONS OF THE ENDEMIC TREPONEMATOSES
Yaws
Transmission of yaws, caused by T. pallidum subsp. pertenue, occurs by direct skin contact with an infectious lesion and is facilitated by breaks in the skin of traumatic or other etiology (e.g., scratches or scabies) (17, 18). The disease generally occurs in tropical areas with an average annual temperature of ≥27°C (80°F) and heavy rainfall (Fig. 1). Early clinical manifestations are seen primarily in children younger than 15 years of age (with a peak between 6 and 10 years) and living in rural communities with scarce water supply and a lack of sanitation (19, 20). The name likely originated from the African word for berry (yaw) or the Carib word for sore (yaya) (21), but this illness is also known by a variety of alternative names, such as buba (Spanish), pian (French), frambesia (from “framboise,” raspberry in French), and parangi or paru (Malay) (22).
The primary stage of the disease appears after a variable incubation period (mean of 21 days after exposure) as a solitary erythematous papule that can grow into a papilloma of 2 to 5 cm in diameter by peripheral extension or by coalescing with satellite papules (23). The lesion is not painful but may be pruritic, and it is typically covered by a crust that hides an ulcer with raised dark margins and an erythematous moist center, overall resembling a raspberry (hence the African and French names) (Fig. 2A). The primary lesion, called “mother yaw” (buba madre or mamapian) is often found on the lower extremities, but can also occur on the patient's buttock, arm, face, or hand (24–26). Teeming with treponemes, the mother yaw is highly contagious and can persist for weeks or months before healing spontaneously, often leaving a hypopigmented or depressed area delimited by a dark border (27). Groups of small dry papules have been reported as the initial manifestation instead of a mother yaw, but the lack of observable primary lesion is quite rare (28). At this stage, regional lymphadenopathy and arthralgia may also occur (25, 29). In the majority of cases, the primary lesion heals spontaneously before the onset of yaws' secondary manifestations, whose appearance is due to the pathogen's systemic dissemination during early infection. In 9 to 15% of the patients, however, the mother yaw is still present when the “daughter yaws” appear. The development of secondary lesions can, however, also be delayed as long as 1 to 2 years from the initial exposure. Secondary papules (pianomas or framboesias), although smaller, may resemble the mother yaw or may appear as scaly maculae of irregular shape (Fig. 2B and C, respectively). The daughter lesions can also expand and ulcerate, releasing a highly infectious fluid. Flies (Hippelates pallipes) are attracted to the oozing fluid, and their involvement in transmission has been proposed but not definitively demonstrated (30–35). Because of central healing, the papules may appear ring shaped (tinea yaws). Other secondary manifestations include condylomata lata in moist crevices such as the axilla and groin and a measles-like eruption (25). Hyperkeratotic plaques on palms and soles are common at this stage, with the latter referred to as “crab yaws” because of the painful crab-like gait they induce (Fig. 2D) (36). The nature of the skin lesions is influenced by climatological conditions: in the warm rainy season, lesions are more numerous and florid than during drier periods of the year, when patients might present with atypical scanty macular lesions. Persons living in areas of endemicity at higher altitudes or in drier climates frequently will have “attenuated” or small, dry lesions on the extremities, with more exuberant lesions being seen only in moist folds of the body (37). During the early stages, periostitis and osteitis may affect the bones of the upper and lower limbs (tibia, fibula, and forearm) and the proximal phalanges of fingers and toes, resulting in bone pain and digital swelling (Fig. 2E) (38). At this stage, fever and malaise are common. The secondary lesions also heal spontaneously within weeks or months, and the patient enters the latent stage of the infection, which can be recognized only through serological tests and will, unless treated, last for a lifetime. Recurrences (generally one or two) of secondary manifestations might be seen up to 5 years after the initial infection, although relapses after 10 years have been reported (23, 29). Approximately 10% of untreated patients will develop tertiary yaws, characterized by subcutaneous gummatous nodules, chronic periostitis that can cause apparent bowing of the tibia (i.e., saber shin) (Fig. 2F), and destructive processes leading to saddle nose and perforation/collapse of the palate and nasal septum (i.e., gangosa) (29, 39). Bilateral hypertrophic periostitis of the paranasal maxilla and nasal bridge causes the clinical manifestation known as goundou (40, 41).
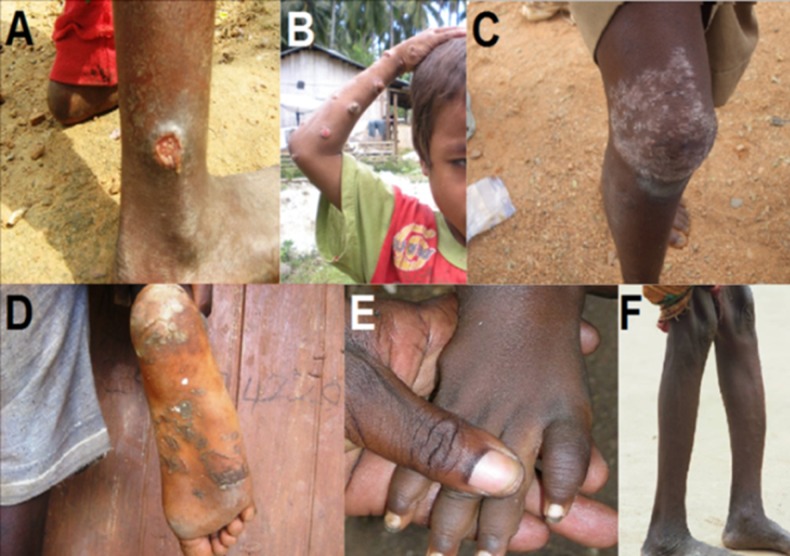
FIG 2.
Clinical manifestations of yaws. (A) Early (primary) yaws: infectious skin lesion known as “mother yaw” on the ankle. (B) Early (secondary) yaws: infectious ulcero-papillomatous lesions on the arm. (C) Early (secondary) yaws: scaly maculae of irregular shape on the knee. (D) Early (secondary) yaws: plantar hyperkeratosis with painful cracks and fissures. (E) Fusiform swelling of the fourth digit in a yaws patient with dactylitis. (F) Saber shin, caused by chronic osteitis. (Panels A, C, and D are courtesy of Cynthia Kwakye and the Ghana National Yaws Eradication Program, reproduced with permission. Panel B is courtesy of Christina Widaningrum-Mkes and the Ministry of Health of Indonesia, reproduced with permission. Panels E and F were originally taken by Laurent Ferradini and are reprinted from reference 336, published under a Creative Commons license.)
In contrast to syphilis, yaws is reported to affect neither the cardiovascular system nor the central nervous system (CNS), nor to be vertically transmitted to the fetus. These statements are challenged by several studies, including those of Edington (42) and those reviewed by Roman and Roman (43–45). Edington identified a syphilis-like aortitis as a major cause of cardiovascular death in persons from a region of the Gold Coast (now Ghana) where yaws is endemic (42). Roman and Roman reviewed the literature on CNS, cardiovascular, visceral, and congenital infection in yaws patients and concluded that there is ample evidence to support the involvement of the CNS, cardiovascular system, eyes, and fetus during yaws infection (43). The striking similarity between T. pallidum subsp. pallidum and T. pallidum subsp. pertenue infections, and resulting uncertainty, is exemplified by the Haiti B strain, which was isolated from a “typical frambesiform yaws” lesion on the lower abdomen of an 11-year-old boy in Haiti in the 1950s (5). This strain was shown to fail to cross the placenta in a guinea pig model of congenital syphilis, and these results were stated to confirm the lack of placental invasion by T. pallidum subsp. pertenue strains (46). Once molecular signatures for the subspecies had been identified, however, it was shown that the Haiti B strain is really a T. pallidum subsp. pallidum strain, rather than T. pallidum subsp. pertenue, reminding us that different clinical characteristics may not be absolutely related to subspecies but may be affected by the nature of the strains, by the individual host, or by other undefined environmental variables. A milder or “attenuated” form of yaws, characterized by smaller and fewer papillomas of shorter duration localized in the moist skin folds, has been described in areas where disease incidence was reduced by mass treatment campaigns (37, 47). In these settings, a reduction in the occurrence of the most severe tertiary manifestations was also noted. This phenomenon might be due, however, to improved sanitation or health care in these areas, which would allow for reduced transmission and earlier diagnosis and treatment, rather than to a less virulent agent.
Bejel (Endemic Syphilis)
Bejel is the Arabic name for endemic (or nonvenereal) syphilis and is caused by T. pallidum subsp. endemicum. Acute infection is seen primarily in children between 2 and 15 years of age in dry, arid climates (Fig. 1). Additional names for the disease are njovera (Zimbabwe), belesh or bishel (Saudi Arabia), and dichuchwa (Botswana) (48–50). Although the mode of transmission has not been adequately studied, it is believed to occur through mucosal and skin contact or the sharing of eating utensils or drinking vessels. The fomite hypothesis is supported mainly by microscopic visualization of spirochetes on a recently used drinking vessel, described by Grin (51). Given that there are many spirochetes in the mouth that may be impossible to differentiate by microscopy, this mode of transmission of T. pallidum subsp. endemicum remains unproven.
In contrast to the other treponematoses, bejel's primary lesion is often unobserved. When seen, however, it appears as a small and painless mucous papule or ulcer that develops in the oral cavity or nasopharynx. A primary lesion was also reported on the nipple of a nursing woman and in the genital regions of adults (51–53), as has been commonly reported for venereal syphilis. Secondary lesions are very similar to those of venereal syphilis and may manifest as mucous patches on the oral mucosa (Fig. 3A), tonsils, tongue (Fig. 3B), lips, and nasopharynx. Split papules at the labial commissures (angular stomatitis, as in yaws patients) (Fig. 3C), nonitchy skin eruptions, generalized lymphadenopathy, and laryngitis are common manifestations (54). Secondary skin lesions include condylomata lata in intertriginous body areas, comparable to those in yaws and syphilis. Maculopapular or papulosquamous lesions, as well as a nonpruritic generalized papular rash, can be observed in a minority of patients with bejel (54). As in yaws, osteitis and periostitis of the long bones and hands may occur, causing nocturnal bone pain. Secondary manifestations heal in 6 to 9 months, and the disease enters latency (27). The tertiary stage might manifest earlier (55) than for yaws (6 months to several years) but, as in yaws, is characterized by gummatous lesions of the skin, mucosa, and bone that may progress to destructive ulcers (52). Skin lesions resolve in time and leave characteristic depigmented scars surrounded by hyperpigmentation. Destructive lesions of the palate and nasal septum (i.e., gangosa, as in yaws) are associated with difficulties in swallowing and talking (56) (Fig. 3D). Although bone changes (i.e., saber tibia) are common, they have been described as less severe than in yaws. Neurological and cardiac involvement, as well as congenital transmission, have been reported as being rare in bejel. In 1989, however, Tabbara et al. (57), found evidence of uveitis in 13 of 17 patients with tertiary bejel, signs of optic atrophy in six patients, and choroidal atrophic scars or healed chorioretinitis in another six. Additionally, Grin (51) described second trimester miscarriage (as seen in congenital syphilis) in bejel-infected women occurring at rates significantly higher than those for healthy women (in whom miscarriage typically occurs during the first trimester). An attenuated form of bejel was described in 1984 by Pace and Csonka in Saudi Arabia (56, 58). In these patients, early lesions are reduced in number, severity, and duration, and the disease is often manifest as leg pain with radiological evidence of osteoperiostitis (56, 58).
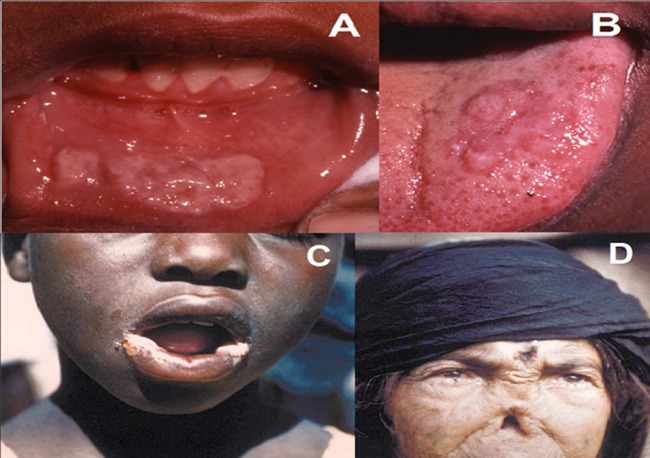
FIG 3.
Clinical manifestations of bejel. (A) Early bejel: labial mucosal plaques. (B) Early bejel: lingual plaques. (C) Angular stomatitis of early bejel, which also may occur in yaws. (D) Late bejel rhinopharyngitis mutilans (gangosa), which also occurs in yaws. (Panels A and B are courtesy of Emmanuel Galoo, reprinted from reference 337 with permission. Panel C is reprinted from reference 23 with permission of the publisher. Panel D is reprinted from reference 26 with permission.)
Pinta
Pinta (from pintar, to paint) is also known as mal de pinto (in Mexico and Cuba), enfermedad azul (blue illness, in Chile and Peru), and carate or cute (in Venezuela and Colombia). Caused by T. carateum, it is regarded as the mildest of the treponematoses, in that its lesions are limited to the skin and there is no evidence of systemic involvement or vertical transmission (44, 59–61). The disease is found focally in tropical Central and South America (Fig. 1). Because no laboratory strain of this pathogen is currently available for studies, T. carateum is the least characterized of the agents of the human treponematoses and is still classified independently from the other T. pallidum subspecies, whose genetic and antigenic relatedness has been experimentally demonstrated. The precise mechanism of transmission is unknown, although repeated skin-to-skin contact appears to be the most plausible (62). Young adults (≤15 years old) with chronic skin lesions are considered to be the disease's main reservoir (54, 59, 62). Pinta also appears to be transmitted to infants from their mothers by close contact. As in the other treponematoses, early and late stages can be identified, although in this case overlapping stages are not uncommon (23, 59). The primary lesion manifests as a papule or an erythemato-squamous plaque on exposed parts of the body, after an incubation period varying from 1 week to 2 months. Satellite lesions may be present. With time, the papules increase in size and coalesce to form patches with a pale center (Fig. 4A) (61). After months, many of the patches become hypochromic or acquire a light-blue/grayish pigmentation, with the color more marked at the center of the lesion. The initial lesions may either heal, leaving a slightly pigmented or hypochromic area, or persist for years and become indistinguishable from the secondary lesions (61). Regional lymphadenopathy is common at this stage. After months or years, small disseminated secondary lesions (called pintids) may appear in the form of scaly papules that will again enlarge and coalesce in psoriasiform plaques (Fig. 4B). These plaques might become hypo- or hyperchromic, as well as erythematous or desquamative (62). Different types of pintids might be present at the same time in the same individual. This stage usually lasts 2 to 4 years, during which some patches will heal and others will persist and enlarge. The late stage usually develops 2 to 4 years after initial infection and is characterized by the appearance of pigmentary changes, skin atrophy, and hyperkeratosis. Symmetric depigmented lesions can appear over wrists, elbows, and ankles. Early pinta lesions contain a large number of treponemes, and treponemes persist in later lesions. With the exception of late depigmented lesions (Fig. 4C), pinta lesions are considered infectious (54).
FIG 4.
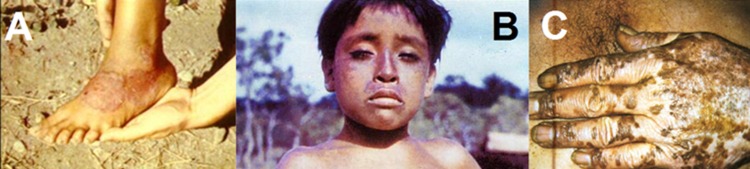
Clinical manifestations of pinta. (A) Early pinta: erythematous-squamous plaque. (B) Early pinta: hyperpigmented lesions of pinta. (C) Late pinta: depigmented lesions on hand. (Panels A and B are reprinted from reference 23 with permission of the publisher. Panel C is reprinted from http://itg.content-e.eu/Generated/pubx/173/treponematoses/pinta.htm with permission from the Institute of Tropical Medicine-Antwerp).
Histopathology of Endemic Treponematoses
The inflammatory infiltrate of early yaws lesions is composed mainly of plasma cells and lymphocytes and less commonly neutrophils and eosinophils (63). In contrast with venereal syphilis, the blood vessels show limited or no endothelial proliferation. With the aid of silver staining, treponemes can be visualized in the intercellular spaces of the epidermis, among inflammatory cells, and within the dermis. The histopathology of late yaws lesions resembles that of tertiary syphilis, with the presence of granulomas associated with necrotic areas (64, 65).
The histopathology of early bejel lesions is reported to resemble closely that of venereal syphilis. The dermal inflammatory infiltrate is located perivascularly and is composed of lymphocytes and plasma cells. Granulomas consisting of epithelioid cells and multinuclear giant cells might be present (25, 65). With regard to the histopathological picture of late bejel lesions, one study of three cases of late mutilating disease showed parakeratosis, acanthosis, spongiosis, and an inflammatory infiltrate of epithelioid cells, lymphocytes, and plasma cells (48), again similar to syphilis.
Primary and secondary pinta lesions are histopathologically similar and are characterized by mild acanthosis and spongiosis (25). The inflammatory infiltrate localizes in the upper dermis around enlarged blood vessels and is composed of lymphocytes, plasma cells, and neutrophils. Melanophages can also infiltrate the dermis (25, 62, 65). The histopathology of the pintids differs according to their appearance. Parakeratosis and acanthosis are more evident in psoriasiform lesions. Late pinta lesions are characterized by pigmentary changes due to a decrease of melanin in the basal cell layer of the epidermis. Hyperchromic lesions show pigment deposition in the dermis, epidermal atrophy, and accumulation of melanophages and lymphocytes in the dermis (66). Hypochromic and leukodermic lesions show epidermal atrophy and lack of melanin in the epidermis. Perivascular lymphocytic infiltrates are common in late pinta. Treponemes have been demonstrated in all other types of lesions except late depigmented lesions (25).
Treponemal Infections and HIV
The interaction of T. pallidum and human immunodeficiency virus (HIV) in coinfected individuals is poorly understood and has been examined only in the setting of syphilis. Rompalo et al. reported that HIV coinfection may have a limited effect on the clinical manifestations of primary and secondary syphilis: patients with primary syphilis and HIV tended to exhibit multiple ulcers at the site of inoculation more frequently than HIV-negative patients, and HIV-infected individuals presented more often with unhealed primary ulcers along with secondary manifestations (67). The cutaneous rash that is commonly associated with secondary syphilis has occasionally been reported to be atypical in HIV-infected patients (68, 69). The prevalence of neurosyphilis was shown to be higher in HIV-infected patients with low CD4+ counts than in HIV-negative individuals with syphilis (70, 71). Several reports of false-positive and false-negative results on serological tests for syphilis in HIV-infected patients might suggest that the specificity and sensitivity of these diagnostics methods could be altered in these individuals (72–77), although this is not a common finding. Furthermore, according to study by Ghanem et al., HIV-infected patients treated for syphilis may be at higher risk for serological failure (78). Nothing is known about the effect of concurrent HIV infection on the clinical manifestations or response to treatment of yaws and endemic syphilis, although HIV does exist in countries where the endemic treponematoses are found (79). Given the similarities between venereal syphilis and the endemic treponematoses, health care providers involved in diagnosis and treatment of these diseases should be aware that HIV infection might affect the manifestations, diagnosis, and treatment of yaws and bejel.
ON THE ORIGIN OF TREPONEMATOSES
Three main hypotheses currently address the origin, evolution, and global spread of syphilis and the endemic treponematoses: the Columbian, pre-Columbian, and Unitarian hypotheses.
The Columbian Hypothesis
The Columbian hypothesis is the most widely known and asserts that syphilis originated in North America and was brought to Europe by Columbus and his crew following their journey to the New World in 1492. This hypothesis finds its strongest advocates in paleopathologists who claim that the absence of skeletal evidence of syphilis infection in continental Europe prior to Columbus' journey excludes Europe as the site of origin of syphilis (80–83). Based on the analysis of skeletal remains of Homo erectus, for example, Rothschild et al. proposed that treponematoses originated in East Africa during the Pleistocene era (1.5 million years ago) in the form of yaws (83, 84). According to his hypothesis, the other two T. pallidum subspecies evolved from the yaws treponeme during its dissemination to Asia, where bejel first appeared, and then to North America, where approximately 8,000 years ago syphilis evolved from bejel (83). Reported cases of osseous signs of syphilis infection in the pre-Columbian Old World (85–87) have generally been met by “Columbianist” paleopathologists with skepticism regarding the accuracy of the diagnosis and epidemiological context (83), although some investigators find this evidence convincing of the existence of syphilis in the Old World before Columbus' time (88). In 1988, Baker and Armelagos contested the presence of specific signs of syphilis infection in the New World and proposed a variant of the Columbian hypothesis, according to which venereal syphilis quickly evolved in the Old World from a nonvenereal infection carried to Europe by Columbus' crew (89).
The Pre-Columbian Hypothesis
Supporters of the pre-Columbian hypothesis believe that syphilis existed in the Old World prior to 1493 but that it was misdiagnosed as leprosy or other illnesses (88, 90). With regard to disease evolution, Hackett, perhaps the most renowned “pre-Columbianist,” proposed that because of its longest period of infectiousness, pinta was the earliest of the known treponematoses to appear in Africa or Asia, possibly as a zoonotic infection. According to Hackett, pinta was estimated to have reached global distribution (with the exception of Europe and part of northern Asia) by ∼15,000 BC (91) but was subsequently isolated in the Americas as the result of the flooding of the Bering Strait. In parallel, the warmer and moister environmental conditions in Africa and Asia would have favored the emergence of yaws from pinta around 10,000 BC. Bejel developed approximately 3,000 years later (7,000 BC), to then spin off syphilis at around 3,000 BC in Southwest Asia (91). According to Hackett, syphilis reached North Africa and Europe between then and the first century BC but spread widely through the rest of the world as a consequence of the intensive European exploration and colonization initiatives of the 16th century, which were also responsible for dissemination of yaws to the Americas and part of Asia (91).
The Unitarian Hypothesis
Proposed by Hudson in 1963 on the basis of skeletal signs of disease, the Unitarian hypothesis states that all venereal and nonvenereal treponematoses are caused by the same pathogen and that the distinct clinical manifestations associated with these diseases are due primarily to differences in climatic conditions of the area where the disease is contracted and to social habits of the residing population (93, 94). According to Hudson, treponematoses originated in the humid and hot climate of sub-Saharan Africa during the Paleolithic era, primarily affecting children and being transmitted through skin-to-skin contact. In this area, the disease would manifest with the symptoms that we classically associate with yaws. Migrating populations carried the infection to drier areas, inducing the clinical manifestations to localize mainly in the moist body folds (e.g., mouth, armpits, and crotch), as typically seen in endemic syphilis. In the tropical areas of the Americas, the disease would again manifest as “yaws.” Since ∼4,000 BC, the improvements in personal and community hygiene that followed urbanization allowed more and more individuals to reach sexual maturity without prior exposure to treponemes, so that the sexual route became an important mode of transmission, resulting in the manifestations that we commonly associate with venereal syphilis (93, 94).
Despite being initially supported by the apparent lack of genetic and antigenic diversity among the human treponemes, the Unitarian hypothesis was progressively abandoned as genomic investigation of a limited number of existing strains permitted the identification of subspecies-specific genetic signatures (see also “Direct Detection Methods” below). It was hypothesized that these genetic differences among subspecies might be related to the different clinical manifestations of these diseases and support the classification of the T. pallidum subspecies into distinct groups (95–99). Phylogenetic analysis using genomic data only partially helped to solve the complex problem of the origin of treponematoses. In 2006, a study by Gray et al. (97) that evaluated the role of gene conversion in the evolution of six genes encoding putative virulence factors in T. pallidum subspecies, all members of the T. pallidum repeat (tpr) gene family, did not support a dramatically older origin of yaws compared to syphilis, as is required by Rothschild's theory (83). In this study, Gray et al. emphasized that their results were more consistent with Hackett's hypothesis of a nearly coincident evolution of these pathogens (∼12,000 years for the agent of pinta to evolve into the agent of syphilis), in contrast to the longer evolutionary pathway proposed by Rothschild (∼1.5 million years for the agent of yaws to evolve into the agent of syphilis). Furthermore, the analysis by Gray et al. suggested that the degree of genetic variability seen among T. pallidum subsp. pallidum strains was too great to support evolution of syphilis within the last ∼500 years (97), as proposed by the alternative Columbian hypothesis of Baker and Armelagos (89). This conclusion was also supported by de Melo et al., who, by combining the analysis of genomic data and osteological evidence of treponematoses prior to the European arrival to the New World, dated the appearance of syphilis between 16,500 and 5,000 years ago, though without providing any hypothesis on the place of origin (100). Although this finding was not in conflict with Rothschild's proposed timeline for syphilis appearance, de Melo et al. rejected Rothschild's hypothesis that the treponematoses as we know them today arose more than a million years ago in the Pleistocene and postulated that the treponematosis diagnosed in H. erectus (84) was caused by an ancestral treponemal pathogen (100).
Harper et al. reported a phylogenetic analysis based on 20 polymorphic regions in the T. pallidum genome (96). By combining genetic data and geographic information about the strains (including two yaws strains from Guyana that, however, were only partially sequenced because of nucleic acid degradation), the authors postulated that T. pallidum arose in the Old World as a nonvenereal disease similar to yaws and then spread to the Middle East and Eastern Europe, where it evolved into bejel, and then to the Americas as a New World yaws, with the last representing the progenitor of modern syphilis. T. pallidum's reintroduction into the Old World was attributed to the European explorations of the Americas (96). The theory of Harper et al., clearly more similar to Rothschild's, lacks evidence of the existence of venereal syphilis in the Dominican Republic (and the New World in general) at the time of Columbus' arrival, despite Rothschild's claimed certainty of it (102). The work by Harper et al. was criticized by Mulligan et al., who, upon reanalysis of Harper's published data, argued that no evolutionary order could be inferred, in that strains of all three subspecies branched at the same time from the tree's most basal branch (103). Furthermore, Mulligan et al. received with skepticism the hypothesis that syphilis evolved from New World yaws, given that this theory was based solely upon the homology of two single nucleotide polymorphisms (SNPs) in the degraded Guyana samples with the group of T. pallidum subsp. pallidum strains (103). The dilemma of the origin and evolution of treponematoses might find additional clarity when whole-genome sequences of T. pallidum strains are analyzed instead of subsets of genes or polymorphic genomic regions. The feasibility of this approach is becoming more concrete, given the accumulation of treponemal genome sequences made possible by modern high-throughput sequencing technologies.
Genetic Diversity and Pathogenesis of Human Treponematoses
Aside from establishing phylogenetic relationships among treponemal species and subspecies, the aid of comparative genomics was also sought to explain the lower degree of virulence associated with T. pallidum subsp. pertenue than with T. pallidum subsp. pallidum. Unlike syphilis, yaws is said not to be vertically transmitted or to affect the central nervous system, rather being limited to skin, joints, soft tissues, and bones. The very limited genetic diversity between these pathogens was already established in the 1980s, when hybridization experiments were performed using DNA from yaws and syphilis strains (1). The results of these experiments suggested that the degree of genomic diversity between these pathogens was lower than the limit of resolution of the technique (2% of genomic diversity), and this led to the reclassification of these pathogens into subspecies instead of distinct species, as they had been previously, based upon clinical manifestations and epidemiology (2). Antigenic (and therefore genetic) diversity between these pathogens was supported, however, by the evidence that experimental rabbit infection with a given pathogenic treponeme will induce protective immunity against reinfection with many strains of the homologous subspecies but not with a different treponemal species or subspecies (5). Although genetic signatures specific for yaws and syphilis treponemes have been described over the last 20 years (see “Direct Detection Methods” below) (99, 104–109), it is only recently that the genome sequences of three T. pallidum subsp. pertenue strains (Gauthier, CDC-2, and Samoa D) were determined and compared to the then-available genomes of T. pallidum subsp. pallidum strains (Nichols, DAL-1, SS14, and Chicago) (110). Soon, additional strains of T. pallidum subsp. pallidum (Mexico A and Sea 81-4) and T. pallidum subsp. endemicum (Bosnia A) will be available for extensive comparative analysis to improve our fragmentary knowledge on this aspect of human treponematoses (110–116; L. Giacani and A. Centurion-Lara [unpublished data] and B. Štaudová, L. Giacani, and D. Šmajs [unpublished data]).
The published work showed that genomes of yaws and syphilis treponemes share 99.8% overall identity, as well as identical organization and nearly identical sizes (110). This evidence induced Cejkova et al. (110) to propose that small genetic changes in key genes among these organisms could be responsible for the reported differences in disease pathogenesis. Compared to syphilis strains, “yaws-specific” nonsynonymous mutations (able to induce amino acid substitutions and/or major sequence changes in the coded protein) were identified in a total of 297 genes, 34 of which were predicted to code for proteins with 6 or more substitutions and/or major sequence changes (110). Among the annotated functional genes that showed the highest number of sequence changes between yaws and syphilis subspecies, interest was drawn by the following: the TPE0136 gene, reported to code in T. pallidum subsp. pallidum for an outer membrane protein (OMP) involved in attachment to the fibronectin component of the host extracellular matrix; the TPE0488 gene, known to code for a methyl-accepting chemotaxis protein (Mcp); and the recQ helicase gene, which is important in genome maintenance and repair. The TPE0326 gene, which encodes a treponemal β-barrel assembly machinery A (BamA) ortholog, and the arp gene, shown to contain a 60-bp repetitive sequence in its central region and to code for a fibronectin-binding protein, were also identified as worthy of further investigation (110, 117, 118). The arp gene had been previously reported by Harper et al. (95) to contain up to four different types of 60-bp repetitive sequences (named types I, II, II-III, and III) in T. pallidum subsp. pallidum isolates, while T. pallidum subsp. pertenue and T. pallidum subsp. endemicum carry only type I repeats, suggesting a role for this protein in disease pathogenesis.
Among the genes showing ≥6 amino acid substitutions and/or major sequence changes, eight of the 12 tpr genes (tprA, -C, -D, -F, -I, -J, -K, and -L) were found. The Tpr proteins represent candidate virulence factors that have been the focus of intense investigation since their discovery in the late 1980s. These antigens may play an essential role during infection, although their specific functions are yet to be determined. Several studies showed that the Tpr antigens are expressed during infection and are able to elicit marked humoral and cellular immune responses in the infected host (119–124). Of the encoded Tpr antigens, TprA, -B, -C, -D, -F, -I, -E, -J, and -K are predicted to be OMPs (123, 125, 126), and surface exposure of these antigens is supported by the evidence that anti-TprK immune sera, for example, can opsonize T. pallidum subsp. pallidum (125). Immunization experiments with recombinant TprK, TprC/TprD, and TprF/TprI peptides resulted in marked attenuation of lesion development following infectious challenge (121, 125, 127–129). TprK undergoes antigenic variation during infection, and newly arisen variants are selected by the adaptive host immune response as infection progresses, which is strongly suggestive of a role for this antigen in immune evasion (130–135). A recent detailed study by Centurion-Lara et al., highlighting the remarkable sequence diversity in the tpr gene family among and within T. pallidum species, subspecies, and strains, underscored the possible implications for the distinct clinical manifestations of human treponematoses (99).
Although the question of the contribution of genetic differences to the pathogenesis of the human treponematoses is far from being answered, additional comparative genomics studies are certainly warranted, particularly as T. pallidum subsp. endemicum genomes become available (Štaudová et al., unpublished data). Comparative genomics studies have already been performed to address the issue of host specificity among treponemal species and have focused on the comparison between T. pallidum subsp. pallidum strains and T. paraluiscuniculi, the causative agent of rabbit venereal syphilis (115, 136). T. paraluiscuniculi is reportedly not infectious to humans, despite its genetic relatedness to syphilis isolates (137, 138). Upon sequencing of the T. paraluiscuniculi (Cuniculi A strain) genome, hypotheses were drawn on the likely role of genome decay and pseudogene accumulation (compared to syphilis strains) in determining T. paraluiscuniculi's loss of infectivity for humans (115). Remarkably, in the Cuniculi A strain, five out of 10 tpr paralogs were found to be pseudogenes, again highlighting the likely importance of these putative virulence factors in host adaptation and disease pathogenesis (115, 139).
A Critical Assessment of the Unitarian Hypothesis
As mentioned above, the Unitarian hypothesis on the origin of treponematoses states that the agents of syphilis, yaws, bejel, and pinta are in reality the same microorganism and that differences in disease manifestations reflect climatic and social differences inherent to the region in which the disease is contracted (93, 94). Could the very low genomic diversity (∼0.2%) among the syphilis and yaws strains examined to date be used to argue that, indeed, these pathogens are the same? Overall, the same genomic studies that aimed to identify pathogenesis-related differences between yaws and syphilis isolates concluded that the classification of these treponemes into distinct subspecies is based on very subtle differences (110, 117). At the genomic level, even T. paraluiscuniculi differs by <2% from T. pallidum subsp. pallidum isolates, which could argue for its reclassification as a T. pallidum subspecies, despite its different host specificity (115).
Debates have focused on whether the modes of transmission and clinical manifestations that accompany yaws and syphilis are really different. Syphilis is said to be transmitted sexually, but many examples demonstrate that alternative routes of infection are also used by T. pallidum subsp. pallidum. Such examples, in the past century, include digital infection of dentists via direct contact with oral lesions of patients with syphilis (140), infection of the nipple in wet nurses via oral lesions in infected infants, and reports of infants and young children infected by syphilitic caregivers through close contact, common use of eating utensils, mouth-to-mouth transfer of prechewed food, or, in one case, contact with a contaminated object (i.e., a used condom found in a park). Although sexual abuse cannot be ruled out with absolute certainty, nonsexual routes of transmission were considered most likely in these cases (141–147). Yaws is generally transmitted by skin-to-skin contact during childhood, and infectious lesions usually resolve before sexual maturity; thus, sexual transmission might be possible but is not frequently described. That said, a review of “sibbens” in Scotland, which is thought to be very similar to yaws, contains many descriptions of lesions in many anatomical sites, including the genitals (148). The similarities between “sibbens” and syphilis lesions have been remarked upon since the late 1790s by Foderé (149), McBride (150), and Hibbert (151). Grin's monograph on endemic syphilis in Bosnia (51) reveals an infection that can be manifest as genital lesions resulting from sexual transmission, as well as being passed from one child to another through skin-to-skin contact or from mother to child via oral lesions. Further, Grin reported higher rates of second-trimester spontaneous abortion in women with bejel than in uninfected women. Although no analysis of fetal tissue samples or sera was performed to ascertain the presence of treponemes or antitreponemal antibodies in the study by Grin (51), this finding could be suggestive of transplacental transmission of bejel. As clearly stated by Mulligan et al., “mode of transmission appears to be defined by opportunity, rather than biology” (i.e., genomic differences between subspecies) (103).
With regard to clinical manifestations, it is well recognized that the lesions of primary and secondary syphilis can vary tremendously in size and appearance, from barely visible macular lesions to the numerous ulcers characteristic of “malignant syphilis.” Although the skin lesions of yaws are typically described as raised “berry-like” lesions, compared to the ulcer or maculo-papular rash most typical of syphilis infection, reports of less severe yaws lesions in patients living in drier climates can be found (37). Condylomata lata, which arise in moist body folds, are seen in endemic syphilis and yaws and are indistinguishable from venereal syphilis. These observations support the notion that lesion type is dependent upon the anatomical microclimate rather than the causative subspecies. Further, gummatous destruction of bone and cartilage is well recognized in the tertiary stages of all human treponematoses except pinta.
The most highly cited differences between venereal syphilis and the endemic treponematoses involve dissemination to the CNS and the fetus. It is commonly stated that only syphilis can lead to CNS involvement and congenital infection; however, numerous pieces of evidence for CNS, cardiovascular, and congenital infection in yaws were reviewed by Roman and Roman (43). As mentioned above, Grin (51) provided evidence for congenital infection in bejel. It is important to remember that the endemic treponematoses are found largely “at the end of the road,” where medical expertise is limited (152). Is it likely that the association between a childhood infection that is extremely common in some communities, and is one of many concurrent infections in this population, would be recognized as a cause of such rare sequelae as dementia many years later? Certainly, lumbar punctures for cerebrospinal fluid (CSF) examination would be unlikely to be conducted in these settings. Virtually all of our current clinical knowledge about the endemic treponematoses is a remnant of investigations conducted decades ago. Although there are ongoing yaws eradication programs today, there have been virtually no well-controlled studies of etiologically confirmed clinical manifestations or modern examinations for findings such as CSF abnormalities or visual and hearing loss, as are seen in some persons with syphilis in developed countries. Even in developed countries, CSF and more subtle neurological abnormalities are neither sought nor recognized in most syphilis patients.
An example of the imprecision of etiological assignment based on clinical manifestations is given by the Haiti B strain mentioned above, which was originally identified as a yaws strain based upon clinical manifestations and epidemiological factors (5) but was subsequently demonstrated to carry multiple genetic signatures consistent with T. pallidum subsp. pallidum, rather than T. pallidum subsp. pertenue (105, 108, 153). This finding clearly demonstrates that clinical characteristics do overlap among infections caused by the T. pallidum subspecies and cannot be strictly assigned to one subspecies. This “blending” of characteristics among the subspecies is also seen at the genomic level, with the Mexico A strain as an example. This strain was isolated from a typical primary chancre in 1953 (5), and it was thus thought to be a T. pallidum subsp. pallidum strain. We had shown that this strain (along with another T. pallidum subsp. pallidum strain, Sea 81-3) has a tprC (TP0117) molecular signature in common with T. pallidum subsp. endemicum strains (109) and unlike that in other T. pallidum subsp. pallidum and T. pallidum subsp. pertenue strains. More recently, we showed that the Mexico A strain shares a tprG locus (TP0317) hybrid gene with T. pallidum subsp. pertenue, compared to the other subspecies (99). Following the elucidation of the T. pallidum subsp. pallidum Mexico A strain genome, Pětrošová et al. noted two other genes, TPaMA0326 (tp92) and TPaMA0488 (mcp2-1), which combine T. pallidum subsp. pallidum- and T. pallidum subsp. pertenue-specific nucleotide sequences (114). The observed mosaic character of these genes prompted them to examine a series of circumstances that might have resulted in the appearance of such gene sequences, and the authors suggested that these hybrid loci are likely the result of interstrain horizontal gene transfer between T. pallidum subsp. pallidum and T. pallidum subsp. pertenue strains during concurrent infection of a single host (114). DNA acquisition by lateral gene transfer (LGT) is an important mechanism for natural variation among prokaryotes, and recent advances in the phylogenetic analysis of microbial genomes reveals a substantial impact of LGT during microbial genome evolution (154, 155). However, remarkable physical and genomic barriers exist to restrict DNA transfer between cells and its integration into the recipient's chromosome, thus limiting the acquisition of new genes (156, 157). To date there are still not enough data to support any of the four known prokaryotic LGT mechanisms (conjugation, transduction, gene transfer agents, and transformation) (156, 158–160) in pathogenic treponemes.
The recent evidence presented by Centurion-Lara et al. (99) that there is significant variability in some tpr gene sequences among strains of the same subspecies and, conversely, that some unusual “hybrid” tpr alleles can be found in multiple subspecies discloses a genetic elasticity that is inconsistent with the existing nomenclature. As more strains are examined, more “exceptions to the rule” are identified. Accumulating evidence suggests that, rather than belonging to discrete categories of pathogens, the agents of human treponematoses might be placed in a single group, comprising agents able to cause disease with a broad range of clinical manifestations distributed over a conceptual continuum, from dermal lesions alone to destructive multiorgan involvement. This kind of disease spectrum has long been recognized in syphilis (“the great imitator”), and the inclusion of the “nonvenereal” treponematoses within that spectrum is possible. The clinical outcome in an infected individual may be influenced by a combination of the specific genetic traits of the infectious strain, coupled with genetic and other factors of the host, including mutations in immune response genes, age at infection, mode of transmission, climate, and concurrent infections. Although this is highly speculative, we do not exclude that in the future, following the elucidation of a significantly higher number of treponemal genomes (including those of T. carateum strains), we might be able to locate each individual strain in a more precise position in such a continuum of disease.
In conclusion, it appears to some that the clinical, epidemiological, and even genomic criteria used to differentiate the agents of yaws and syphilis are rather arguable and that perhaps Hudson's Unitarian hypothesis was too quickly dismissed in the excitement that followed the advent of the treponemal genomic era in 1998 (111). We will leave it to individual readers to ponder whether the evidence above warrants a reconsideration of the Unitarian hypothesis.
DIAGNOSTIC TOOLS FOR ENDEMIC TREPONEMATOSES
In the absence of laboratory confirmation, the diagnosis of syphilis, yaws, bejel, or pinta has traditionally been based upon the careful analysis of the patient's symptoms and signs, in combination with knowledge of the epidemiological context of human treponematoses. In the laboratory, the available diagnostic tools for endemic treponematoses and venereal syphilis are limited to two generic categories: direct detection of treponemes in biological specimens (including molecular assays) and serological tests. Although serology remains the most common diagnostic method for treponemal infection, none of the available serological tests is able to differentiate among the agents of these diseases. This ability is currently limited to molecular methods that target genetic signatures thought to be specific to each subspecies. The practical utility of most of these diagnostic tests is further complicated by the lack of advanced laboratory services in the regions where the endemic treponematoses are most common. A brief overview of the diagnostic tools available for human treponematoses is given below, considering their applicability in resource-limited settings.
Serological Tests
Serological tests for syphilis (and the endemic treponematoses) are divided into so-called “nontreponemal” and treponemal tests, based on the antigens recognized during the reaction (161). Alone, neither type of test is considered to be sufficient for diagnosis, and the use of a nontreponemal test (for screening purposes) needs to be combined with a treponemal test to confirm the initial result. Nontreponemal tests use a mixture of lipid antigens to detect antibodies present in sera of patients with treponemal infections. The most common nontreponemal tests include rapid plasma reagin (RPR) and, decreasingly, the Venereal Disease Research Laboratory (VDRL) test. Both are flocculation tests whose results are read with the aid of a microscope (VDRL) or with the naked eye (RPR). Although these tests are inexpensive and widely available, their limitations include false-positive reactions (generally associated with increased age, unrelated infections, or autoimmune disorders) (161, 162), as well as false-negative results in very early disease, and the possibility of prozone reaction (161, 163). Quantitation of these tests (by evaluating serum dilutions to determine a reactivity endpoint) permits determination of an antibody titer to monitor treatment response: a 4-fold decrease in antibody titer following therapy indicates successful treatment, while a 4-fold rise in serum titer is usually associated with reinfection or treatment failure (161).
Treponemal tests are based on antibody reactivity toward native or recombinant T. pallidum subsp. pallidum antigens and are generally used in the United States and in many other countries to confirm a reactive nontreponemal test. However, because treponemal tests may remain reactive for the patient's lifetime, even following treatment, they are not recommended to evaluate response to therapy and cannot distinguish between active and past treated infection. Traditional treponemal tests include indirect immunofluorescence assays such as the fluorescent treponemal antibody-absorbed test (FTA-ABS) (161, 164), agglutination assays (e.g., T. pallidum hemagglutination assay [TPHA] and T. pallidum particle agglutination [TPPA]) (165–168), Western and dot blot assays with native and recombinant antigens (169, 170), and the more recent automated enzyme immunoassays (EIAs) (171), chemiluminescent immunoassays (CIAs) (172, 173), and multiplex flow immunoassay (MFI) (174). Performing both treponemal and nontreponemal tests in a resource-limited environment is challenging, due to the need for venipuncture, equipment for storage of reagents and sample processing, and trained personnel to conduct and interpret test results. Nonetheless, some modification of nontreponemal tests have made their use in resource limited settings easier (e.g., use of plasma versus serum, heat-stable antigens) (175, 176). Using a reference laboratory, these tests were used to evaluate the impact of mass treatment campaigns against yaws in the late 1980s by Autier et al. (177). These investigators reported that in the Sahelian zone of Mali, 16% of children were reactive when tested by quantitative VDRL (serum dilution, ≥1:8), while 18.7% were TPHA positive prior to mass treatment. One year after the intervention, the percentage of children reactive in the quantitative VDRL (≥1:8) had decreased to 5.7%, with no difference in the percentage of children reactive by TPHA (177).
Recently, several “user-friendly” Treponema-specific rapid tests or point-of-care (POC) tests have become available (178, 179). These tests generally consist of immunochromatographic nitrocellulose strips that carry one or more T. pallidum subsp. pallidum recombinant antigens mounted on a plastic microfluidic cassette. Following binding of antigen, antibodies in the patient's serum are revealed by dye-labeled anti-immunoglobulins, which trigger the appearance of a dark line on the nitrocellulose. Unlike the tests previously described, POC tests require minimal equipment and training and can be performed using microliters of whole blood (generally from a fingerstick) as well as serum or plasma, and the results can be visualized within minutes. A WHO study commissioned to compare the sensitivity and specificity for syphilis of eight point-of care tests with TPPA and TPHA as reference standards reported sensitivities ranging between 84.5 and 97.7% and specificities of 92 to 98% (178), although lower sensitivity was found for whole blood than for the serum or plasma fractions and when tests were performed in the field rather than in the laboratory (179–181). Despite their lower specificity compared to standard treponemal tests, the use of POC tests will confer an advantage over standard tests for hard-to-reach populations and where lab facilities are not available. However, as for all treponemal tests, POC treponemal tests cannot discriminate between treated or untreated disease, and unless a nontreponemal test is performed, unnecessary treatment may occur. Recently, a dual POC test for syphilis was developed for simultaneous detection of antibodies against treponemal and nontreponemal antigens. Castro et al., upon evaluating their sensitivity and specificity in comparison to those of TPPA and RPR, concluded that dual tests could be used for both screening and confirmatory purposes for the diagnosis of syphilis (and presumably of endemic treponematoses) in resource-poor settings (182, 183). Furthermore, a study performed with the same rapid dual test on patients with syphilis in China showed that test sensitivity and specificity are not affected by the nature of the clinical specimen (whole blood, plasma, or serum) or by testing outside traditional clinical settings (184). This dual POC test is currently being evaluated in a mass treatment program for yaws in Papua New Guinea (O. Mitja, personal communication).
Despite those remarkable improvements, the goal of a serological test able to differentiate between the agents of syphilis and the endemic treponematoses is yet to come. Prior to the advent of comparative genomics, the lack of significant antigenic differences among T. pallidum subsp. pallidum and other pathogenic treponemes was mainly supported by immunoblot studies (185–188), by unsuccessful efforts to identify subspecies-specific epitopes using synthetic peptides (189), and by the inability to produce subspecies-specific monoclonal antibodies (190–192). Most of these studies agreed on the existence of only very subtle antigenic differences among these pathogens. Baker-Zander and Lukehart (185), for example, probed Western blot antigen strips prepared with whole T. pallidum subsp. pallidum and T. pallidum subsp. pertenue with sera from syphilis- and yaws-infected rabbits. Although electrophoretic separation of the pathogens' proteins revealed differences in the banding pattern between the agent of yaws and syphilis, because these differences were detected with antisera raised against either pathogen, the authors concluded that these molecules must contain antigenic determinants shared by the two subspecies of treponemes. A study by Thornburg and Baseman (186), contemporary to that of Baker-Zander and Lukehart, aimed to compare protein profiles of T. pallidum subsp. pallidum and T. pallidum subsp. pertenue using one- and also two-dimensional gel electrophoresis (2D-GE), radioimmunoprecipitation (RIP), and surface iodination. In this study, no differences between the subspecies' antigens could be appreciated using Western blots or RIP, even though comparison of 2D protein profiles revealed differences in few minor (i.e., poorly expressed) treponemal molecules, some of which are more highly expressed in T. pallidum subsp. pallidum than in T. pallidum subsp. pertenue but also vice versa. Surface iodination experiments showed differences in the high-molecular-mass proteins (95 to 50 kDa), with shared antigens generally more strongly labeled in T. pallidum subsp. pallidum than in T. pallidum subsp. pertenue. Interestingly, Thornburg and Baseman could not help but conclude their work by saying that the classically accepted differences in the clinical manifestations of syphilis and yaws “are far overshadowed by the extensive biological, chemical, and immunological similarities between these treponemes” and suggesting that the importance of host and environmental factors in determining these different disease phenotypes should not be underestimated (186). Fohn et al. (188) examined the specificity of antibodies from pinta patients for T. pallidum subsp. pallidum antigens, highlighting a very high degree of antigenic relatedness between the two treponemal species. These studies also suggested that in pinta patients, development of humoral responses against T. carateum antigens seems to follow a pattern similar to that in patients with syphilis, where increasing antibody reactivity against T. pallidum subsp. pallidum antigens is seen with duration of infection in patients with active early disease and reactivity decreases with longer duration of the latent stage (193–195). This evidence suggested that similar antigens might be expressed in similar ways by these two pathogens during infection.
In recent work that focuses on the comparative analysis of the tpr gene sequences among treponemal species, subspecies, and strains, however, Centurion-Lara et al. presented evidence that the tprL gene sequences of the analyzed T. pallidum subsp. pallidum and T. pallidum subsp. endemicum strains differ from those of the T. pallidum subsp. pertenue strains (99). According to Centurion-Lara et al., a tprL gene of 1,806 bp is present in T. pallidum subsp. pallidum and T. pallidum subsp. endemicum strains, while a deletion of 278 bp creates an alternative gene start site for tprL in T. pallidum subsp. pertenue, resulting in a shorter gene of 1,668 bp. Such differences in coding sequences yield two different predicted proteins: a shorter T. pallidum subsp. pertenue TprL variant, with a 44-amino-acid unique amino terminus, and a longer TprL antigen in the remaining species/subspecies with 90 unique amino acids in the mature TprL protein (including the 25-amino-acid predicted signal peptide) (99). This finding is currently being evaluated in our laboratory by T. Brinck et al. (unpublished data) for its potential to be developed into a serological test that can differentiate between yaws, and syphilis or bejel infection.
Direct Detection Methods
Dark-field (DF) microscopy and the rabbit infectivity test (RIT) are historically the best-recognized methods for direct identification of treponemes in biological specimens. Lesion exudates contain elevated number of spirochetes that can be visualized under a DF microscope but not under a conventional light microscope because of the spirochetes' small cellular diameter (0.2 μm). DF microscopy is generally useful in patients with active lesions, particularly in those who have not yet developed an immune response against the disease, which makes serological diagnosis premature. This method, however, needs to be performed immediately after specimen collection, and its limited sensitivity in healing lesions may result in false-negative outcomes (161). In the mid-1980s, an alternative to the use of DF microscopy was offered by the employment of fluorescently labeled monoclonal antibodies to detect the syphilis agent in lesion exudates (196–198). This approach was shown to be equal to DF microscopy in sensitivity and specificity for the diagnosis of early syphilis, and although it could theoretically relieve health care personnel from the need to perform DF microscopy, it would necessarily require the availability of an epifluorescence microscope. These monoclonal antibodies, however, could not differentiate the T. pallidum subspecies.
The RIT takes advantage of the rabbit's (Oryctolagus cuniculus) high susceptibility to infection with T. pallidum subspecies. Biological material suspected to contain treponemes is injected intratesticularly into the rabbit, and the animal is monitored for seroconversion over a 3-month period. Although seroconversion is sufficient to prove the presence of infectious spirochetes in the original sample, tissue may also be obtained for direct identification of treponemes using DF microscopy or molecular methods (161). The need for injecting the specimen into a recipient rabbit within 1 h of collection, the time necessary to complete the test, the need for an animal facility, and the costs of animal use and husbandry all make this test impractical for diagnosis, even in modern laboratory settings. RIT is therefore used today as a research tool to assess the efficacy of antibiotic treatments, to determine the effectiveness of vaccine candidates, and to isolate new treponemal strains from clinical samples. It should be noted, nonetheless, that RIT sensitivity is comparable to that of molecular detection methods (199, 200).
A remarkable number of amplification-based methods have been developed over the last 2 decades for detection of T. pallidum subsp. pallidum nucleic acids in biological specimens, even though none of them is currently commercially available (201–205). These tests generally employ qualitative or quantitative PCR alone or PCR followed by amplicon detection with a labeled probe to increase the sensitivity and specificity of the assay. Although designed to diagnose syphilis infection, many of these tests were also reported to be equally efficacious in detecting T. pallidum subsp. pertenue DNA. The analysis of the available T. pallidum and non-T. pallidum strain genomes (98, 110–112, 114) confirmed that these methods could also be applied to detect nucleic acids from all of these treponemal strains, but without differentiating them. Examples of molecular tests include the amplification of the tmpA (TP0768) and the 4D genes (also known as tpf-1 [TP1038]), used by Hay et al. (201, 207) or the amplification of the bmp gene (TP1016) used by Noordhoek et al. (202). The use of qualitative or quantitative PCR targeting the 47-kDa lipoprotein (TP0574) gene was also evaluated to diagnose syphilis (199, 203, 208–213). Since the early 2000s, the T. pallidum subsp. pallidum polA gene (TP0105) has been widely used as a target for detection of the syphilis agent according to the protocols of Liu et al. (204) and Behrhorf et al. (214). Several examples of quantitative amplification tests targeting polA are also available (215–217). An alternative approach to DNA detection was proposed in 1997 by Centurion-Lara et al., who developed a reverse transcriptase PCR (RT-PCR) assay followed by probe hybridization to detect T. pallidum subsp. pallidum 16S rRNA, with the rationale that multiple copies of the 16S rRNA per treponemal cell would increase the sensitivity of the assay compared to that of amplification-based methods targeting single-copy genes in the pathogen's genome (205).
Multiple efforts have been made to identify genetic signatures specific for each of the agents of human treponematoses to use in differential molecular diagnosis. In the pregenomics era, Noordhoek et al. were the first to identify a single nucleotide polymorphism (SNP) within the TP1038 gene that was thought to differentiate between syphilis and yaws strains (104), although this signature was later shown not to be a specific trait for either subspecies (153). In 1998, Centurion-Lara et al. reported the use of a restriction fragment length polymorphism (RFLP) assay based on a single nucleotide difference in the 5′ flanking region of the TP0170 gene (encoding the 15-kDa membrane lipoprotein) to differentiate T. pallidum subsp. pallidum from the remaining subspecies (105), but this was unable to discriminate between the agents of yaws and bejel. A similar RFLP-based method, also lacking discriminatory power for the agents of endemic treponematoses, was developed by Cameron et al. using an SNP within the glycerophosphodiester phosphodiesterase enzyme-coding gene (gpd [TP0257]) (107). In the decade that followed the above findings, the accumulation of genetic information on the agents of human treponematoses permitted development of a molecular method capable of differentiating among the available syphilis, yaws, and bejel strains. This method relies on the analysis of the tprC (TP0117) and tprI (TP0620) genes (109). As mentioned, tprI and tprC are members of the T. pallidum repeat (tpr) family of genes, a group of 12 paralogs (named tprA to -L) that became the object of intensive investigation following their discovery (111, 125). Most importantly, evidence that polymorphic tprC, -D, -G, -J, -A, and -L alleles could be found among T. pallidum subsp. pallidum strains (99, 106, 121–123, 218) prompted extension of the investigations of these genes to subspecies other than T. pallidum subsp. pallidum. In 2006, Centurion-Lara et al. showed, using two subsequent RFLP assays targeting differences located within the tprI and tprC genes, respectively, that the three T. pallidum subspecies can be distinguished (109). More recently, Pillay et al. reported the use of the RFLP analysis of the tprI gene by Centurion-Lara et al. in combination with sequencing of the fliG-hlyB intergenic region 19 (IGR19) to identify T. pallidum subsp. pertenue-specific sequences in a patient with active yaws (219), while Harper et al. used the sequence variation in the arp gene (TP0433) to perform a relatively straightforward differentiation of T. pallidum subsp. pallidum from nonvenereal subspecies (95, 220). A nice example of the clinical use of molecular tools to diagnose an imported case of endemic syphilis in Canada is represented by the recent work of Fanella et al. They first targeted bmp, polA, and TP0574 by PCR to detect treponemal DNA in a sample harvested from a patient's oral lesion and subsequently used both arp sequencing and the tprC/tprI RFLP assays to identify the etiological agent as T. pallidum subsp. endemicum (221).
Performing these molecular identification assays is limited to laboratories equipped with the appropriate instruments, resources, and trained personnel. An additional limitation of molecular testing for the treponematoses is that the sensitivity of DNA detection in a biological sample varies by the type of sample analyzed (e.g., lesion exudates versus blood samples) and, likely, the fluctuations in the bacterial burden in the host that accompany these multistage diseases (211). Furthermore, only a limited number of yaws and bejel strains has been analyzed using these methods, and no definitive proof of the stability of these signatures among subspecies is currently available. Despite the fact that the diagnosis of endemic treponematoses in resource-limited settings will continue to rely on clinical evaluation and serological tests rather than molecular assays for the time being, more comparative genomics studies of the agents of human treponematoses are highly warranted, in that they hold the potential for the identification of genetic signatures that might improve molecular and serological assays to differentiate among the T. pallidum subspecies. In any case, even in the absence of differential diagnosis, any ascertained case of treponematosis should be promptly treated to avoid the possibility of severe sequelae.
A molecular test of pivotal importance for those involved in the diagnosis and treatment of human treponemal infections is represented by the assay to detect the point mutations in the 23S rRNA genes known to confer resistance to azithromycin (and macrolides in general), the antibiotic that WHO is planning to use in the new eradication campaign for yaws and endemic treponematoses (discussed in more detail below). Conventionally, detection of the two known mutations associated with macrolide resistance in T. pallidum subsp. pallidum relies on a nested PCR approach followed by RFLP and DNA visualization after agarose gel electrophoresis (222). As this approach is time-consuming and labor-intensive, development of a faster (but equally sensitive and specific) test that can help assess macrolide susceptibility among T. pallidum strains is definitely desirable, even though these assays will still need to be performed in selected reference laboratories. Toward this end, a real-time PCR assay using fluorescence resonance energy transfer (FRET) probes and melting curve analysis was developed for one of the two known point mutations (223), while a real-time multiplex PCR assay for detection of both mutations has been recently published by Chen et al. (224).
Differential Diagnosis of Endemic Treponematoses from Other Diseases
The clinical manifestations associated with the endemic treponematoses may also need to be differentiated from those of a variety of other diseases. Illnesses that might hinder accurate the diagnosis of early yaws include impetigo (caused by Staphylococcus aureus), ecthyma (a pyogenic disease of the skin caused by Pseudomonas species, Streptococcus pyogenes, and Staphylococcus aureus), leprosy, cutaneous leishmaniasis, scabies, molluscum contagiosum, planar warts, tropical ulcers (a polymicrobial infection), tungiasis (infection with the parasite Tunga penetrans), chromomycosis (fungal infections caused by members of the family Dematiaceae), sarcoidosis, and psoriasis. Extragenital lesions caused by Haemophilus ducreyi have been reported from the Western Pacific (338–340). Bone lesions can resemble those of syphilis and bejel, tuberculosis, African histoplasmosis (caused by Histoplasma duboisii), bacterial osteomyelitis (caused by pyogenic bacteria or mycobacteria), and sickle cell anemia (29, 225). Rhinopharyngeal lesions may resemble those of rhinosporidiosis (an infection caused by Rhinosporidium seeberi), rhinoscleroma (granulomatous lesions on the nose caused by Klebsiella pneumoniae subsp. rhinoscleromatis), mucocutaneous leishmaniasis, tuberculosis, and leprosy (29, 225).
Early skin manifestations of bejel can be confused with those of other dermatoses such as psoriasis, pityriasis rosea, and also eczema, herpes simplex, lichen planus, leprosy, mycoses, condylomata acuminata, perlèche (angular cheilitis), aphthae, and vitamin deficiencies (25, 225). Destructive lesions of the nasopharynx seen in late yaws and bejel can also be associated with cutaneous leishmaniasis, rhinoscleroma, histoplasmosis (caused by the fungus Histoplasma capsulatum), tuberculosis, and rhinosporidiosis (25, 58, 225, 226). Late-stage bejel can be reminiscent of malignancies (such as squamous cell carcinoma) but also lupus vulgaris, bromoderma tuberosum, iododerma, and facial granuloma (225).
Pinta skin manifestations also need to be distinguished from vitiligo, pityriasis versicolor, pityriasis alba, chloasma, discoid lupus erythematous, and erythema dyschromicum perstans, all diseases characterized by changes in pigmentation. Furthermore, diagnosis could be difficult in the presence of the dermatophytosis tinea corporis, pellagra, eczema, psoriasis, and leprosy (25, 225, 226).
PAST ERADICATION PROGRAMS
First WHO-UNICEF Control Program
When the World Health Organization (WHO) was established in 1948, endemic treponematoses were among the first public health problems addressed by the new agency, together with malaria, tuberculosis, and venereal infections (227). At that time, it was estimated that there were about 50 million cases of yaws worldwide (although some reports estimated up to 150 million cases) (32), possibly a million endemic syphilis cases, and a lesser number of pinta cases, while about 20 million individuals globally were thought to suffer from venereal syphilis (228). Venereal and nonvenereal treponematoses were considered public health problems also because of the significant economic losses in terms of manpower resulting from disease-associated disability in both industrialized countries (from syphilis) and agricultural tropical countries (mostly from yaws) (228). Based on the resolution adopted during the second World Health Assembly in 1949, WHO in collaboration with the United Nations International Children's Fund (UNICEF) led a worldwide campaign to control and eventually eradicate yaws and the endemic treponematoses (228). As the result of this unprecedented effort, vertical control programs were brought to 46 developing countries (229, 230) with the goals of screening the entire target population, treating individuals with evidence of active and latent infection with long-acting penicillin, treating family and community contacts, and implementing periodic surveys to identify missed, new, and imported cases. Between 1952 and 1964 approximately 160 million individuals underwent initial surveys, and over 50 million were treated. About 300 million follow-up examinations were conducted during resurveys. By the end of the campaign, the global burden of cases of endemic treponematoses was estimated to be reduced by 95% (229, 230), to a mere 2.5 million cases. The WHO-UNICEF control campaigns were also key to establishing or strengthening primary health care systems in the affected areas. Unfortunately, these remarkable achievements also induced WHO to gradually dismantle the targeted control programs in favor of their integration into the newly established primary care systems, confident that these would suffice to identify and treat the remaining 5% of cases. In the end, the waning of commitment and resources led to the resurgence of the endemic treponematoses in many countries and to the necessity for new control efforts and strategies (231).
Necessity for Renewed Control Efforts and Strategies in the 1970s and 1980s
Following the adoption of a new resolution on control of endemic treponematoses in 1978 (232), new programs were implemented in the 1980s in seven western African countries (Benin, Burkina Faso, Côte d'Ivoire, Ghana, Mali, Niger, and Togo). Unfortunately, the results were disappointing, mostly due to lack of resources and weak commitment from local governments (233). Not even the organization of an international symposium on yaws and endemic treponematoses, with multiple regional meetings, helped to revive the global interest in control and eradication of these diseases (32). In 1995, according to the last official WHO estimate, approximately 2.5 million cases of endemic treponematoses (mostly yaws) still remained worldwide. Of these cases, 460,000 were estimated to be infectious, mostly localized in western and central Africa (400,000 cases), Southeast Asia (50,000 cases), and other tropical regions (10,000 cases) (234).
CURRENT EPIDEMIOLOGICAL SITUATION OF YAWS, BEJEL, AND PINTA
Yaws
With the exception of the Southeast Asia Region of WHO, which has kept yaws control a priority in its public health agenda with an eradication goal originally set for 2012, surveillance and reporting of yaws cases have been sporadic. In 1993, Louis et al. reported that indigenous populations in Central African Republic were infected with yaws, and they emphasized the risk for the spreading of the disease among settled communities because of the trend of these people to abandon their nomadic life style (24). Similar findings were reported in 1992 by Hervé et al. (235). In 1994, Edorh et al. reported about 5,000 annual yaws cases among children in Togo (236), and a yaws focus was reported in northeastern Nigeria a few years later by Akogun (237), despite the official position that yaws had been eradicated in Nigeria. A more recent study by Nnoruka, however, failed to identify any yaws-affected individuals among 2,871 consecutive patients at the dermatology clinic of the University of Nigeria in Enugu (approximately 500 miles from the more rural area of Garkida, where Akogun [237] conducted his research) (238). In this study, however, most of the patients were adults, diagnosis was based on the patient's history and physical examination, and no tests to specifically identify treponemes or treponemal infection were performed (238). In 2007 Touré et al. reported that yaws prevalence was 5 per thousand inhabitants in Côte d'Ivoire, with only 27% of diagnosed patients with a history of medical treatment at the time of the study (239). Between 2008 and 2011, seven African countries have reported cases of yaws (Table 2), with the majority of cases reported in Ghana and Côte d'Ivoire (20,525, and 3,704, respectively) (240). Although it is known to be a country where yaws is endemic (240), no data were available for Benin between 2008 and 2011. More recently, however, data on yaws prevalence limited to school children of the Toffo, Allada, and ZE health districts of Benin (covering approximately 1.4% of the country area) were presented at the WHO consultation on yaws eradication in March 2013 in Geneva, Switzerland, showing prevalences of clinical and serological yaws of 4.11%, 1.77%, and 1.38% for the three regions, respectively (courtesy of Gilbert Ayelo, Centre de Dépistage et de Traitement de l'Ulcère de Buruli d'Allada, Benin).
TABLE 2.
Countries with available information on yaws, 2008 to 2011a
| WHO region and country | Yr of report | No. of reported cases |
|---|---|---|
| WHO African Region | ||
| Beninb | NAc | NDd |
| Cameroon | 2010 | 789 |
| Central African Republic | 2008 | 243 |
| Côte d'Ivoire | 2010 | 3,704 |
| Democratic Republic of the Congo | 2011 | 383 |
| Ghana | 2010 | 20,525 |
| Republic of the Congo | 2011 | 167 |
| Togo | 2010 | 15 |
| WHO Southeast Asia Region | ||
| Indonesia | 2011 | 5,319 |
| Timor-Lesteb | NA | ND |
| WHO Western Pacific Region | ||
| Papua New Guinea | 2011 | 34,628 |
| Solomon Islands | 2010 | 20,635 |
| Vanuatu | 2010 | 1,574 |
Adapted from http://www.who.int/yaws/epidemiology/en/.
Country where yaws is known to be endemic but with no data available between 2008 and 2011.
NA, not applicable.
ND, no data.
In the Western Pacific Region of WHO, yaws was highly prevalent prior to mass treatment campaigns carried out in the 1950s and 1960s (241). Following mass treatment, however, the number of reported cases declined significantly, to the point that yaws was considered eliminated in most areas (242, 243). Since the late 1970s, however, reports of suspected cases of yaws from several areas of Papua New Guinea (244–250), the Solomon Islands (242, 251–253), and Vanuatu (254, 255) started to appear in the literature, suggesting that elimination was not achieved. In 2011, Papua New Guinea reported 34,628 yaws cases, followed by the Solomon Islands (20,635 cases in 2010) and Vanuatu (1,574 cases in 2010) (240) (Table 2).
There is no recent information on the epidemiological situation of yaws in the WHO Region of the Americas, where since 1977 this disease has no longer been identified as a public health problem (256). As always, it is not known whether a lack of reported cases is the result of true disease elimination or lack of surveillance. In Ecuador, for example, no clinical cases were detected between 1993 and 1998, suggesting elimination of yaws (257, 258). In Guyana, however, following the implementation of a control program in 2000, a resurvey conducted the following year showed a residual prevalence of yaws skin lesions of 1.6% (259).
In the Southeast Asia Region, Indonesia reported 5,319 cases in 2011, and no data are available from Timor-Leste (240), although this is known to be a country where yaws is endemic (Table 2). Great success was achieved in India, where no cases of yaws have been reported since 2003 and elimination was officially declared on 19 September 2006 (260). This success is the result of an active government-backed elimination program launched in 1996 and based on active case finding with treatment of infectious and latent cases and contacts with benzathine penicillin (260). In India, toward the final stage of the campaign, a cash reward system was established to encourage voluntary reporting of any suspected case. The equivalent of US$100 was given to health workers for a confirmed case and approximately US$10 to the first person reporting the case to the health authorities (240). The successful Indian elimination campaign showed the world that yaws prevalence in a defined area can be brought to zero through deliberate efforts and demonstrated that yaws eradication (i.e., the reduction to zero of yaws prevalence on a global scale) is an attainable goal if sufficient resources and political commitment are available. It is currently estimated that at least 100 million children are at risk of acquiring yaws globally (25, 261).
Bejel (Endemic Syphilis)
Bejel was previously endemic in northern Europe, in the Balkans, Russia, Mongolia, the Near East and eastern Mediterranean, and in southern and northern Africa among poor rural populations. Social and economic improvements in many of these areas resulted in a natural recession of the disease, even prior to the advent of mass treatment campaigns. Nonetheless, foci of endemic syphilis have been reported by several investigators in the last 2 decades, mostly in the Near East and Sahelian Africa. Csonka and Pace reported bejel cases among the nomadic communities living in rural Saudi Arabia (52, 56, 58). A clinical and serological survey for endemic syphilis carried out by Gazin and Meynard in 1986 in Burkina Faso revealed that 7.5% of 5- to 14-year-old children had clinical lesions and 22% had anti-Treponema antibodies (262). A similar survey carried out 5 years earlier in the same region showed very similar results. In 1989, the prevalence of endemic syphilis in the Sahelian area of Mali was reported to equal that prior to the eradication campaigns of the 1950s and 1960s (6% and 19% of 5- to 14-year-old children had lesions and anti-Treponema antibodies, respectively) (177). In the 1990s, cases of bejel were reported in Sahelo-Sudanian Niger and Mauritania (263, 264). In Niger approximately 12% of the children under 5 years had serological evidence of Treponema infection (263), while in Mauritania a focus of bejel was identified by Galoo and Shmoor (264). Previously, in Mauritania, a survey to assess frequency and distribution of serological evidence of treponemal infection examined 6,049 subjects between 6 and 18 years old, showing that 33.4% of the screened individuals were serologically reactive in treponemal tests. Of these 6,049 patients, 102 showed clinical evidence of bejel infection (266). It is notable that although bejel is diagnosed in the drier countries of Burkina Faso, Mali, Niger, and Mauritania, these countries share borders with Côte d'Ivoire, Ghana, Togo, and Benin, where yaws is known to be endemic. Are the etiological agents actually different, or are the different clinical manifestations due to regional climatic conditions?
In Turkey, where no cases of bejel had been reported for more than 3 decades, the disease was diagnosed in 1995 in three children and their father in Malatya (265). In 2006, a case report described two cases of bejel in children in Maputo, Mozambique (267), although a clear differential diagnosis from yaws could not be made. The most recent published case report (2013) of bejel is from southwest Iran (268).
Pinta
In former years, Colombia and Mexico were the countries in the world where pinta was most highly endemic. Today this disease is believed to exist only in very circumscribed parts of Latin America, although surveillance is meager at best. Known recent foci are represented by the Tikuna tribes living in the Amazon Trapeze, a land corridor between the territories of Colombia, Brazil, and Peru, and the inhabitants of a Panamanian village screened for clinical and serological evidence of infection (188, 269–271). In Mexico, a recently reported case of secondary syphilis was questioned by Italian physicians who argued with the initial diagnosis, claiming that the reported findings were more consistent with the clinical and histological signs of infection with T. carateum rather than T. pallidum subsp. pallidum (272, 273). In 1999, an early pinta lesion was identified in a female resident of Cuba who was visiting Austria, although the last case of pinta in Cuba was reported in 1975 (274).
Imported Cases of Endemic Treponematoses
In 1989, early yaws was diagnosed in the Netherlands in a 9-year-old girl from Ghana who had immigrated 6 months earlier (275). It was assumed that the patient contracted yaws shortly before leaving her native country. This case, which mirrored the one described in 1966 by Fry and Rodin in England (276), suggests that health care providers should still be aware of possible encounters with yaws cases in immigrants from regions of endemicity. In 1999, Vabres et al. reported a case of secondary bejel in a 5-year-old patient from Mali with painless stomatitis and enlarged cervical nodes (277). The primary stage of the disease was thought to have occurred in Mali, where the patient had lived before moving to France a few months earlier. Recently, Fanella et al. reported a case of endemic syphilis in Winnipeg in a 1-year-old patient born to a couple who had lived in a refugee camp in Senegal for 20 years before immigrating to Canada (221). Further assessment of all family members (parents and eight siblings) revealed that all of the couple's children were affected. Despite such examples, an accurate estimate of the real extent of the burden of imported cases of endemic treponematoses in Western countries is currently unavailable (278).
GOAL OF YAWS ERADICATION BY 2020
In 2007, WHO launched a new global initiative aimed at yaws eradication, hoping that the successful example given by India (279) could be repeated on a global scale. Since then, over 100,000 cases have been treated with long-acting benzathine penicillin G (BPG), which is recommended for endemic treponematoses (26, 280). For the past 30 years, WHO has recommended a single intramuscular dose of 1.2 million units (MU) of BPG for adults with active or latent yaws, while children less than 10 years of age should receive 0.6 MU (26, 280). It is unclear why these doses, which are half that used for syphilis, have been recommended for the endemic treponematoses. Although cases of BPG treatment failure with yaws have been reported from Papua New Guinea and Ecuador (249, 258), there are not sufficient data to rule out reinfection in such individuals, and testing of isolated strains for resistance under controlled conditions has not been published. It is stated that, after over 60 years of continuous use, treponemes have failed to develop true genetic resistance to beta-lactam antibiotics. The probability of treponemes developing such resistance in the future is currently regarded as remote, as discussed by investigators focusing on the study of a T. pallidum subsp. pallidum penicillin-binding protein (281, 282). Development of penicillin resistance would, according to these authors, require a significantly rare multistep mutational process. Disadvantages of using BPG, however, include the need for a cold chain, needles and syringes, and trained personnel.
Recently, an open-label, noninferiority, randomized trial conducted on 250 children with yaws in the Lihir island of Papua New Guinea showed that a single oral dose of azithromycin is as effective as BPG for treatment of yaws (283). This finding was welcomed as a major advancement in the control of this disease and further contributed to reviving interest in a global eradication campaign against yaws and endemic treponematoses. In 2012, WHO officially launched a road map to accelerate work to eradicate several neglected tropical diseases (NTDs) (279). The goal for yaws eradication is currently set as 2020, which implies an interruption of transmission by 2017 and a 3-year period in which no new cases are reported through clinical and serological surveillance. In March 2012, WHO organized a first consultation in Morges (Switzerland) to prepare a strategy for the eradication of yaws. At the meeting, Kingsley Asiedu, director of the yaws eradication program in the Department of Control of NTDs, acknowledged that “a new era of eradication of yaws and other endemic treponematoses was beginning, which, this time, should succeed” (240).
Favorable Conditions and Challenges for Yaws Eradication
For a complete list of favorable conditions for yaws eradication as well as operational challenges, we recommend the summary report of the Morges consultation (240). It seems, however, worthwhile to comment on the burst of confidence ignited by the potential use of azithromycin to fight yaws, tempered by the recent scientific evidence suggesting that primates might serve as a natural reservoir of yaws treponemes in some of the areas where the new control programs will be implemented.
A single oral dose of azithromycin (30 mg/kg, with a maximum of 2 g) is now considered by WHO as equivalent to BPG for treatment of yaws (240). Azithromycin has the advantage of being administered orally rather than requiring needles, syringes, and highly trained personnel, as for BPG. However, resistance to azithromycin may ultimately limit its utility. Azithromycin is a macrolide antibiotic with a long tissue half-life that has already been shown to be effective against chlamydial urethritis and cervicitis (284), uncomplicated gonorrhea (285), and chancroid (286). Promising results on the use of azithromycin in lieu of BPG for treatment of early venereal syphilis were reported from several studies throughout the 1990s (287–289) and from large-scale studies performed in Tanzania (290) and Uganda (291), as well as an equivalence trial that involved multiple clinical sites in North America and Madagascar (292). Mitigating the enthusiasm for azithromycin as the new “magic bullet” against yaws, however, is the overall experience with this antibiotic for the treatment of early syphilis. Cases of azithromycin treatment failure were initially recognized among syphilis patients in 2002 (222, 293–295), which led to the findings that genetic resistance to azithromycin in T. pallidum subsp. pallidum is associated with a single point mutation (either A2058G or A2059G) in the 23S rRNA gene (222, 296, 297) and that this newly developed resistance is selected by antibiotic pressure (298). Also concerning is the evidence that in San Francisco and Seattle, the prevalence of azithromycin-resistant T. pallidum subsp. pallidum has steadily increased over time, from 4% and 9% in 2002 to 77% and >80% in 2005 (293, 298–301), and that the mutation has also become nearly universal among T. pallidum subsp. pallidum strains from primary syphilis cases in Shanghai, China (302). Although similar studies have shown that these mutations are relatively rare or absent in Taiwan, Madagascar, Tanzania, and Uganda (290, 291, 303–305), this phenomenon could be attributed to the limited use of macrolides in these countries and the fact that most of these studies were conducted a number of years ago. In light of the high genetic relatedness between T. pallidum subsp. pallidum and the other T. pallidum subspecies (110), the risk of selection of azithromycin-resistant T. pallidum subsp. pertenue and T. pallidum subsp. endemicum strains should not be ignored when a program that heavily relies on the use of macrolides is implemented. Lab surveillance of clinical samples from patients being treated initially with azithromycin or those suspected of treatment failure is recommended to detect the emergence of known or new mutations that might confer macrolide resistance. The use of a lower dose of azithromycin for mass treatment for trachoma eradication among the same populations that have yaws may, in fact, hasten macrolide resistance in treponemes. The present time, then, may represent a limited window of opportunity for the yaws eradication program to utilize azithromycin, and countries should rapidly develop and implement their yaws eradication plans before resistance develops.
Cocirculation of Syphilis and Yaws
The human treponematoses were earlier believed not to overlap within geographical areas, and this observation fueled speculation that infection with one T. pallidum subspecies protected against infection with another. Studies in the rabbit model, however, have shown that cross-protection among subspecies is partial at best. Thus, it is quite possible that syphilis and yaws, for example, could exist in the same community. The increasing mobility of persons makes this possibility more likely. Several studies have reported the presence of syphilis in some countries where yaws is endemic (e.g., Papua New Guinea) (306–311), and it is unclear how the coexistence of venereal and nonvenereal transmission of treponemal infections might affect surveillance and assessment of eradication program success. This topic seems particularly relevant given the facts that patients in remote areas may not have access to sophisticated molecular techniques and that current serological tests are unable to discriminate among the agents of the human treponematoses. If venereal and nonvenereal treponemal infections do coexist in a population, the common practice of endemic treponematosis control programs to screen only children for infectious lesions might result in missed infections in adults. If syphilis goes undiagnosed, it could have detrimental impacts on the health of the community. If mass serological screening is conducted in these settings, however, both venereal and nonvenereal infections would be identified and treated, and control of both infections may result from a treatment program intended for just one.
Animal Reservoirs
Another condition considered favorable for yaws eradication is the long-held tenet that humans are the only reservoir for infection. Already questioned in the past (312), this statement was recently challenged again by Zobaníková and coworkers, who, upon analysis of the full genome sequence of the simian treponemal strain called Fribourg-Blanc (98), proposed to rename it T. pallidum subsp. pertenue strain Fribourg-Blanc. This strain was isolated from the popliteal lymph nodes of a yellow baboon (Papio cynocephalus cynocephalus) from Guinea (313, 314) and was later reported to cause symptoms in experimentally infected human subjects (44). If nonhuman primates are found to harbor pathogenic treponemes similar to T. pallidum subsp. pertenue that are readily transmissible to humans, it could impact the design, implementation, and probability of success of the new WHO yaws eradication campaign, particularly in those areas where primate treponematoses have been described (315–322). The genetic and ecological relationship between treponemal infections in humans and primates will necessarily need to be clarified.
GENETIC CHARACTERIZATION OF Treponema ISOLATES FROM FREE-RANGE PRIMATES IN AFRICA
Evidence of Treponemal Infection in African Primates
Evidence that free-range African primates are infected with T. pallidum first appeared in the early 1960s, following a series of studies conducted by Fribourg-Blanc, Niel, and Mollaret (191, 313, 314) in Guinea and Senegal. These studies showed that, in these countries where yaws or bejel was known to be endemic, most of the examined troops of yellow baboon (Papio cynocephalus cynocephalus) tested positive for treponemal antibodies, with a seroprevalence ≥60% in certain areas (313). No clinical signs of the infection were reported in these animals. Similar studies by the same investigators also showed the presence of treponemal antibodies in a very small cohort of yellow baboons in Cameroon but failed to show any serological evidence of treponematoses among a few hundred baboons captured in Kenya (191, 313, 314). In the early 1970s, Baylet et al. reported that a high percentage of Guinea baboons (Papio papio papio) in the Casamance region of Senegal were reactive in treponemal serological tests and that clinical signs of the disease, if present, appeared localized on the animal's muzzle or near the armpit as small ulcers teeming with treponemes and as enlarged lymph nodes (321, 322). “Yaws” has also been reported in wild gorilla (Gorilla gorilla gorilla) and chimpanzee (Pan troglodytes troglodytes) populations of the Democratic Republic of Congo, Gabon, Cameroon, and Guinea, based on the presence of yaws-like lesions on the faces of gorillas or analysis of gorilla and chimpanzees skeletal samples conserved at the Powell-Cotton Museum in Birchington, United Kingdom. These findings, however, have not been confirmed by laboratory tests (317, 318, 320, 323).
Altogether, these studies suggest that primate treponematoses were more common in western African countries rather than in eastern ones (e.g., Kenya), despite the presence of yaws also in eastern Africa (18). In 1989, however, several troops of olive baboons (Papio hamadryas anubis) at Gombe Stream National Park in Tanzania were reported to be affected by yaws (324). In this case, unlike the mild manifestations seen in western Africa yellow baboons, lesions were mostly localized in the genital areas of sexually active baboons, and in a small number of animals, the lesions were so severe as to cause death by obstruction of the urinary tract. Based on this evidence, scientists favored sexual transmission as the most likely route for treponemal infection within this troop. In 1994, at Lake Manyara National Park (LMNP) (also in Tanzania, but about 450 miles east of Gombe), severe anogenital lesions were described in olive baboons (325). Even though the etiological agent of the disease was not investigated at that time, observations indicated that the disease solely affected primates of the genus Papio and that it closely resembled the manifestations of human syphilis. More recently, using a combination of molecular and histopathological methods, Knauf et al. confirmed that LMNP baboons with moderate to severe genital ulcerations are infected with a strain of T. pallidum carrying four genetic signatures specific for T. pallidum subsp. pertenue (316). These findings raised the question of whether wild baboons could function as a reservoir for human yaws or, vice versa, humans could be responsible for primate infection. A third possibility was that the human and primate diseases are caused by different, although related, strains. To shed further light on the subject of treponemal infections of wild baboons in eastern Africa, Harper et al. mapped the distribution of T. pallidum infection among olive baboons in LMNP, as well as four other national parks or conservation areas in Tanzania, and collected additional data on the clinical manifestations of the disease and the treponemal strains circulating in these areas (319). In the same study, serum samples collected from six sites in Kenya (in proximity to the Tanzanian border) from several hundred olive baboons were also used to investigate treponemal infection in these animals. Harper and collaborators found that only one site in Kenya was populated with Treponema-infected baboons, in agreement with Fribourg-Blanc's previous finding, while baboons with clinical or serological signs of infection were found in all but one of the sites in Tanzania (319).
Genetic Characterization and Phylogenetic Analysis of Treponemal Samples from Baboons
For their phylogenetic analysis, Harper et al. amplified and sequenced six genomic loci known to carry a total of 25 polymorphisms among treponemal strains and included the Fribourg-Blanc isolate, three strains isolated from LMNP (which turned out to be genetically identical in these loci and therefore were treated as a single strain), one strain from the Serengeti National Park (SeNP) in Tanzania, two T. pallidum subsp. endemicum strains, nine T. pallidum subsp. pertenue strains, nine T. pallidum subsp. pallidum strains, and three strains of the rabbit pathogen T. paraluiscuniculi (319). The results of their analyses suggested that the strain collected in SeNP and the Fribourg-Blanc isolate appeared to diverge prior to the clade containing the human treponemes, while the LMNP strain fell within the polytomy containing T. pallidum subsp. pertenue strains. Despite these findings, Harper and coworkers cautiously highlight that such results could be affected by the limited number of polymorphisms analyzed and that, rather than being a T. pallidum subsp. pertenue isolate, the LMNP strain could represent a strain that shares the most recent common ancestor with yaws-causing treponemes in humans (319). Equally interesting is the evidence that the Fribourg-Blanc isolate clustered separately from the treponemal strains isolated from humans as well as from the LMNP and SeNP strains. This result is in agreement with several earlier genetic studies in which partial sequences of the Fribourg-Blanc isolate were compared with homologous sequences from the agents of syphilis, yaws, and bejel (95–97, 105, 107, 108, 118, 326–328). Although several of these studies showed that sequences from Fribourg-Blanc were more closely related to T. pallidum subsp. pertenue strains than those of agents of syphilis or bejel, none of them concluded that Fribourg-Blanc was a T. pallidum subsp. pertenue strain, as proposed following the sequencing of the genome of the Fribourg-Blanc strain by Zobaníková et al. (98). The similarity between the Fribourg-Blanc isolate and T. pallidum subsp. pertenue was recognized long before the advent of comparative genomics. For example, studies by Sepetjian et al. (329) compared lesion appearance and evolution and development of antitreponemal antibodies in Asian rhesus monkeys (Macaca mulatta) inoculated with the Fribourg-Blanc strain, T. pallidum subsp. pallidum, and T. pallidum subsp. pertenue. Although in these experiments, the lesion type, time of appearance, and evolution were more similar in animals inoculated with the Fribourg-Blanc strain and T. pallidum subsp. pertenue than in those inoculated with T. pallidum subsp. pallidum, the appearance of antitreponemal antibodies was more delayed in Fribourg-Blanc-infected than in T. pallidum subsp. pertenue-infected animals (329), suggesting that despite their clear genetic relatedness, additional factors should be considered before reclassifying the Fribourg-Blanc isolate as a T. pallidum subsp. pertenue strain. Additionally, recent work from our laboratory showed that while Fribourg-Blanc shares several tpr gene signatures with T. pallidum subsp. pertenue strains, it differs from T. pallidum subsp. pertenue and is like T. pallidum subsp. endemicum at the tprG locus (TP0317) (99).
The implications of the possible presence of an animal reservoir of yaws-like organisms for the new WHO yaws control campaign are certainly important, in that it would imply that mass treatment of humans alone might not be sufficient for disease elimination in these areas. This concern is relevant, however, only if the nonhuman primate strains are infectious for humans, and this issue is not resolved. The possibility of treating animals over a wide geographical region is daunting, although it was done in a small area by Wallis and Lee for the animals of Gombe by feeding them penicillin-laced foods (324) or by a more conventional intramuscular (i.m.) injection of penicillin. To investigate the possible presence of an animal reservoir for human infection, the mode of transmission between primates and humans would need to be defined. A possible role for sucking flies (Hippelates pallipes), suspected to inoculate the pathogen through wounds, was proposed in the past but was not definitively demonstrated (31, 33–35, 318). Exposure of villagers in African countries to nonhuman primates is not rare, as shown by studies conducted in Cameroon that helped explain how HIV crossed species to infect humans (330, 331). Most certainly, however, additional comparative studies, possibly involving whole-genome sequences rather than small discrete genomic regions, are needed for an accurate classification of treponemal strains in nonhuman primates. Aside from these more compelling questions, it would be interesting to clarify the reasons for the differences in clinical manifestations associated with treponemal infections in western and eastern Africa baboons and the lack of a clear geographical trend differentiating affected versus nonaffected sites, such as those in proximity to the Tanzanian and Kenyan border (319).
CONCLUSIONS
Exciting times await those involved in the study of endemic treponematoses and in the ongoing WHO eradication effort against these diseases. The probability of finally succeeding in an endeavor that, with alternate fortune, has been in the WHO agenda for over 50 years is greatly increased by the availability of new diagnostic tools, a new, easier, and effective treatment option, a remarkably improved knowledge of the agents of the human treponematoses and the pathogenesis of these diseases, and new molecular tests for the identification of genetic resistance to azithromycin. At the same time, great challenges will be represented by the necessity to screen and treat populations difficult to reach, as well as the possibility that macrolide-resistant treponemes will be selected by azithromycin treatment and the possibility that animal reservoirs for human treponemal pathogens may exist. All considered, however, if the new eradication effort finds the necessary support and commitment, we might soon welcome a world devoid of the burden of the endemic treponematoses.
ACKNOWLEDGMENTS
We are grateful to Rebecca Gray, Research Fellow at the University of Oxford (Oxford, United Kingdom), for her enlightened opinions and helpful conversation about treponemal phylogeny and evolution. We are also grateful to Emmanuel Galoo, Cynthia Kwakye, and the Ghana National Yaws Eradication Program, to Laurent Ferradini, to Christina Widaningrum-Mkes and the Ministry of Health of Indonesia, and to the World Health Organization for granting us permission to use their photographs of cases of endemic treponematoses. We are particularly grateful to reviewer 4 for a very thoughtful and comprehensive review of the manuscript.
This work was supported by the National Institute for Allergy and Infectious Diseases of the National Institutes of Health under award numbers R03AI096129 (to L.G.) and R01AI42143 and R01AI63940 (to S.A.L.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Biographies

Lorenzo Giacani is an Assistant Professor of Medicine, Division of Allergy and Infectious Diseases, at the University of Washington. He received a B.S. in molecular biology in 1998 and a Ph.D. in medical biotechnology in 2005, from the University of Bologna in Italy. He began his studies on pathogenic treponemes in 2001. Following postdoctoral training at the University of Washington, he joined the faculty. Dr. Giacani's research interests include regulation of gene expression in pathogenic treponemes and molecular mechanisms for immune evasion and persistence of treponemes.

Sheila Lukehart is Professor of Medicine and Global Health, Associate Dean for Research and Graduate Education, and Adjunct Professor of Microbiology at the University of Washington School of Medicine in Seattle, WA. She received her bachelor's degree in biology at the University of California, San Diego, and her Ph.D. in microbiology and immunology at the University of California, Los Angeles. Following postdoctoral training at the University of California, San Diego, in immunopathology, she moved to the University of Washington, where she joined the faculty. Dr. Lukehart is a recognized expert on syphilis and Treponema pallidum, and her research has been actively supported by the National Institutes of Health for over 30 years. Dr. Lukehart has focused her research on the molecular pathogenesis of syphilis and the host immune responses to Treponema pallidum. Although her work is largely laboratory based, she has conducted several clinically relevant investigations, most notably on neurosyphilis, syphilis-HIV interactions, and antibiotic resistance in T. pallidum.
REFERENCES
- 1.Miao RM, Fieldsteel AH. 1980. Genetic relationship between Treponema pallidum and Treponema pertenue, two noncultivable human pathogens. J. Bacteriol. 141:427–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paster BJ. 2010. Phylum XV. Spirochaetes Garrity and Holt 2001, p 471–566 In Krieg NR, Ludwig W, Whitman WB, Hedlund BP, Paster BJ, Staley JT, Ward N, Brown D, Parte A. (ed), Bergey's manual of systematic bacteriology. Springer, New York, NY [Google Scholar]
- 3.Magnuson HJ, Thomas EW, Olansky S, Kaplan BI, DeMello L, Cutler JC. 1956. Inoculation syphilis in human volunteers. Medicine 35:33–82. 10.1097/00005792-195602000-00002 [DOI] [PubMed] [Google Scholar]
- 4.Magnuson HJ, Eagle H, Fleischman R. 1948. The minimal infectious inoculum of Spirochaeta pallida (Nichols Strain), and a consideration of its rate of multiplication in vivo. Am. J. Syphilis 32:1–18 [PubMed] [Google Scholar]
- 5.Turner TB, Hollander DH. 1957. Biology of the treponematoses. World Health Organization, Geneva, Switzerland: [PubMed] [Google Scholar]
- 6.Sell S, Norris SJ. 1983. The biology, pathology, and immunology of syphilis. Int. Rev. Exp. Pathol. 24:203–276 [PubMed] [Google Scholar]
- 7.Van Voorhis WC, Barrett LK, Koelle DM, Nasio JM, Plummer FA, Lukehart SA. 1996. Primary and secondary syphilis lesions contain mRNA for Th1 cytokines. J. Infect. Dis. 173:491–495. 10.1093/infdis/173.2.491 [DOI] [PubMed] [Google Scholar]
- 8.Van Voorhis WC, Barrett LK, Nasio JM, Plummer FA, Lukehart SA. 1996. Lesions of primary and secondary syphilis contain activated cytolytic T cells. Infect. Immun. 64:1048–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leader BT, Godornes C, Van Voorhis WC, Lukehart SA. 2007. CD4+ Lymphocytes and gamma interferon predominate in local immune responses in early experimental syphilis. Infect. Immun. 75:3021–3026. 10.1128/IAI.01973-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schell RF. 1983. Rabbit and hamster models of treponemal infection, p 121–135 In Schell RF, Musher DM. (ed), Pathogenesis and immunology of treponemal infection. Marcel Dekker, Inc., New York., NY [Google Scholar]
- 11.Pierce CS, Wicher K, Nakeeb S. 1983. Experimental syphilis: guinea pig model. Br. J. Vener Dis. 59:157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wicher K, Wicher V. 1989. Experimental syphilis in guinea pig. Crit. Rev. Microbiol. 16:181–234. 10.3109/10408418909104471 [DOI] [PubMed] [Google Scholar]
- 13.Gueft B, Rosahn PD. 1948. Experimental mouse syphilis, a critical review of the literature. Am. J. Syph. 32:59–88 [PubMed] [Google Scholar]
- 14.Kuhn USG, Varela G, Chandler FW, Osuna GG. 1968. Experimental pinta in the chimpanzee. JAMA 206:829. 10.1001/jama.206.4.829 [DOI] [PubMed] [Google Scholar]
- 15.Kuhn US, III, Medina R, Cohen PG, Vegas M. 1970. Inoculation pinta in chimpanzees. Br. J. Vener. Dis. 46:311–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandler FW, Jr, Kaufmann AF, Kuhn US., III 1972. The histopathology of experimental pinta in the chimpanzee. J. Invest. Dermatol. 58:103–108. 10.1111/1523-1747.ep12538894 [DOI] [PubMed] [Google Scholar]
- 17.Hackett CJ. 1984. On the epidemiology of yaws in African miners (1942). Trans. R. Soc. Trop. Med. Hyg. 78:536–538. 10.1016/0035-9203(84)90077-4 [DOI] [PubMed] [Google Scholar]
- 18.Hackett CJ. 1953. Extent and nature of the yaws problem in Africa. Bull. World Health Organ. 8:129–182 [PMC free article] [PubMed] [Google Scholar]
- 19.Engelkens HJ, Niemel PL, van der Sluis JJ, Stolz E. 1991. The resurgence of yaws. World-wide consequences. Int. J. Dermatol. 30:99–101 [DOI] [PubMed] [Google Scholar]
- 20.Widy-Wirski R. 1985. Surveillance and control of resurgent yaws in the African region. Rev. Infect. Dis. 7(Suppl 2):S227–S232. 10.1093/clinids/7-Supplement_2.S227 [DOI] [PubMed] [Google Scholar]
- 21.Harper D. Yaws. In Online etymology dictionary. http://www.etymonline.com/index.php?search=yaws [Google Scholar]
- 22.Nagreh DS. 1986. Yaws. Cutis 38:303–305 [PubMed] [Google Scholar]
- 23.Perine PL, Hopkins DR, Niemel PLA, St John R, Causse G, Antal GM. 1984. Handbook of endemic treponematoses: yaws, endemic syphilis, and pinta. World Health Organization, Geneva, Switzerland [Google Scholar]
- 24.Louis FJ, Miailhes P, Trébucq A, Maubert B, Louis JP. 1993. Le pian chez les Pygmées, indicateur d'une régression de l'accès aux soins en Afrique Centrale. Cahier Santé 1993:128–132 [Google Scholar]
- 25.Koff AB, Rosen T. 1993. Nonvenereal treponematoses: yaws, endemic syphilis, and pinta. J. Am. Acad. Dermatol. 29:519–535. 10.1016/0190-9622(93)70217-H [DOI] [PubMed] [Google Scholar]
- 26.Antal GM, Lukehart SA, Meheus AZ. 2002. The endemic treponematoses. Microbes Infect. 4:83–94. 10.1016/S1286-4579(01)01513-1 [DOI] [PubMed] [Google Scholar]
- 27.Sehgal VN. 1990. Leg ulcers caused by yaws and endemic syphilis. Clin. Dermatol. 8:166–174. 10.1016/0738-081X(90)90055-6 [DOI] [PubMed] [Google Scholar]
- 28.Powell A. 1923. Framboesia: history of its introduction into India; with personal observations of over 200 initial lesions. Proc. R. Soc. Med. 16:15–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engelkens HJ, Judanarso J, Oranje AP, Vuzevski VD, Niemel PL, van der Sluis JJ, Stolz E. 1991. Endemic treponematoses. I. Yaws Int. J. Dermatol. 30:77–83. 10.1111/j.1365-4362.1991.tb04215.x [DOI] [PubMed] [Google Scholar]
- 30.Hackett CJ. 1957. The transmission of yaws in nature. J. Trop. Med. Hyg. 60:159–168 [PubMed] [Google Scholar]
- 31.Brack M. 1987. Agents transmissible from simians to man. Springer-Verlag, Berlin, Germany [Google Scholar]
- 32.Burke JP, Hopkins DR, Hume JC, Perine PL, St John R. 1985. International Symposium on Yaws and Other Endemic Treponematoses. Rev. Infect. Dis. 7(Suppl 2):S217–S351. 10.1093/clinids/7-Supplement_2.S217 [DOI] [PubMed] [Google Scholar]
- 33.Langley PA. 1975. Pathogen transmission in relation to feeding and digestion by haematophagous arthropods. Acta Trop. 32:116–124 [PubMed] [Google Scholar]
- 34.Sanches FG, Mazzotti L, Salenas EG. 1961. Hippelates pallipes as the vector of yaws. Salud. Publ. Mex. 3:183 [Google Scholar]
- 35.Kumm HW. 1935. The natural infection of Hippelates pallipes loew with the spirochaetes of yaws. Trans. R. Soc. Trop. Med. Hyg. 29:265–272. 10.1016/S0035-9203(35)90089-X [DOI] [Google Scholar]
- 36.Gip LS. 1989. Yaws revisited. Med. J. Malaysia 44:307–311 [PubMed] [Google Scholar]
- 37.Vorst FA. 1985. Clinical diagnosis and changing manifestations of treponemal infection. Rev. Infect. Dis. 7(Suppl 2):S327–S331. 10.1093/clinids/7-Supplement_2.S327 [DOI] [PubMed] [Google Scholar]
- 38.Vanthournout I, Quinet B, Neuenschwander S. 1991. Pediatric yaws osteoperiostitis. Pediatr. Radiol. 21:303. 10.1007/BF02018632 [DOI] [PubMed] [Google Scholar]
- 39.Whittet HB, Quiney RE. 1988. Nasal manifestations of yaws. J. Laryngol. Otol. 102:1147–1149. 10.1017/S0022215100107558 [DOI] [PubMed] [Google Scholar]
- 40.Mafart B. 2002. Goundou: a historical form of yaws. Lancet 360:1168–1170. 10.1016/S0140-6736(02)11205-0 [DOI] [PubMed] [Google Scholar]
- 41.Martinez SA, Mouney DF. 1982. Treponemal infections of the head and neck. Otolaryngol. Clin. North Am. 15:613–620 [PubMed] [Google Scholar]
- 42.Edington GM. 1954. Cardiovascular disease as a cause of death in the Gold Coast African. Trans. R. Soc. Trop. Med. Hyg. 48:419–425. 10.1016/0035-9203(54)90143-1 [DOI] [PubMed] [Google Scholar]
- 43.Roman GC, Roman LN. 1986. Occurrence of congenital, cardiovascular, visceral, neurologic, and neuro-ophthalmologic complications in late yaws: a theme for future research. Rev. Infect. Dis. 8:760–770. 10.1093/clinids/8.5.760 [DOI] [PubMed] [Google Scholar]
- 44.Lawton-Smith L, David NJ, Indgin S, Israel CW, Levine BM, Justice JJ, McCrary JA, Medina R, Paez P, Santana E, Sarkar M, Schatz NJ, Spitzer ML, Spitzer WO, Walter EK. 1971. Neuro-ophthalmological study of late yaws and pinta. II. The Caracas Project. Br. J. Vener Dis. 47:226–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohamed KN. 1990. Late yaws and optic atrophy. Ann. Trop. Med. Parasitol. 84:637–639 [DOI] [PubMed] [Google Scholar]
- 46.Wicher K, Wicher V, Abbruscato F, Baughn RE. 2000. Treponema pallidum subsp. pertenue displays pathogenic properties different from those of T. pallidum subsp. pallidum. Infect. Immun. 68:3219–3225. 10.1128/IAI.68.6.3219-3225.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niemel PL, Brunings EA, Menke HE. 1979. Attenuated yaws in Surinam. Br. J. Vener Dis. 55:99–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanan MW, Abbas M, Girgis HY. 1971. Late mutilating bejel in the nomadic Bedouins of Kuwait. Dermatologica 143:277–287. 10.1159/000252218 [DOI] [PubMed] [Google Scholar]
- 49.Willcox RR. 1960. Evolutionary cycle of the treponematoses. Br. J. Vener Dis. 36:78–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chaglassian HT, Bustani N, Anderson HH. 1952. Endemic treponematosis (balash or bejel) in Saudi Arabia. Am. J. Trop. Med. Hyg. 1:826–830 [DOI] [PubMed] [Google Scholar]
- 51.Grin EI. 1952. Endemic syphilis in Bosnia; clinical and epidemiological observations on a successful mass-treatment campaign. Bull. World Health Organ. 7:1–74 [PMC free article] [PubMed] [Google Scholar]
- 52.Pace JL, Csonka GW. 1988. Late endemic syphilis: case report of bejel with gummatous laryngitis. Genitourin. Med. 64:202–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erdelyi RL, Molla AA. 1984. Burned-out endemic syphilis (Bejel): facial deformities and defects in Saudi Arabia. Plast. Reconstr. Surg. 74:589–602. 10.1097/00006534-198411000-00001 [DOI] [PubMed] [Google Scholar]
- 54.Lukehart SA. 2012. Endemic treponematoses, p 1389–1391 In Longo DL, Fauci AS, Kasper DL, Hauser S, Jameson JL, Loscalzo J. (ed), Harrison's principles of internal medicine, 18th ed. McGraw Hill, Inc., New York, NY [Google Scholar]
- 55.Basset A, Maleville J, Basset M. 1969. Aspects of endemic syphilis among the Tuaregs of Niger. Bull. Soc. Pathol. Exot. Filiales. 62:80–92 [PubMed] [Google Scholar]
- 56.Pace JL, Csonka GW. 1984. Endemic non-venereal syphilis (bejel) in Saudi Arabia. Br. J. Vener. Dis. 60:293–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tabbara KF, al Kaff AS, Fadel T. 1989. Ocular manifestations of endemic syphilis (bejel). Ophthalmology 96:1087–1091. 10.1016/S0161-6420(89)32781-3 [DOI] [PubMed] [Google Scholar]
- 58.Csonka G, Pace J. 1985. Endemic nonvenereal treponematosis (bejel) in Saudi Arabia. Rev. Infect. Dis. 7(Suppl 2):S260–S265. 10.1093/clinids/7-Supplement_2.S260 [DOI] [PubMed] [Google Scholar]
- 59.Kerdel-Vegas F. 1986. Pinta, p 878–883 In Rook A, Wilkinson DS, Ebling FJG. (ed), Textbook of dermatology, 4th ed. Blackwell Scientific Publications, Oxford, United Kingdom [Google Scholar]
- 60.Luger AFH. 1989. Endemic treponematoses, p 32–58 In Parish LC, Gschnait F. (ed), Sexually transmitted diseases: a guide for clinicians. Springer-Verlag, Berlin, Germany [Google Scholar]
- 61.Edmundson WF, Demis DJ, Bejarano G. 1967. A clinico-serologic study of pinta in the Alto Beni region, Bolivia. Dermatol. Int. 6:64–76. 10.1111/j.1365-4362.1967.tb05242.x [DOI] [PubMed] [Google Scholar]
- 62.Marquez F, Rein CR, Arias O. 1955. Mal del pinto in Mexico. Bull. World Health Organ. 13:299–322 [PMC free article] [PubMed] [Google Scholar]
- 63.Engelkens HJ, Vuzevski VD, Judanarso J, van Lier JB, van der Stek J, van der Sluis JJ, Stolz E. 1990. Early yaws: a light microscopic study. Genitourin. Med. 66:264–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams HU. 1935. Pathology of yaws, especially the relation of yaws to syphilis. Arch. Pathol. 8:596–630 [Google Scholar]
- 65.Hasselmann CM. 1957. Comparative studies on the histo-pathology of syphilis, yaws, and pinta. Br. J. Vener. Dis. 33:5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pecher SA, Azevedo EB. 1987. Histopathological aspects of tertiary pinta. Med. Cutan Ibero Lat Am. 15:239–242 [PubMed] [Google Scholar]
- 67.Rompalo AM, Joesoef MR, O'Donnell JA, Augenbraun M, Brady W, Radolf JD, Johnson R, Rolfs RT. 2001. Clinical manifestations of early syphilis by HIV status and gender: results of the syphilis and HIV study. Sex. Transm. Dis. 28:158–165. 10.1097/00007435-200103000-00007 [DOI] [PubMed] [Google Scholar]
- 68.Liotta EA, Turiansky GW, Berberian BJ, Sulica VI, Tomaszewski MM. 2000. Unusual presentation of secondary syphilis in 2 HIV-1 positive patients. Cutis 66:383–386 [PubMed] [Google Scholar]
- 69.Glover RA, Piaquadio DJ, Kern S, Cockerell CJ. 1992. An unusual presentation of secondary syphilis in a patient with human immunodeficiency virus infection. A case report and review of the literature. Arch. Dermatol. 128:530–534 [PubMed] [Google Scholar]
- 70.Marra CM, Maxwell CL, Smith SL, Lukehart SA, Rompalo AM, Eaton M, Stoner BP, Augenbraun M, Barker DE, Corbett JJ, Zajackowski M, Raines C, Nerad J, Kee R, Barnett SH. 2004. Cerebrospinal fluid abnormalities in patients with syphilis: association with clinical and laboratory features. J. Infect. Dis. 189:369–376. 10.1086/381227 [DOI] [PubMed] [Google Scholar]
- 71.Ghanem KG, Moore RD, Rompalo AM, Erbelding EJ, Zenilman JM, Gebo KA. 2009. Lumbar puncture in HIV-infected patients with syphilis and no neurologic symptoms. Clin. Infect. Dis. 48:816–821. 10.1086/597096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Erbelding EJ, Vlahov D, Nelson KE, Rompalo AM, Cohn S, Sanchez P, Quinn TC, Brathwaite W, Thomas DL. 1997. Syphilis serology in human immunodeficiency virus infection: evidence for false-negative fluorescent treponemal testing. J. Infect. Dis. 176:1397–1400. 10.1086/517330 [DOI] [PubMed] [Google Scholar]
- 73.Rompalo AM, Cannon RO, Quinn TC, Hook EW., III 1992. Association of biologic false-positive reactions for syphilis with human immunodeficiency virus infection. J. Infect. Dis. 165:1124–1126. 10.1093/infdis/165.6.1124 [DOI] [PubMed] [Google Scholar]
- 74.Augenbraun MH, DeHovitz JA, Feldman J, Clarke L, Landesman S, Minkoff HM. 1994. Biological false-positive syphilis test results for women infected with human immunodeficiency virus. Clin. Infect. Dis. 19:1040–1044. 10.1093/clinids/19.6.1040 [DOI] [PubMed] [Google Scholar]
- 75.Rusnak JM, Butzin C, McGlasson D, Blatt SP. 1994. False-positive rapid plasma reagin tests in human immunodeficiency virus infection and relationship to anti-cardiolipin antibody and serum immunoglobulin levels. J. Infect. Dis. 169:1356–1359. 10.1093/infdis/169.6.1356 [DOI] [PubMed] [Google Scholar]
- 76.Gregory N, Sanchez M, Buchness MR. 1990. The spectrum of syphilis in patients with human immunodeficiency virus infection. J. Am. Acad. Dermatol. 22:1061–1067. 10.1016/0190-9622(90)70153-9 [DOI] [PubMed] [Google Scholar]
- 77.Hicks CB, Benson PM, Lupton GP, Tramont EC. 1987. Seronegative secondary syphilis in a patient infected with the human immunodeficiency virus (HIV) with Kaposi sarcoma. A diagnostic dilemma. Ann. Intern. Med. 107:492–495. 10.7326/0003-4819-107-4-492 [DOI] [PubMed] [Google Scholar]
- 78.Ghanem KG, Erbelding EJ, Wiener ZS, Rompalo AM. 2007. Serological response to syphilis treatment in HIV-positive and HIV-negative patients attending sexually transmitted diseases clinics. Sex. Transm. Infect. 83:97–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.WHO Global Health Observatory Data Repository. Data on the size of the HIV/AIDS epidemic: number of people (all ages) living with HIV by country. WHO, Geneva, Switzerland: http://apps.who.int/gho/data/node.main.620?lang=en [Google Scholar]
- 80.Harrison LW. 1959. The origin of syphilis. Br. J. Vener. Dis. 35:1–7 [PMC free article] [PubMed] [Google Scholar]
- 81.Crosby AW. 1969. An early hystory of syphilis: a reapprisal. Am. Anthropologist 71:218–227. 10.1525/aa.1969.71.2.02a00020 [DOI] [Google Scholar]
- 82.Dennie CW. 1962. A history of syphilis. Thomas, Springfield, IL [Google Scholar]
- 83.Rothschild BM. 2005. History of syphilis. Clin. Infect. Dis. 40:1454–1463. 10.1086/429626 [DOI] [PubMed] [Google Scholar]
- 84.Rothschild BM, Hershkovitz I, Rothschild C. 1995. Origin of yaws in the Pleistocene. Nature 378:343–344. 10.1038/378343a0 [DOI] [PubMed] [Google Scholar]
- 85.Blondiaux J, Bagousse AA. 1994. A treponematosis dated from the late Roman Empire in Normandie, France, p 99–100 In Dutour O, Pa'lfi G, Berato J. (ed), L'origine de la syphilis en Europe: avant ou apre's 1493? Centre Archeologique du Var, Toulon, France [Google Scholar]
- 86.Trembly DL. 1998. Treponematosis in pre-Spanish Western Micronesia. Int. J. Osteoarchaeol. 6:397–402 [Google Scholar]
- 87.Henneberg M, Henneberg RJ. 1994. Treponematosis in an ancient Greek colony of Metaponto, southern Italy, 580-250 BCE, p 92–99 In Dutour O, Pa'lfi G, Berato J. (ed), Centre Archeologique du Var, Toulon, France [Google Scholar]
- 88.Meyer C, Jung C, Kohl T, Poenicke A, Poppe A, Alt KW. 2002. Syphilis 2001—a paleopathological reappraisal. Homo 53:39–58. 10.1078/0018-442X-00037 [DOI] [PubMed] [Google Scholar]
- 89.Baker BJ, Armelagos GJ. 1988. The origin and antiquity of syphilis: paleopathological diagnosis and interpretation. Curr. Anthropol. 29:703–738. 10.1086/203691 [DOI] [PubMed] [Google Scholar]
- 90.Cockburn TA. 1961. The origin of the treponematoses. Bull. World Health Organ. 24:221–228 [PMC free article] [PubMed] [Google Scholar]
- 91.Hackett CJ. 1963. On the origin of the human treponematoses (pinta, yaws, endemic syphilis and venereal syphilis). Bull. World Health Organ. 29:7–41 [PMC free article] [PubMed] [Google Scholar]
- 92. Reference deleted.
- 93.Hudson EH. 1963. Treponematosis and anthropology. Ann. Intern. Med. 58:1037–1048. 10.7326/0003-4819-58-6-1037 [DOI] [PubMed] [Google Scholar]
- 94.Hudson EH. 1965. Treponematosis in perspective. Bull. World Health Organ. 32:735–748 [PMC free article] [PubMed] [Google Scholar]
- 95.Harper KN, Liu H, Ocampo PS, Steiner BM, Martin A, Levert K, Wang D, Sutton M, Armelagos GJ. 2008. The sequence of the acidic repeat protein (arp) gene differentiates venereal from nonvenereal Treponema pallidum subspecies, and the gene has evolved under strong positive selection in the subspecies that causes syphilis. FEMS Immunol. Med. Microbiol. 53:322–332. 10.1111/j.1574-695X.2008.00427.x [DOI] [PubMed] [Google Scholar]
- 96.Harper KN, Ocampo PS, Steiner BM, George RW, Silverman MS, Bolotin S, Pillay A, Saunders NJ, Armelagos GJ. 2008. On the origin of the treponematoses: a phylogenetic approach. PLoS Negl. Trop. Dis. 2:e148 10.1371/journal.pntd.0000148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gray RR, Mulligan CJ, Molini BJ, Sun ES, Giacani L, Godornes C, Kitchen A, Lukehart SA, Centurion-Lara A. 2006. Molecular evolution of the tprC, D, I, K, G, and J. genes in the pathogenic genus Treponema. Mol. Biol. Evol. 23:2220–2233. 10.1093/molbev/msl092 [DOI] [PubMed] [Google Scholar]
- 98.Zobaníková M, Strouhal M, Mikalová L, DČejková Ambrožová L, Pospíšilová P, Fulton LL, Chen L, Sodergren E, Weinstock GM, DŠmajs 2013. Whole genome sequence of the Treponema Fribourg-Blanc: unspecified simian isolate is highly similar to the yaws subspecies. PLoS Negl. Trop. Dis 7:e2172 10.1371/journal.pntd.0002172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Centurion-Lara A, Giacani L, Godornes C, Molini BJ, Brinck Reid T, Lukehart SA. 2013. Fine analysis of genetic diversity of the tpr gene family among treponemal species, subspecies and strains. PLoS Negl. Trop. Dis. 16:e2222 10.1371/journal.pntd.0002222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de Melo FL, de Mello JC, Fraga AM, Nunes K, Eggers S. 2010. Syphilis at the crossroad of phylogenetics and paleopathology. PLoS Negl. Trop. Dis. 4:e575 10.1371/journal.pntd.0000575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Reference deleted.
- 102.Rothschild BM, Calderon FL, Coppa A, Rothschild C. 2000. First European exposure to syphilis: the Dominican Republic at the time of Columbian contact. Clin. Infect. Dis. 31:936–941. 10.1086/318158 [DOI] [PubMed] [Google Scholar]
- 103.Mulligan CJ, Norris SJ, Lukehart SA. 2008. Molecular studies in Treponema pallidum evolution: toward clarity? PLoS Negl. Trop. Dis. 2:e184 10.1371/journal.pntd.0000184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Noordhoek GT, Hermans PW, Paul AN, Schouls LM, van der Sluis JJ, van Embden JD. 1989. Treponema pallidum subspecies pallidum (Nichols) and Treponema pallidum subspecies pertenue (CDC 2575) differ in at least one nucleotide: comparison of two homologous antigens. Microb. Pathog. 6:29–42. 10.1016/0882-4010(89)90005-3 [DOI] [PubMed] [Google Scholar]
- 105.Centurion-Lara A, Castro C, Castillo R, Shaffer JM, Van Voorhis WC, Lukehart SA. 1998. The flanking region sequences of the 15-kDa lipoprotein gene differentiate pathogenic treponemes. J. Infect. Dis. 177:1036–1040. 10.1086/515247 [DOI] [PubMed] [Google Scholar]
- 106.Stamm LV, Greene SR, Bergen HL, Hardham JM, Barnes NY. 1998. Identification and sequence analysis of Treponema pallidum tprJ, a member of a polymorphic multigene family. FEMS Microbiol. Lett. 169:155–163. 10.1111/j.1574-6968.1998.tb13312.x [DOI] [PubMed] [Google Scholar]
- 107.Cameron CE, Castro C, Lukehart SA, Van Voorhis WC. 1999. Sequence conservation of glycerophosphodiester phosphodiesterase among Treponema pallidum strains. Infect. Immun. 67:3168–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cameron CE, Lukehart SA, Castro C, Molini B, Godornes C, Van Voorhis WC. 2000. Opsonic potential, protective capacity, and sequence conservation of the Treponema pallidum subspecies pallidum Tp92. J. Infect. Dis. 181:1401–1413. 10.1086/315399 [DOI] [PubMed] [Google Scholar]
- 109.Centurion-Lara A, Molini BJ, Godornes C, Sun E, Hevner K, Van Voorhis WC, Lukehart SA. 2006. Molecular differentiation of Treponema pallidum subspecies. J. Clin. Microbiol. 44:3377–3380. 10.1128/JCM.00784-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cejkova D, Zobanikova M, Chen L, Pospisilova P, Strouhal M, Qin X, Mikalova L, Norris SJ, Muzny DM, Gibbs RA, Fulton LL, Sodergren E, Weinstock GM, Smajs D. 2012. Whole genome sequences of three Treponema pallidum ssp. pertenue strains: yaws and syphilis treponemes differ in less than 0.2% of the genome sequence. PLoS Negl. Trop. Dis. 6:e1471 10.1099/jmm.0.050658-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fraser CM, Norris SJ, Weinstock GM, White O, Sutton GG, Dodson R, Gwinn M, Hickey EK, Clayton R, Ketchum KA, Sodergren E, Hardham JM, McLeod MP, Salzberg S, Peterson J, Khalak H, Richardson D, Howell JK, Chidambaram M, Utterback T, McDonald L, Artiach P, Bowman C, Cotton MD, Venter JC. 1998. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 281:375–388. 10.1126/science.281.5375.375 [DOI] [PubMed] [Google Scholar]
- 112.Giacani L, Jeffrey BM, Molini BJ, Le HT, Lukehart SA, Centurion-Lara A, Rockey DD. 2010. Complete genome sequence and annotation of the Treponema pallidum subsp. pallidum Chicago strain. J. Bacteriol. 192:2645–2646. 10.1128/JB.00159-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zobaniková M, Mikolka P, Cejkova D, Pospisilova P, Chen L, Strouhal M, Qin X, Weinstock GM, Smajs D. 2012. Complete genome sequence of Treponema pallidum strain DAL-1. Stand. Genomic Sci. 7:12–21. 10.4056/sigs.2615838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Petrosova H, Zobanikova M, Cejkova D, Mikalova L, Pospisilova P, Strouhal M, Chen L, Qin X, Muzny DM, Weinstock GM, Smajs D. 2012. Whole genome sequence of Treponema pallidum ssp. pallidum, strain Mexico A, suggests recombination between yaws and syphilis strains. PLoS Negl. Trop. Dis. 6:e1832 10.1371/journal.pntd.0001832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smajs D, Zobanikova M, Strouhal M, Cejkova D, Dugan-Rocha S, Pospisilova P, Norris SJ, Albert T, Qin X, Hallsworth-Pepin K, Buhay C, Muzny DM, Chen L, Gibbs RA, Weinstock GM. 2011. Complete genome sequence of Treponema paraluiscuniculi, strain Cuniculi A: the loss of infectivity to humans is associated with genome decay. PLoS One 6:e20415 10.1371/journal.pone.0020415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Matejkova P, Strouhal M, Smajs D, Norris SJ, Palzkill T, Petrosino JF, Sodergren E, Norton JE, Singh J, Richmond TA, Molla MN, Albert TJ, Weinstock GM. 2008. Complete genome sequence of Treponema pallidum ssp. pallidum strain SS14 determined with oligonucleotide arrays. BMC Microbiol. 8 10.1186/1471-2180-8-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Smajs D, Norris SJ, Weinstock GM. 2012. Genetic diversity in Treponema pallidum: implications for pathogenesis, evolution and molecular diagnostics of syphilis and yaws. Infect. Genet. Evol. 12:191–202. 10.1016/j.meegid.2011.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mikalova L, Strouhal M, Cejkova D, Zobanikova M, Pospisilova P, Norris SJ, Sodergren E, Weinstock GM, Smajs D. 2010. Genome analysis of Treponema pallidum subsp. pallidum and subsp. pertenue strains: most of the genetic differences are localized in six regions. PLoS One 5:e15713 10.1371/journal.pone.0015713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Morgan CA, Molini BJ, Lukehart SA, Van Voorhis WC. 2002. Segregation of B and T cell epitopes of Treponema pallidum repeat protein K to variable and conserved regions during experimental syphilis infection. J. Immunol. 169:952–957 [DOI] [PubMed] [Google Scholar]
- 120.Leader BT, Hevner K, Molini BJ, Barrett LK, Van Voorhis WC, Lukehart SA. 2003. Antibody responses elicited against the Treponema pallidum repeat proteins differ during infection with different isolates of Treponema pallidum subsp. pallidum. Infect. Immun. 71:6054–6057. 10.1128/IAI.71.10.6054-6057.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sun ES, Molini BJ, Barrett LK, Centurion-Lara A, Lukehart SA, Van Voorhis WC. 2004. Subfamily I Treponema pallidum repeat protein family: sequence variation and immunity. Microbes Infect. 6:725–737. 10.1016/j.micinf.2004.04.001 [DOI] [PubMed] [Google Scholar]
- 122.Giacani L, Hevner K, Centurion-Lara A. 2005. Gene organization and transcriptional analysis of the tprJ, tprI, tprG and tprF loci in the Nichols and Sea 81-4 Treponema pallidum isolates. J. Bacteriol. 187:6084–6093. 10.1128/JB.187.17.6084-6093.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Giacani L, Molini B, Godornes C, Barrett L, Van Voorhis WC, Centurion-Lara A, Lukehart SA. 2007. Quantitative analysis of tpr gene expression in Treponema pallidum isolates: differences among isolates and correlation with T-cell responsiveness in experimental syphilis. Infect. Immun. 75:104–112. 10.1128/IAI.01124-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Smajs D, McKevitt M, Howell JK, Norris SJ, Cai WW, Palzkill T, Weinstock GM. 2005. Transcriptome of Treponema pallidum: gene expression profile during experimental rabbit infection. J. Bacteriol. 187:1866–1874. 10.1128/JB.187.5.1866-1874.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Centurion-Lara A, Castro C, Barrett L, Cameron C, Mostowfi M, Van Voorhis WC, Lukehart SA. 1999. Treponema pallidum major sheath protein homologue Tpr K is a target of opsonic antibody and the protective immune response. J. Exp. Med. 189:647–656. 10.1084/jem.189.4.647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cox DL, Luthra A, Dunham-Ems S, Desrosiers DC, Salazar JC, Caimano MJ, Radolf JD. 2010. Surface immunolabeling and consensus computational framework to identify candidate rare outer membrane proteins of Treponema pallidum. Infect. Immun. 78:5178–5194. 10.1128/IAI.00834-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Giacani L, Sambri V, Marangoni A, Cavrini F, Storni E, Donati M, Corona S, Lanzarini P, Cevenini R. 2005. Immunological evaluation and cellular location analysis of the TprI antigen of Treponema pallidum subsp. pallidum. Infect. Immun. 73:3817–3822. 10.1128/IAI.73.6.3817-3822.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morgan CA, Lukehart SA, Van Voorhis WC. 2002. Immunization with the N-terminal portion of Treponema pallidum repeat protein K attenuates syphilitic lesion development in the rabbit model. Infect. Immun. 70:6811–6816. 10.1128/IAI.70.12.6811-6816.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Morgan CA, Lukehart SA, Van Voorhis WC. 2003. Protection against syphilis correlates with specificity of antibodies to the variable regions of Treponema pallidum repeat protein K. Infect. Immun. 71:5605–5612. 10.1128/IAI.71.10.5605-5612.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Centurion-Lara A, Godornes C, Castro C, Van Voorhis WC, Lukehart SA. 2000. The tprK gene is heterogeneous among Treponema pallidum strains and has multiple alleles. Infect. Immun. 68:824–831. 10.1128/IAI.68.2.824-831.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Centurion-Lara A, LaFond RE, Hevner K, Godornes C, Molini BJ, Van Voorhis WC, Lukehart SA. 2004. Gene conversion: a mechanism for generation of heterogeneity in the tprK gene of Treponema pallidum during infection. Mol. Microbiol. 52:1579–1596. 10.1111/j.1365-2958.2004.04086.x [DOI] [PubMed] [Google Scholar]
- 132.LaFond RE, Centurion-Lara A, Godornes C, Rompalo AM, Van Voorhis WC, Lukehart SA. 2003. Sequence diversity of Treponema pallidum subsp. pallidum tprK in human syphilis lesions and rabbit-propagated isolates. J. Bacteriol. 185:6262–6268. 10.1128/JB.185.21.6262-6268.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.LaFond RE, Centurion-Lara A, Godornes C, Van Voorhis WC, Lukehart SA. 2006. TprK sequence diversity accumulates during infection of rabbits with Treponema pallidum subsp. pallidum Nichols strain. Infect. Immun. 74:1896–1906. 10.1128/IAI.74.3.1896-1906.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.LaFond RE, Molini BJ, Van Voorhis WC, Lukehart SA. 2006. Antigenic variation of TprK V regions abrogates specific antibody binding in syphilis. Infect. Immun. 74:6244–6251. 10.1128/IAI.00827-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Giacani L, Molini BJ, Kim EY, Godornes BC, Leader BT, Tantalo LC, Centurion-Lara A, Lukehart SA. 2010. Antigenic variation in Treponema pallidum: TprK sequence diversity accumulates in response to immune pressure during experimental syphilis. J. Immunol. 184:3822–3829. 10.4049/jimmunol.0902788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Strouhal M, Smajs D, Matejkova P, Sodergren E, Amin AG, Howell JK, Norris SJ, Weinstock GM. 2007. Genome differences between Treponema pallidum subsp. pallidum strain Nichols and T. paraluiscuniculi strain Cuniculi A. Infect. Immun. 75:5859–5866. 10.1128/IAI.00709-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Graves S, Downes J. 1981. Experimental infection of man with rabbit-virulent Treponema paraluis-cuniculi. Br. J. Vener. Dis. 57:7–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Levaditi C, Marie A, Nicolau S. 1921. Virulence pour l'homme du spirochete de la spirillose spontanee du lapin. C. R. Hebd. Seances Acad. Sci. 172:1542–1543 [Google Scholar]
- 139.Giacani L, Sun ES, Hevner K, Molini BJ, Van Voorhis WC, Lukehart SA, Centurion-Lara A. 2004. Tpr homologs in Treponema paraluiscuniculi Cuniculi A strain. Infect. Immun. 72:6561–6576. 10.1128/IAI.72.11.6561-6576.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Alexander WN. 1975. Venereal disease and the dentist. J. Acad. Gen. Dent. 23:14–18 [PubMed] [Google Scholar]
- 141.Krivatkin SL, Krivatkina EV. 1997. Secondary syphilis transmitted through non-sexual contact. J. Eur. Acad. Dermatol. Venereol. 9:S224. 10.1016/S0926-9959(97)89778-4 [DOI] [Google Scholar]
- 142.Long FQ, Wang QQ, Jiang J, Zhang JP, Shang SX. 2012. Acquired secondary syphilis in preschool children by nonsexual close contact. Sex. Transm. Dis. 39:588–590. 10.1097/OLQ.0b013e3182515764 [DOI] [PubMed] [Google Scholar]
- 143.Ozturk F, Gurses N, Sancak R, Bay A, Baris S. 1998. Acquired secondary syphilis in a 6-year-old girl with no history of sexual abuse. Cutis 62:150–151 [PubMed] [Google Scholar]
- 144.Zhou P, Qian Y, Lu H, Guan Z. 2009. Nonvenereal transmission of syphilis in infancy by mouth-to-mouth transfer of prechewed food. Sex. Transm. Dis. 36:216–217. 10.1097/OLQ.0b013e3181901c79 [DOI] [PubMed] [Google Scholar]
- 145.Hofmann B, Schuppe HC, Ruzicka T, Kuhn A, Lehmann P. 1998. Acquired syphilis II in early childhood: reappearance of syphilis brephotrophica. J. Am. Acad. Dermatol. 38:638–639. 10.1016/S0190-9622(98)70135-5 [DOI] [PubMed] [Google Scholar]
- 146.Murrel M, Scott M. 1947. Acquired syphilis in children. Br. Med. J. 9:206–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Echols SK, Shupp DL, Schroeter AL. 1990. Acquired secondary syphilis in a child. J. Am. Acad. Dermatol. 22:313–314. 10.1016/S0190-9622(08)80768-2 [DOI] [PubMed] [Google Scholar]
- 148.Morton RS. 1967. The sibbens of Scotland. Med. Hist. 11:374–380. 10.1017/S0025727300012515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fodere' FE. 1799. Les lois eclairees par les sciences physique; ou traite de medecine-legale et d'hygiene publique. Croullebois P. ed, Paris, France [Google Scholar]
- 150.McBride P. 1884. Syphilis or framboesia? Edinb. Clin. Pathol. J. 1:921–924 [Google Scholar]
- 151.Hibbert S. 1927. Remarques historiques sur l'yaws et le sibbens, consideres comme des affections identiques. Ann. Méd. Physiol. 11:367–368 [Google Scholar]
- 152.Suvanasara B, Hill KR. 1953. Extent and nature of the yaws problem. Bull. World Health Organ. 8:205–210 [PMC free article] [PubMed] [Google Scholar]
- 153.Noordhoek GT, Wieles B, van der Sluis JJ, van Embden JD. 1990. Polymerase chain reaction and synthetic DNA probes: a means of distinguishing the causative agents of syphilis and yaws? Infect. Immun. 58:2011–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ochman H, Lawrence JG, Groisman EA. 2000. Lateral gene transfer and the nature of bacterial innovation. Nature 405:299–304. 10.1038/35012500 [DOI] [PubMed] [Google Scholar]
- 155.Mirkin BG, Fenner TI, Galperin MY, Koonin EV. 2003. Algorithms for computing parsimonious evolutionary scenarios for genome evolution, the last universal common ancestor and dominance of horizontal gene transfer in the evolution of prokaryotes. BMC Evol. Biol. 3:2. 10.1186/1471-2148-3-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen I, Christie PJ, Dubnau D. 2005. The ins and outs of DNA transfer in Bacteria. Science 310:1456–1460. 10.1126/science.1114021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Thomas CM, Nielsen KM. 2005. Mechanisms of, and barriers to, horizontal gene transfer between Bacteria. Nat. Rev. Microbiol. 3:711–721. 10.1038/nrmicro1234 [DOI] [PubMed] [Google Scholar]
- 158.Norman A, Hansen LH, Sorensen SJ. 2009. Conjugative plasmids: vessels of the communal gene pool. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364:2275–2289. 10.1098/rstb.2009.0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wozniak RA, Waldor MK. 2010. Integrative and conjugative elements: mosaic mobile genetic elements enabling dynamic lateral gene flow. Nat. Rev. Microbiol. 8:552–563. 10.1038/nrmicro2382 [DOI] [PubMed] [Google Scholar]
- 160.Lang AS, Beatty JT. 2007. Importance of widespread gene transfer agent genes in alpha-proteobacteria. Trends Microbiol. 15:54–62. 10.1016/j.tim.2006.12.001 [DOI] [PubMed] [Google Scholar]
- 161.Larsen SA. 1999. Manual of tests for syphilis. American Public Health Association, Washington, DC [Google Scholar]
- 162.Hook EW, III, Marra CM. 1992. Acquired syphilis in adults. N. Engl. J. Med. 326:1060–1069. 10.1056/NEJM199204163261606 [DOI] [PubMed] [Google Scholar]
- 163.Berkowitz K, Baxi L, Fox HE. 1990. False-negative syphilis screening: the prozone phenomenon, nonimmune hydrops, and diagnosis of syphilis during pregnancy. Am. J. Obstet. Gynecol. 163:975–977. 10.1016/0002-9378(90)91107-N [DOI] [PubMed] [Google Scholar]
- 164.Hunter EF, Deacon WE, Meyer PE. 1964. An improved FTA test for syphilis, the absorption procedure (FTA-Abs). Public Health Rep. 79:410–412. 10.2307/4592145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rathlev T. 1967. Haemagglutination test utilizing pathogenic Treponema pallidum for the sero-diagnosis of syphilis. Br. J. Vener Dis. 43:181–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rudolph AH. 1976. The microhemagglutination assay for Treponema pallidum antibodies (MHA-TP), a new treponemal test for syphilis: where does it fit? J. Am. Vener. Dis. Assoc. 3:3–8 [PubMed] [Google Scholar]
- 167.Tomizawa T. 1966. Hemagglutination tests for diagnosis of syphilis. A preliminary report. Jpn. J. Med. Sci. Biol. 19:305–308 [DOI] [PubMed] [Google Scholar]
- 168.Deguchi M, Hosotsubo H, Yamashita N, Ohmine T, Asari S. 1994. Evaluation of gelatin particle agglutination method for detection of Treponema pallidum antibody. Kansenshogaku Zasshi 68:1271–1277 [DOI] [PubMed] [Google Scholar]
- 169.Byrne RE, Laska S, Bell M, Larson D, Phillips J, Todd J. 1992. Evaluation of a Treponema pallidum western immunoblot assay as a confirmatory test for syphilis. J. Clin. Microbiol. 30:115–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ebel A, Vanneste L, Cardinaels M, Sablon E, Samson I, De Bosschere K, Hulstaert F, Zrein M. 2000. Validation of the INNO-LIA syphilis kit as a confirmatory assay for Treponema pallidum antibodies. J. Clin. Microbiol. 38:215–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Veldkamp J, Visser AM. 1975. Application of the enzyme-linked immunosorbent assay (ELISA) in the serodiagnosis of syphilis. Br. J. Vener Dis. 51:227–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Marangoni A, Sambri V, Accardo S, Cavrini F, D'Antuono A, Moroni A, Storni E, Cevenini R. 2005. Evaluation of LIAISON Treponema Screen, a novel recombinant antigen-based chemiluminescence immunoassay for laboratory diagnosis of syphilis. Clin. Diagn. Lab. Immunol. 12:1231–1234. 10.1128/CDLI.12.10.1231-1234.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yoshioka N, Deguchi M, Kagita M, Kita M, Watanabe M, Asari S, Iwatani Y. 2007. Evaluation of a chemiluminescent microparticle immunoassay for determination of Treponema pallidum antibodies. Clin. Lab. 53:597–603 [PubMed] [Google Scholar]
- 174.Gomez E, Jespersen DJ, Harring JA, Binnicker MJ. 2010. Evaluation of the Bio-Rad BioPlex 2200 syphilis multiplex flow immunoassay for the detection of IgM- and IgG-class antitreponemal antibodies. Clin. Vaccine Immunol. 17:966–968. 10.1128/CVI.00086-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Perine PL, Nelson JW, Lewis JO, Liska S, Hunter EF, Larsen SA, Agadzi VK, Kofi F, Ofori JA, Tam MR. 1985. New technologies for use in the surveillance and control of yaws. Rev. Infect. Dis. 7(Suppl 2):S295–S299. 10.1093/clinids/7-Supplement_2.S295 [DOI] [PubMed] [Google Scholar]
- 176.Larsen SA, Pettit DE, Perryman MW, Hambie EA, Mullally R, Whittington W. 1983. EDTA-treated plasma in the rapid plasma reagin card test and the toluidine red unheated serum test for serodiagnosis of syphilis. J. Clin. Microbiol. 17:341–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Autier P, Delcambe JF, Sangare D, Lamine D, Kessler W, Goemaere E, Dallemagne G. 1989. Serological and clinical studies of endemic treponematosis in the Republic of Mali. Ann. Soc. Belg. Med. Trop. 69:319–329 [PubMed] [Google Scholar]
- 178.Herring AJ, Ballard RC, Pope V, Adegbola RA, Changalucha J, Fitzgerald DW, Hook EW, III, Kubanova A, Mananwatte S, Pape JW, Sturm AW, West B, Yin YP, Peeling RW. 2006. A multi-centre evaluation of nine rapid, point-of-care syphilis tests using archived sera. Sex. Transm. Infect. 82(Suppl 5):v7–v12. 10.1136/sti.2006.022707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mabey D, Peeling RW, Ballard R, Benzaken AS, Galban E, Changalucha J, Everett D, Balira R, Fitzgerald D, Joseph P, Nerette S, Li J, Zheng H. 2006. Prospective, multi-centre clinic-based evaluation of four rapid diagnostic tests for syphilis. Sex. Transm. Infect. 82(Suppl 5):v13–v16. 10.1136/sti.2006.022467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Benzaken AS, Sabido M, Galban EG, Pedroza V, Vasquez F, Araujo A, Peeling RW, Mayaud P. 2008. Field evaluation of the performance and testing costs of a rapid point-of-care test for syphilis in a red-light district of Manaus, Brazil. Sex. Transm. Infect. 84:297–302. 10.1136/sti.2007.029462 [DOI] [PubMed] [Google Scholar]
- 181.Mishra S, Naik B, Venugopal B, Kudur P, Washington R, Becker M, Kenneth J, Jayanna K, Ramesh BM, Isac S, Boily MC, Blanchard JF, Moses S. 2010. Syphilis screening among female sex workers in Bangalore, India: comparison of point-of-care testing and traditional serological approaches. Sex. Transm. Infect. 86:193–198. 10.1136/sti.2009.038778 [DOI] [PubMed] [Google Scholar]
- 182.Castro AR, Esfandiari J, Kumar S, Ashton M, Kikkert SE, Park MM, Ballard RC. 2010. Novel point-of-care test for simultaneous detection of nontreponemal and treponemal antibodies in patients with syphilis. J. Clin. Microbiol. 48:4615–4619. 10.1128/JCM.00624-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Castro AR, Mody HC, Parab SY, Patel MT, Kikkert SE, Park MM, Ballard RC. 2010. An immunofiltration device for the simultaneous detection of non-treponemal and treponemal antibodies in patients with syphilis. Sex. Transm. Infect. 86:532–536. 10.1136/sti.2010.042937 [DOI] [PubMed] [Google Scholar]
- 184.Yin YP, Chen XS, Wei WH, Gong KL, Cao WL, Yong G, Feng L, Huang SJ, Wang DM, Han Y, Chen SC, Mabey D, Peeling RW. 2013. A dual point-of-care test shows good performance in simultaneously detecting nontreponemal and treponemal antibodies in patients with syphilis: a multisite evaluation study in China. Clin. Infect. Dis. 56:659–665. 10.1093/cid/cis928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Baker-Zander SA, Lukehart SA. 1983. Molecular basis of immunological cross-reactivity between Treponema pallidum and Treponema pertenue. Infect. Immun. 42:634–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Thornburg RW, Baseman JB. 1983. Comparison of major protein antigens and protein profiles of Treponema pallidum and Treponema pertenue. Infect. Immun. 42:623–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Martin PM, Cockayne A, Georges AJ, Penn CW. 1990. Immune response to Treponema pertenue and Treponema pallidum Nichols in patients with yaws. Res. Microbiol. 141:181–186. 10.1016/0923-2508(90)90027-N [DOI] [PubMed] [Google Scholar]
- 188.Fohn MJ, Wignall S, Baker-Zander SA, Lukehart SA. 1988. Specificity of antibodies from patients with pinta for antigens of Treponema pallidum subspecies pallidum. J. Infect. Dis. 157:32–37. 10.1093/infdis/157.1.32 [DOI] [PubMed] [Google Scholar]
- 189.Noordhoek GT, Cockayne A, Schouls LM, Meleon RH, Stolz E, van Embden JD. 1990. A new attempt to distinguish serologically the subspecies of Treponema pallidum causing syphilis and yaws. J. Clin. Microbiol. 28:1600–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ito F, Hunter EF, George RW, Pope V, Larsen SA. 1992. Specific immunofluorescent staining of pathogenic treponemes with a monoclonal antibody. J. Clin. Microbiol. 30:831–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fribourg-Blanc A, Niel G, Mollaret HH. 1963. Note sur quelques aspects immunologiques du cynocephale africain. Bull. Soc. Pathol. Exot. 56:474–485 [PubMed] [Google Scholar]
- 192.Marchitto KS, Selland-Grossling CK, Norgard MV. 1986. Molecular specificities of monclonal antibodies directed against virulent Treponema pallidum. Infect. Immun. 51:168–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hanff PA, Fehniger TE, Miller JN, Lovett MA. 1982. Humoral immune response in human syphilis to polypeptides of Treponema pallidum. J. Immunol. 129:1287–1291 [PubMed] [Google Scholar]
- 194.Moskophidis M, Muller F. 1984. Molecular analysis of immunoglobulins M and G immune response to protein antigens of Treponema pallidum in human syphilis. Infect. Immun. 43:127–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Baker-Zander SA, Hook EW, III, Bonin P, Handsfield HH, Lukehart SA. 1985. Antigens of Treponema pallidum recognized by IgG and IgM antibodies during syphilis in humans. J. Infect. Dis. 151:264–272. 10.1093/infdis/151.2.264 [DOI] [PubMed] [Google Scholar]
- 196.Romanowski B, Forsey E, Prasad E, Lukehart S, Tam M, Hook EW., III 1987. Detection of Treponema pallidum by a fluorescent monoclonal antibody test. Sex. Transm. Dis. 14:156–159. 10.1097/00007435-198707000-00007 [DOI] [PubMed] [Google Scholar]
- 197.Hook EW, III, Roddy RE, Lukehart SA, Hom J, Holmes KK, Tam MR. 1985. Detection of Treponema pallidum in lesion exudate with a pathogen-specific monoclonal antibody. J. Clin. Microbiol. 22:241–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lukehart SA, Tam MR, Hom J, Baker-Zander SA, Holmes KK, Nowinski RC. 1985. Characterization of monoclonal antibodies to Treponema pallidum. J. Immunol. 134:585–592 [PubMed] [Google Scholar]
- 199.Grimprel E, Sanchez PJ, Wendel GD, Burstain JM, McCracken GH, Jr, Radolf JD, Norgard MV. 1991. Use of polymerase chain reaction and rabbit infectivity testing to detect Treponema pallidum in amniotic fluid, fetal and neonatal sera, and cerebrospinal fluid. J. Clin. Microbiol. 29:1711–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sanchez PJ, Wendel GD, Jr, Grimprel E, Goldberg M, Hall M, Arencibia-Mireles O, Radolf JD, Norgard MV. 1993. Evaluation of molecular methodologies and rabbit infectivity testing for the diagnosis of congenital syphilis and neonatal central nervous system invasion by Treponema pallidum. J. Infect. Dis. 167:148–157. 10.1093/infdis/167.1.148 [DOI] [PubMed] [Google Scholar]
- 201.Hay PE, Clarke JR, Strugnell RA, Taylor-Robinson D, Goldmeier D. 1990. Use of the polymerase chain reaction to detect DNA sequences specific to pathogenic treponemes in cerebrospinal fluid. FEMS Microbiol. Lett. 56:233–238 [DOI] [PubMed] [Google Scholar]
- 202.Noordhoek GT, Wolters EC, de Jonge ME, van Embden JD. 1991. Detection by polymerase chain reaction of Treponema pallidum DNA in cerebrospinal fluid from neurosyphilis patients before and after antibiotic treatment. J. Clin. Microbiol. 29:1976–1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Burstain JM, Grimprel E, Lukehart SA, Norgard MV, Radolf JD. 1991. Sensitive detection of Treponema pallidum by using the polymerase chain reaction. J. Clin. Microbiol. 29:62–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Liu H, Rodes B, Chen CY, Steiner B. 2001. New tests for syphilis: rational design of a PCR method for detection of Treponema pallidum in clinical specimens using unique regions of the DNA polymerase I gene. J. Clin. Microbiol. 39:1941–1946. 10.1128/JCM.39.5.1941-1946.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Centurion-Lara A, Castro C, Shaffer JM, Van Voorhis WC, Marra CM, Lukehart SA. 1997. Detection of Treponema pallidum by a sensitive reverse transcriptase PCR. J. Clin. Microbiol. 35:1348–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Schaible UE, Wallich R, Kramer MD, Gern L, Anderson JF, Museteanu C, Simon MM. 1993. Immune sera to individual Borrelia burgdorferi isolates or recombinant OspA thereof protect SCID mice against infection with homologous strains but only partially or not at all against those of different OspA/OspB genotype. Vaccine 11:1049–1054. 10.1016/0264-410X(93)90132-H [DOI] [PubMed] [Google Scholar]
- 207.Hay PE, Clarke JR, Taylor-Robinson D, Goldmeier D. 1990. Detection of treponemal DNA in the CSF of patients with syphilis and HIV infection using the polymerase chain reaction. Genitourin. Med. 66:428–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Orle KA, Gates CA, Martin DH, Body BA, Weiss JB. 1996. Simultaneous PCR detection of Haemophilus ducreyi, Treponema pallidum, and herpes simplex virus types 1 and 2 from genital ulcers. J. Clin. Microbiol. 34:49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Palmer HM, Higgins SP, Herring AJ, Kingston MA. 2003. Use of PCR in the diagnosis of early syphilis in the United Kingdom. Sex. Transm. Infect. 79:479–483. 10.1136/sti.79.6.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Castro R, Prieto E, Aguas MJ, Manata MJ, Botas J, Santo I, Azevedo J, Pereira FL. 2007. Detection of Treponema pallidum sp pallidum DNA in latent syphilis. Int. J. STD AIDS 18:842–845. 10.1258/095646207782716901 [DOI] [PubMed] [Google Scholar]
- 211.Grange PA, Gressier L, Dion PL, Farhi D, Benhaddou N, Gerhardt P, Morini JP, Deleuze J, Pantoja C, Bianchi A, Lassau F, Avril MF, Janier M, Dupin N. 2012. Evaluation of a PCR test for detection of Treponema pallidum in swabs and blood. J. Clin. Microbiol. 50:546–552. 10.1128/JCM.00702-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chen CY, Chi KH, George RW, Cox DL, Srivastava A, Rui Silva M, Carneiro F, Lauwers GY, Ballard RC. 2006. Diagnosis of gastric syphilis by direct immunofluorescence staining and real-time PCR testing. J. Clin. Microbiol. 44:3452–3456. 10.1128/JCM.00721-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Scott LJ, Gunson RN, Carman WF, Winter AJ. 2010. A new multiplex real-time PCR test for HSV1/2 and syphilis: an evaluation of its impact in the laboratory and clinical setting. Sex. Transm. Infect. 86:537–539. 10.1136/sti.2009.040451 [DOI] [PubMed] [Google Scholar]
- 214.Behrhof W, Springer E, Brauninger W, Kirkpatrick CJ, Weber A. 2008. PCR testing for Treponema pallidum in paraffin-embedded skin biopsy specimens: test design and impact on the diagnosis of syphilis. J. Clin. Pathol. 61:390–395. 10.1136/jcp.2007.046714 [DOI] [PubMed] [Google Scholar]
- 215.Heymans R, van der Helm JJ, de Vries HJ, Fennema HS, Coutinho RA, Bruisten SM. 2010. Clinical value of Treponema pallidum real-time PCR for diagnosis of syphilis. J. Clin. Microbiol. 48:497–502. 10.1128/JCM.00720-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Koek AG, Bruisten SM, Dierdorp M, van Dam AP, Templeton K. 2006. Specific and sensitive diagnosis of syphilis using a real-time PCR for Treponema pallidum. Clin. Microbiol. Infect. 12:1233–1236. 10.1111/j.1469-0691.2006.01566.x [DOI] [PubMed] [Google Scholar]
- 217.Leslie DE, Azzato F, Karapanagiotidis T, Leydon J, Fyfe J. 2007. Development of a real-time PCR assay to detect Treponema pallidum in clinical specimens and assessment of the assay's performance by comparison with serological testing. J. Clin. Microbiol. 45:93–96. 10.1128/JCM.01578-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Centurion-Lara A, Sun ES, Barrett LK, Castro C, Lukehart SA, Van Voorhis WC. 2000. Multiple alleles of Treponema pallidum repeat gene D in Treponema pallidum isolates. J. Bacteriol. 182:2332–2335. 10.1128/JB.182.8.2332-2335.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Pillay A, Chen CY, Reynolds MG, Mombouli JV, Castro AC, Louvouezo D, Steiner B, Ballard RC. 2011. Laboratory-confirmed case of yaws in a 10-year-old boy from the Republic of the Congo. J. Clin. Microbiol. 49:4013–4015. 10.1128/JCM.01121-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Liu H, Rodes B, George R, Steiner B. 2007. Molecular characterization and analysis of a gene encoding the acidic repeat protein (Arp) of Treponema pallidum. J. Med. Microbiol. 56:715–721. 10.1099/jmm.0.46943-0 [DOI] [PubMed] [Google Scholar]
- 221.Fanella S, Kadkhoda K, Shuel M, Tsang R. 2012. Local transmission of imported endemic syphilis, Canada, 2011. Emerg. Infect. Dis. 18:1002–1004. 10.3201/eid1806.111421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lukehart SA, Godornes C, Molini BJ, Sonnett P, Hopkins S, Mulcahy F, Engelman J, Mitchell SJ, Rompalo AM, Marra CM, Klausner JD. 2004. Macrolide resistance in Treponema pallidum in the United States and Ireland. N. Engl. J. Med. 351:154–158. 10.1056/NEJMoa040216 [DOI] [PubMed] [Google Scholar]
- 223.Pandori MW, Gordones C, Castro L, Engelman J, Siedner M, Lukehart S, Klausner J. 2007. Detection of azithromycin resistance in Treponema pallidum by real-time PCR. Antimicrob. Agents Chemother. 51:3425–3430. 10.1128/AAC.00340-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Chen CY, Chi KH, Pillay A, Nachamkin E, Su JR, Ballard RC. 2013. Detection of the A2058G and A2059G 23S rRNA gene point mutations associated with azithromycin resistance in Treponema pallidum by use of a TaqMan real-time multiplex PCR assay. J. Clin. Microbiol. 51:908–913. 10.1128/JCM.02770-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Engelkens HJ, Vuzevski VD, Stolz E. 1999. Nonvenereal treponematoses in tropical countries. Clin. Dermatol. 17:143–152. 10.1016/S0738-081X(99)00007-3 [DOI] [PubMed] [Google Scholar]
- 226.Engelkens HJ, Niemel PL, van der Sluis JJ, Meheus A, Stolz E. 1991. Endemic treponematoses. II. Pinta and endemic syphilis. Int. J. Dermatol 30:231–238 [DOI] [PubMed] [Google Scholar]
- 227.Shimkin MB. 1946. The World Health Organization. Science 104:281–283. 10.1126/science.104.2700.281 [DOI] [PubMed] [Google Scholar]
- 228.Guthe T. 1960. The treponematoses as a world problem. Br. J. Vener. Dis. 36:67–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Antal GM, Causse G. 1985. The control of endemic treponematoses. Rev. Infect. Dis. 7(Suppl 2):S220–S226. 10.1093/clinids/7-Supplement_2.S220 [DOI] [PubMed] [Google Scholar]
- 230.WHO 1988. Four decades of achievement: 1948-1988. Highlights of the work of WHO. WHO, Geneva, Switzerland [Google Scholar]
- 231.Anonymous. 1980. Yaws again. Br. Med. J. 281:1090–1091. 10.1136/bmj.281.6248.1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.WHO 1978. Control of endemic treponematoses. World Health Assembly resolution WHA 31.58. WHO, Geneva, Switzerland [Google Scholar]
- 233.WHO 1986. Yaws and other endemic treponematoses AFR/CD/58. Regional meeting. World Health Organization, Brazzaville, Africa [Google Scholar]
- 234.WHO 1995. Informal consultation on endemic treponematoses. WHO/EMC/95.3. WHO, Geneva, Switzerland [Google Scholar]
- 235.Herve V, Kassa Kelembho E, Normand P, Georges A, Mathiot C, Martin P. 1992. Resurgence of yaws in Central African Republic. Role of the Pygmy population as a reservoir of the virus. Bull. Soc. Pathol. Exot. 85:342–346 [PubMed] [Google Scholar]
- 236.Edorh AA, Siamevi EK, Adanlete FA, Aflagah EK, Kassankogno Y, Amouzou AB, Lodonou KG. 1994. Resurgence of endemic yaws in Togo. Cause and eradication approach. Bull. Soc. Pathol. Exot. 87:17–18 [PubMed] [Google Scholar]
- 237.Akogun OB. 1999. Yaws and syphilis in the Garkida area of Nigeria. Zentralbl. Bakteriol. 289:101–107. 10.1016/S0934-8840(99)80130-3 [DOI] [PubMed] [Google Scholar]
- 238.Nnoruka EN. 2005. Skin diseases in south-east Nigeria: a current perspective. Int. J. Dermatol. 44:29–33. 10.1111/j.1365-4632.2004.02485.x [DOI] [PubMed] [Google Scholar]
- 239.Toure B, Koffi NM, Assi KP, Ake O, Konan DJ. 2007. Yaws in Cote d'lvoire: health problem forgotten and neglected. Bull. Soc. Pathol. Exot. 100:130–132 [PubMed] [Google Scholar]
- 240.WHO 2012. Summary report of the consultative meeting on eradication of yaws, 5-7 March 2012, Morges, Switzerland. WHO, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/75528/1/WHO_HTM_NTD_IDM_2012.2_eng.pdf [Google Scholar]
- 241.Geizer I. 1986. Yaws in the Western Pacific region: an overview. Southeast Asian J. Trop. Med. Public Health 17:8–13 [PubMed] [Google Scholar]
- 242.Fegan D, Glennon M, Macbride-Stewart G, Moore T. 1990. Yaws in the Solomon Islands. J. Trop. Med. Hyg. 93:52–57 [PubMed] [Google Scholar]
- 243.de Noray G, Capuano C, Abel M. 2003. Campaign to eradicate yaws on Santo Island, Vanuatu in 2001. Med. Trop. (Mars.) 63:159–162 [PubMed] [Google Scholar]
- 244.Reid MS, Talwat EN, McNamara KM, Garner MF. 1983. Fluctuations in antibody levels in infection with Treponema pertenue. A four-year follow-up of Karkar islanders with early yaws. Australas J. Dermatol. 24:71–78 [DOI] [PubMed] [Google Scholar]
- 245.Reid MS. 1985. Yaws in Papua New Guinea: extent of the problem and status of control programs. Rev. Infect. Dis. 7(Suppl 2):S254–S259. 10.1093/clinids/7-Supplement_2.S254 [DOI] [PubMed] [Google Scholar]
- 246.Garner PA, Talwat EN, Hill G, Reid MS, Garner MF. 1986. Yaws reappears. P. N. G. Med. J. 29:247–252 [PubMed] [Google Scholar]
- 247.Talwat E. 1986. Papua New Guinea. Yaws problems assessed. Southeast Asian J. Trop. Med. Public Health 17:59–65 [PubMed] [Google Scholar]
- 248.Duncan LE, Alto W. 1987. An investigation of yaws on the Trobriand Islands, 1985. P. N. G. Med. J. 30:57–61 [PubMed] [Google Scholar]
- 249.Backhouse JL, Hudson BJ, Hamilton PA, Nesteroff SI. 1998. Failure of penicillin treatment of yaws on Karkar Island, Papua New Guinea. Am. J. Trop. Med. Hyg. 59:388–392 [DOI] [PubMed] [Google Scholar]
- 250.Manning LA, Ogle GD. 2002. Yaws in the periurban settlements of Port Moresby, Papua New Guinea. P. N. G. Med. J. 45:206–212 [PubMed] [Google Scholar]
- 251.Eason RJ, Tasman-Jones T, Henry A, Somerfield SD, Jones G. 1985. Resurgent yaws in the Solomon Islands. Aust. N. Z. J. Med. 15:727–730 [PubMed] [Google Scholar]
- 252.Eason RJ, Tasman-Jones T. 1985. Resurgent yaws and other skin diseases in the Western Province of the Solomon Islands. P. N. G. Med. J. 28:247–250 [PubMed] [Google Scholar]
- 253.Alemaena O. 1986. Yaws situation in the Solomon Islands. Southeast Asian J. Trop. Med. Public Health 17:14–18 [PubMed] [Google Scholar]
- 254.Harris M, Nako D, Hopkins T, Powell DM, Kenny C, Carroll C, Carroll K. 1991. Yaws infection in Tanna, Vanuatu 1989. Southeast Asian J. Trop. Med. Public Health 22:113–119 [PubMed] [Google Scholar]
- 255.Fegan D, Glennon MJ, Thami Y, Pakoa G. 2010. Resurgence of yaws in Tanna, Vanuatu: time for a new approach? Trop. Doct. 40:68–69. 10.1258/td.2009.090249 [DOI] [PubMed] [Google Scholar]
- 256.Hopkins DR. 1977. Yaws in the Americas, 1950-1975. J. Infect. Dis. 136:548–554. 10.1093/infdis/136.4.548 [DOI] [PubMed] [Google Scholar]
- 257.Anselmi M, Araujo E, Narvaez A, Cooper PJ, Guderian RH. 1995. Yaws in Ecuador: impact of control measures on the disease in the Province of Esmeraldas. Genitourin. Med. 71:343–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Anselmi M, Moreira JM, Caicedo C, Guderian R, Tognoni G. 2003. Community participation eliminates yaws in Ecuador. Trop. Med. Int. Health 8:634–638. 10.1046/j.1365-3156.2003.01073.x [DOI] [PubMed] [Google Scholar]
- 259.Scolnik D, Aronson L, Lovinsky R, Toledano K, Glazier R, Eisenstadt J, Eisenberg P, Wilcox L, Rowsell R, Silverman M. 2003. Efficacy of a targeted, oral penicillin-based yaws control program among children living in rural South America. Clin. Infect. Dis. 36:1232–1238. 10.1086/374338 [DOI] [PubMed] [Google Scholar]
- 260.WHO 2008. Elimination of yaws in India. Wkly. Epidemiol. Rec. 83:125–132 [PubMed] [Google Scholar]
- 261.Farnsworth N, Rosen T. 2006. Endemic treponematosis: review and update. Clin. Dermatol. 24:181–190. 10.1016/j.clindermatol.2005.11.004 [DOI] [PubMed] [Google Scholar]
- 262.Gazin P, Meynard D. 1988. A clinical and serologic survey of bejel in north Burkina Faso. Bull. Soc. Pathol. Exot. Filiales. 81:827–831 [PubMed] [Google Scholar]
- 263.Julvez J, Michault A, Kerdelhue V. 1998. Serologic studies of non-venereal treponematoses in infants in Niamey, Niger. Med. Trop. (Mars.) 58:38–40 [PubMed] [Google Scholar]
- 264.Galoo E, Schmoor P. 1998. Identification of a focus of bejel in Mauritania. Med. Trop. (Mars.) 58:311–312 [PubMed] [Google Scholar]
- 265.Yakinci C, Ozcan A, Aslan T, Demirhan B. 1995. Bejel in Malatya, Turkey. J. Trop. Pediatr. 41:117–120. 10.1093/tropej/41.2.117 [DOI] [PubMed] [Google Scholar]
- 266.Monjour L, Niel G, Palminteri R, Sidat M, Daniel-Ribeiro C, Alfred C, Tselentis I, Gentilini M. 1984. A screening of serological syphilis in Mauritania. Acta Trop. 41:81–86 [PubMed] [Google Scholar]
- 267.Clyti E, dosSantos RB. 2007. Endemic treponematoses in Maputo, Mozambique. Bull. Soc. Pathol. Exot. 100:107–108 [PubMed] [Google Scholar]
- 268.Abdolrasouli A, Croucher A, Hemmati Y, Mabey D. 2013. A case of endemic syphilis, Iran. Emerg. Infect. Dis. 19:162–163. 10.3201/eid1901.120756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Pecher SA, Croce J. 1988. Immunology of tertiary pinta. Med. Cutan. Ibero Lat. Am. 16:111–114 [PubMed] [Google Scholar]
- 270.Hopkins DR, Florez D. 1977. Pinta, yaws, and venereal syphilis in Colombia. Int. J. Epidemiol. 6:349–355. 10.1093/ije/6.4.349 [DOI] [PubMed] [Google Scholar]
- 271.Gosset Osuna IG. 1969. Endemic pinta. Present situation in Mexico. Salud Publica Mex. 11:211–215 [PubMed] [Google Scholar]
- 272.Giuliani M, Latini A, Palamara G, Maini A, Di Carlo A. 2005. The clinical appearance of pinta mimics secondary syphilis: another trap of treponematosis? Clin. Infect. Dis. 40:1548. 10.1086/429729 [DOI] [PubMed] [Google Scholar]
- 273.Kim SC, Guerrero R, Gonzalez R. 2004. A 23-year-old pregnant woman with left-foot and left-ankle ulceration. Clin. Infect. Dis. 39:81–82. 10.1086/421560 [DOI] [PubMed] [Google Scholar]
- 274.Woltsche-Kahr I, Schmidt B, Aberer W, Aberer E. 1999. Pinta in Austria (or Cuba?): import of an extinct disease? Arch. Dermatol. 135:685–688. 10.1001/archderm.135.6.685 [DOI] [PubMed] [Google Scholar]
- 275.Engelkens HJ, Oranje AP, Stolz E. 1989. Early yaws, imported in The Netherlands. Genitourin. Med. 65:316–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Fry L, Rodin P. 1966. Early yaws. Br. J. Vener. Dis. 42:28–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Vabres P, Roose B, Berdah S, Fraitag S, Prost YD. 1999. Bejel: an unusual cause of stomatitis in the child. Ann. Dermatol. Venereol. 126:49–50 [PubMed] [Google Scholar]
- 278.Vabres P. 1999. Endemic treponemal infections in international adoptees and immigrant children: how common are they? Pediatr. Dermatol. 28:214–215 [DOI] [PubMed] [Google Scholar]
- 279.WHO 2012. Accelerating work to overcome the global impact of neglected tropical diseases: a roadmap for implementation-executive summary. WHO/HTM/NTD/2012.1. WHO, Geneva, Switzerland [Google Scholar]
- 280.WHO 2007. Yaws: a forgotten disease. WHO, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs316/en/ [Google Scholar]
- 281.Weigel LM, Radolf JD, Norgard MV. 1994. The 47-kDa major lipoprotein immunogen of Treponema pallidum is a penicillin-binding protein with carboxypeptidase activity. Proc. Natl. Acad. Sci. U. S. A. 91:11611–11615. 10.1073/pnas.91.24.11611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Cha JY, Ishiwata A, Mobashery S. 2004. A novel beta-lactamase activity from a penicillin-binding protein of Treponema pallidum and why syphilis is still treatable with penicillin. J. Biol. Chem. 279:14917–14921. 10.1074/jbc.M400666200 [DOI] [PubMed] [Google Scholar]
- 283.Mitja O, Hays R, Ipai A, Penias M, Paru R, Fagaho D, de Lazzari E, Bassat Q. 2012. Single-dose azithromycin versus benzathine benzylpenicillin for treatment of yaws in children in Papua New Guinea: an open-label, non-inferiority, randomised trial. Lancet 379:342–347. 10.1016/S0140-6736(11)61624-3 [DOI] [PubMed] [Google Scholar]
- 284.Martin DH, Mroczkowski TF, Dalu ZA, McCarty J, Jones RB, Hopkins SJ, Johnson RB. 1992. A controlled trial of a single dose of azithromycin for the treatment of chlamydial urethritis and cervicitis. The Azithromycin for Chlamydial Infections Study Group. N. Engl. J. Med. 327:921–925 [DOI] [PubMed] [Google Scholar]
- 285.Handsfield HH, Dalu ZA, Martin DH, Douglas JM, Jr, McCarty JM, Schlossberg D. 1994. Multicenter trial of single-dose azithromycin vs. ceftriaxone in the treatment of uncomplicated gonorrhea. Azithromycin Gonorrhea Study Group. Sex. Transm. Dis. 21:107–111 [DOI] [PubMed] [Google Scholar]
- 286.Martin DH, Sargent SJ, Wendel GD, Jr, McCormack WM, Spier NA, Johnson RB. 1995. Comparison of azithromycin and ceftriaxone for the treatment of chancroid. Clin. Infect. Dis. 21:409–414. 10.1093/clinids/21.2.409 [DOI] [PubMed] [Google Scholar]
- 287.Verdon MS, Handsfield HH, Johnson RB. 1994. Pilot study of azithromycin for treatment of primary and secondary syphilis. Clin. Infect. Dis. 19:486–488. 10.1093/clinids/19.3.486 [DOI] [PubMed] [Google Scholar]
- 288.Mashkilleyson AL, Gomberg MA, Mashkilleyson N, Kutin SA. 1996. Treatment of syphilis with azithromycin. Int. J. STD AIDS 7(Suppl 1):13–15. 10.1258/0956462961917726 [DOI] [PubMed] [Google Scholar]
- 289.Hook EW, III, Stephens J, Ennis DM. 1999. Azithromycin compared with penicillin G benzathine for treatment of incubating syphilis. Ann. Intern. Med. 131:434–437. 10.7326/0003-4819-131-6-199909210-00007 [DOI] [PubMed] [Google Scholar]
- 290.Riedner G, Rusizoka M, Todd J, Maboko L, Hoelscher M, Mmbando D, Samky E, Lyamuya E, Mabey D, Grosskurth H, Hayes R. 2005. Single-dose azithromycin versus penicillin G benzathine for the treatment of early syphilis. N. Engl. J. Med. 353:1236–1244. 10.1056/NEJMoa044284 [DOI] [PubMed] [Google Scholar]
- 291.Kiddugavu MG, Kiwanuka N, Wawer MJ, Serwadda D, Sewankambo NK, Wabwire-Mangen F, Makumbi F, Li X, Reynolds SJ, Quinn TC, Gray RH. 2005. Effectiveness of syphilis treatment using azithromycin and/or benzathine penicillin in Rakai, Uganda. Sex. Transm. Dis. 32:1–6. 10.1097/01.olq.0000148297.48590.d8 [DOI] [PubMed] [Google Scholar]
- 292.Hook EW, III, Behets F, Van Damme K, Ravelomanana N, Leone P, Sena AC, Martin D, Langley C, McNeil L, Wolff M. 2010. A phase III equivalence trial of azithromycin versus benzathine penicillin for treatment of early syphilis. J. Infect. Dis. 201:1729–1735. 10.1086/652239 [DOI] [PubMed] [Google Scholar]
- 293.Mitchell SJ, Engelman J, Kent CK, Lukehart SA, Godornes C, Klausner JD. 2006. Azithromycin-resistant syphilis infection: San Francisco, California, 2000-2004. Clin. Infect. Dis. 42:337–345. 10.1086/498899 [DOI] [PubMed] [Google Scholar]
- 294.CDC 2004. Azithromycin treatment failures in syphilis infections—San Francisco, California, 2002-2003. MMWR Morb. Mortal. Wkly. Rep. 53:197–198 [PubMed] [Google Scholar]
- 295.Chen JC. 2004. Update on emerging infections: news from the Centers for Disease Control and Prevention. Brief report: azithromycin treatment failures in syphilis infections—San Francisco, California, 2002-2003. Ann. Emerg. Med. 44:232–234. 10.1016/j.annemergmed.2004.06.002 [DOI] [PubMed] [Google Scholar]
- 296.Stamm LV, Bergen HL. 2000. A point mutation associated with bacterial macrolide resistance is present in both 23S rRNA genes of an erythromycin-resistant Treponema pallidum clinical isolate. Antimicrob. Agents Chemother. 44:806–807. 10.1128/AAC.44.3.806-807.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Matejkova P, Flasarova M, Zakoucka H, Borek M, Kremenova S, Arenberger P, Woznicova V, Weinstock GM, Smajs D. 2009. Macrolide treatment failure in a case of secondary syphilis: a novel A2059G mutation in the 23S rRNA gene of Treponema pallidum subsp. pallidum. J. Med. Microbiol. 58:832–836. 10.1099/jmm.0.007542-0 [DOI] [PubMed] [Google Scholar]
- 298.Marra CM, Colina AP, Godornes C, Tantalo LC, Puray M, Centurion-Lara A, Lukehart SA. 2006. Antibiotic selection may contribute to increases in macrolide-resistant Treponema pallidum. J. Infect. Dis. 194:1771–1773. 10.1086/509512 [DOI] [PubMed] [Google Scholar]
- 299.Katz KA, Pillay A, Ahrens K, Kohn RP, Hermanstyne K, Bernstein KT, Ballard RC, Klausner JD. 2010. Molecular epidemiology of syphilis—San Francisco, 2004-2007. Sex. Transm. Dis. 37:660–663 [DOI] [PubMed] [Google Scholar]
- 300.Katz KA, Klausner JD. 2008. Azithromycin resistance in Treponema pallidum. Curr. Opin. Infect. Dis. 21:83–91. 10.1097/QCO.0b013e3282f44772 [DOI] [PubMed] [Google Scholar]
- 301.Grimes M, Sahi SK, Godornes BC, Tantalo LC, Roberts N, Bostick D, Marra CM, Lukehart SA. 2012. Two mutations associated with macrolide resistance in Treponema pallidum: increasing prevalence and correlation with molecular strain type in Seattle, Washington. Sex. Transm. Dis. 39:954–958. 10.1097/OLQ.0b013e31826ae7a8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Martin IE, Gu W, Yang Y, Tsang RS. 2009. Macrolide resistance and molecular types of Treponema pallidum causing primary syphilis in Shanghai, China. Clin. Infect. Dis. 49:515–521. 10.1086/600878 [DOI] [PubMed] [Google Scholar]
- 303.Muller EE, Paz-Bailey G, Lewis DA. 2012. Macrolide resistance testing and molecular subtyping of Treponema pallidum strains from southern Africa. Sex. Transm. Infect. 88:470–474. 10.1136/sextrans-2011-050322 [DOI] [PubMed] [Google Scholar]
- 304.Van Damme K, Behets F, Ravelomanana N, Godornes C, Khan M, Randrianasolo B, Rabenja NL, Lukehart S, Cohen M, Hook E. 2009. Evaluation of azithromycin resistance in Treponema pallidum specimens from Madagascar. Sex. Transm. Dis. 36:775–776. 10.1097/OLQ.0b013e3181bd11dd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Wu H, Chang SY, Lee NY, Huang WC, Wu BR, Yang CJ, Liang SH, Lee CH, Ko WC, Lin HH, Chen YH, Liu WC, Su YC, Hsieh CY, Wu PY, Hung CC. 2012. Evaluation of macrolide resistance and enhanced molecular typing of Treponema pallidum in patients with syphilis in Taiwan: a prospective multicenter study. J. Clin. Microbiol. 50:2299–2304. 10.1128/JCM.00341-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Hudson BJ, van der Meijden WI, Lupiwa T, Howard P, Tabua T, Tapsall JW, Phillips EA, Lennox VA, Backhouse JL, Pyakalyia T. 1994. A survey of sexually transmitted diseases in five STD clinics in Papua New Guinea. P. N. G. Med. J. 37:152–160 [PubMed] [Google Scholar]
- 307.Tiwara S, Passey M, Clegg A, Mgone C, Lupiwa S, Suve N, Lupiwa T. 1996. High prevalence of trichomonal vaginitis and chlamydial cervicitis among a rural population in the highlands of Papua New Guinea. P. N. G. Med. J. 39:234–238 [PubMed] [Google Scholar]
- 308.Passey M, Mgone CS, Lupiwa S, Suve N, Tiwara S, Lupiwa T, Clegg A, Alpers MP. 1998. Community based study of sexually transmitted diseases in rural women in the highlands of Papua New Guinea: prevalence and risk factors. Sex. Transm. Infect. 74:120–127. 10.1136/sti.74.2.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Mgone CS, Lupiwa T, Yeka W. 2002. High prevalence of Neisseria gonorrhoeae and multiple sexually transmitted diseases among rural women in the Eastern Highlands Province of Papua New Guinea, detected by polymerase chain reaction. Sex. Transm. Dis. 29:775–779. 10.1097/00007435-200212000-00007 [DOI] [PubMed] [Google Scholar]
- 310.Gare J, Lupiwa T, Suarkia DL, Paniu MM, Wahasoka A, Nivia H, Kono J, Yeka W, Reeder JC, Mgone CS. 2005. High prevalence of sexually transmitted infections among female sex workers in the eastern highlands province of Papua New Guinea: correlates and recommendations. Sex. Transm. Dis. 32:466–473. 10.1097/01.olq.0000161177.21639.96 [DOI] [PubMed] [Google Scholar]
- 311.Bruce E, Bauai L, Masta A, Rooney PJ, Paniu M, Sapuri M, Keogh L, Kaldor J, Fairley CK. 2010. A cross-sectional study of reported symptoms for sexually transmissible infections among female sex workers in Papua New Guinea. Sex Health 7:71–76. 10.1071/SH09093 [DOI] [PubMed] [Google Scholar]
- 312.Mollaret H, Fribourg-Blanc A. 1967. Le singe serait-il reservoir du Pian? Med. d'Afrique Noire 14:397–399 [Google Scholar]
- 313.Fribourg-Blanc A, Mollaret HH. 1969. Natural treponematosis of the African primate. Primates Med. 3:113–121 [PubMed] [Google Scholar]
- 314.Fribourg-Blanc A, Mollaret HH, Niel G. 1966. Serologic and microscopic confirmation of treponemosis in Guinea baboons. Bull. Soc. Pathol. Exot. Filiales. 59:54–59 [PubMed] [Google Scholar]
- 315.Felsenfeld O, Wolf RH. 1971. Serological reactions with treponemal antigens in nonhuman primates and the natural history of treponematosis in man. Folia Primatol. (Basel) 16:294–305. 10.1159/000155411 [DOI] [PubMed] [Google Scholar]
- 316.Knauf S, Batamuzi EK, Mlengeya T, Kilewo M, Lejora IA, Nordhoff M, Ehlers B, Harper KN, Fyumagwa R, Hoare R, Failing K, Wehrend A, Kaup FJ, Leendertz FH, Matz-Rensing K. 2012. Treponema infection associated with genital ulceration in wild baboons. Vet. Pathol. 49:292–303. 10.1177/0300985811402839 [DOI] [PubMed] [Google Scholar]
- 317.Levréro F, Gatti S, Gautier-Hion A, Ménard N. 2007. Yaws disease in a wild gorilla population and its impact on the reproductive status of males. Am. J. Phys. Anthropol. 132:568–575. 10.1002/ajpa.20560 [DOI] [PubMed] [Google Scholar]
- 318.Cousins D. 1972. Diseases and injuries in wild and captive gorillas (Gorilla gorilla). Int. Zoo Yearbook 12:211–218. 10.1111/j.1748-1090.1972.tb02330.x [DOI] [Google Scholar]
- 319.Harper KN, Fyumagwa RD, Hoare R, Wambura PN, Coppenhaver DH, Sapolsky RM, Alberts SC, Tung J, Rogers J, Kilewo M, Batamuzi EK, Leendertz FH, Armelagos GJ, Knauf S. 2012. Treponema pallidum infection in the wild baboons of East Africa: distribution and genetic characterization of the strains responsible. PLoS One 7:e50882. 10.1371/journal.pone.0050882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Lovell NC, Jurmain R, Kilgore L. 2000. Skeletal evidence of probable treponemal 20 infection in free-ranging African apes. Primates 41:275–290. 10.1007/BF02557597 [DOI] [PubMed] [Google Scholar]
- 321.Baylet R, Thivolet J, Sepetjian M, Bert J. 1971. Seroepidemiological studies on primate treponematosis in Senegal. Bull. Soc. Pathol. Exot. Filiales. 64:836–841 [PubMed] [Google Scholar]
- 322.Baylet R, Thivolet J, Sepetjian M, Nouhouay Y, Baylet M. 1971. Natural open treponematosis in the Papio papio baboon in Casamance. Bull. Soc. Pathol. Exot. Filiales. 64:842–846 [PubMed] [Google Scholar]
- 323.Cousins D. 1984. Notes on the occurence of skin infections in gorillas (Gorilla gorilla). Zool. Garten N. F. Jena 4:333–338 [Google Scholar]
- 324.Wallis J, Lee DR. 1999. Primate conservation: the prevention of disease transmission. Int. J. Primatol. 20:803–825. 10.1023/A:1020879700286 [DOI] [Google Scholar]
- 325.Mlengeya T. 2004. Distribution pattern of a sexually transmitted disease (STD) of olive baboons in Lake Manyara National Park, Tanzania. College of African Wildlife Management, Moshi, Tanzania [Google Scholar]
- 326.Centurion-Lara A, Arroll T, Castillo R, Shaffer JM, Castro C, Van Voorhis WC, Lukehart SA. 1997. Conservation of the 15-kilodalton lipoprotein among Treponema pallidum subspecies and strains and other pathogenic treponemes: genetic and antigenic analyses. Infect. Immun. 65:1440–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Giacani L, Brandt SL, Puray-Chavez M, Brinck Reid T, Godornes C, Molini BJ, Benzler M, Hartig JS, Lukehart SA, Centurion-Lara A. 2012. Comparative investigation of the genomic regions involved in antigenic variation of the TprK antigen among treponemal species, subspecies, and strains. J. Bacteriol. 194:4208–4225. 10.1128/JB.00863-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Cejkova D, Zobanikova M, Pospisilova P, Strouhal M, Mikalova L, Weinstock GM, Smajs D. 2013. Structure of rrn operons in pathogenic non-cultivable treponemes: sequence but not genomic position of intergenic spacers correlates with classification of Treponema pallidum and Treponema paraluiscuniculi strains. J. Med. Microbiol. 62:196–207. 10.1099/jmm.0.050658-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Sepetjian M, Guerraz FT, Salussola D, Thivolet J, Monier JC. 1969. Contribution a l'etude du treponeme isole du singe par A. Bull. World Health Organ. 40:141–151 [PMC free article] [PubMed] [Google Scholar]
- 330.Wolfe ND, Prosser TA, Carr JK, Tamoufe U, Mpoudi-Ngole E, Torimiro JN, LeBreton M, McCutchan FE, Birx DL, Burke DS. 2004. Exposure to nonhuman primates in rural Cameroon. Emerg. Infect. Dis. 10:2094–2099. 10.3201/eid1012.040062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.LeBreton M, Yang O, Tamoufe U, Mpoudi-Ngole E, Torimiro JN, Djoko CF, Carr JK, Tassy Prosser A, Rimoin AW, Birx DL, Burke DS, Wolfe ND. 2007. Exposure to wild primates among HIV-infected persons. Emerg. Infect. Dis. 13:1579–1582. 10.3201/eid1310.070338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Grin EI. 1956. Endemic syphilis and yaws. Bull. World Health Organ. 15:959–973 [PMC free article] [PubMed] [Google Scholar]
- 333.Harding RD. 1949. A yaws campaign in Sierra Leone. Trans. R. Soc. Trop. Med. Hyg. 42:347–366. 10.1016/0035-9203(49)90083-8 [DOI] [PubMed] [Google Scholar]
- 334.Bendel EG. 1959. A yaws mass campaign in Liberia; organization and some preliminary results. Acta Trop. 16:69–75 [PubMed] [Google Scholar]
- 335.Da Cruz-Ferreira FS, Sterenberg H. 1956. Some aspects of yaws in Liberia. Am. J. Trop. Med. Hyg. 5:1036–1050 [DOI] [PubMed] [Google Scholar]
- 336.Gerstl S, Kiwila G, Dhorda M, Lonlas S, Myatt M, Ilunga BK, Lemasson D, Szumilin E, Guerin PJ, Ferradini L. 2009. Prevalence study of yaws in the Democratic Republic of Congo using the lot quality assurance sampling method. PLoS One 4:e6338. 10.1371/journal.pone.0006338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Morand JJ, Simon F, Garnotel E, Mahé A, Clity E, Morlain B. 2006. Overview of endemic treponematoses. Med. Trop. (Mars.) 66:15–20 [In French.] [PubMed] [Google Scholar]
- 338.Peel TN, Bhatti D, De Boer JC, Stratov I, Spelman DW. 2010. Chronic cutaneous ulcers secondary to Haemophilus ducreyi infection. Med. J. Aust. 192:348–350 [DOI] [PubMed] [Google Scholar]
- 339.McBride WJ, Hannah RC, Le Cornec GM, Bletchley C. 2008. Cutaneous chancroid in a visitor from Vanuatu. Australas. J. Dermatol. 49:98–99. 10.1111/j.1440-0960.2008.00439.x [DOI] [PubMed] [Google Scholar]
- 340.Ussher JE, Wilson E, Campanella S, Taylor SL, Roberts SA. 2007. Haemophilus ducreyi causing chronic skin ulceration in children visiting Samoa. Clin. Infect. Dis. 44:e85–e87. 10.1086/515404 [DOI] [PubMed] [Google Scholar]