Abstract
We have previously reported that some strains belonging to the marine Actinobacteria class, the Pseudoalteromonas genus, the Roseobacter clade, and the Photobacteriaceae and Vibrionaceae families produce both antibacterial and antivirulence compounds, and these organisms are interesting from an applied point of view as fish probiotics or as a source of pharmaceutical compounds. The application of either organisms or compounds requires that they do not cause any side effects, such as toxicity in eukaryotic organisms. The purpose of this study was to determine whether these bacteria or their compounds have any toxic side effects in the eukaryotic organisms Artemia sp. and Caenorhabditis elegans. Arthrobacter davidanieli WX-11, Pseudoalteromonas luteoviolacea S4060, P. piscicida S2049, P. rubra S2471, Photobacterium halotolerans S2753, and Vibrio coralliilyticus S2052 were lethal to either or both model eukaryotes. The toxicity of P. luteoviolacea S4060 could be related to the production of the antibacterial compound pentabromopseudilin, while the adverse effect observed in the presence of P. halotolerans S2753 and V. coralliilyticus S2052 could not be explained by the production of holomycin nor andrimid, the respective antibiotic compounds in these organisms. In contrast, the tropodithietic acid (TDA)-producing bacteria Phaeobacter inhibens DSM17395 and Ruegeria mobilis F1926 and TDA itself had no adverse effect on the target organisms. These results reaffirm TDA-producing Roseobacter bacteria as a promising group to be used as probiotics in aquaculture, whereas Actinobacteria, Pseudoalteromonas, Photobacteriaceae, and Vibrionaceae should be used with caution.
INTRODUCTION
Treatment of microbial diseases is necessary in humans, animals, and plants and, in the past several decades, it has become increasingly challenging due to the rapid development of microbial resistance to antibiotics and other antimicrobials. This leads to extra health care costs and losses in productivity, and the discovery of novel antimicrobial compounds is essential not only to ensure human health but also societal economy (1).
The marine environment represents a promising source of new bioactive compounds (2), and bacteria and fungi associated with marine macroorganisms, such as algae, invertebrates, or sponges, seem to be the most important producers (3, 4). The spectrum of activity of these biological active compounds ranges from pathogenic bacteria, fungi, and viruses to tumor cells (4). During two marine research expeditions (Galathea 3 and LOMROG II), our group isolated bacterial species with antibacterial activities. These species predominantly belonged to the Actinobacteria class, the Pseudoalteromonas genus, Roseobacter clade, and the Photobacteriaceae and Vibrionaceae families (3, 5, 6). Due to their antagonistic activity, live cultures of bacteria from Pseudoalteromonas, Phaeobacter, Ruegeria, and Vibrio genera are not only of interest as sources of novel pharmaceuticals but also as probiotics in fish and shellfish aquaculture systems (7–9).
Although many of the secondary metabolites derived from marine bacteria seem to be promising leads as new antibiotics, little is known about their potential effect on eukaryotes. Some Pseudoalteromonas, Phaeobacter, and Vibrio strains associated with macroalgae have a negative effect on the colonization of the algal surfaces by other bacteria and the settlement of invertebrates, such as barnacles or polychaetes (10). This effect has been related to the production of secondary metabolites, which seem to give an ecological advantage to the producing bacteria both against other microorganisms but also against bacterivorous eukaryotic predators (10–12). Consequently, the application of the producing bacteria or the bioactive compounds might cause adverse effects on the organisms to be treated (e.g., humans, animals, or plants) or, in the case of aquaculture, negatively affect the other organisms involved in fish or shellfish culture such as algae or live prey (e.g., rotifers and Artemia sp.). Hence, the toxicity of live bacterial cultures and bioactive compounds on the target organisms should be tested and any adverse effect ruled out before they can be applied. A complete toxicology assessment is a significant task, and we therefore, as a preassessment, determined the effect of the marine bacteria and their bioactive products in two model eukaryotic systems: Caenorhabditis elegans and Artemia sp.
C. elegans is a bacterivorous nematode that has a short life cycle, is easy to cultivate, and is one of the most commonly used organisms in biological research and toxicology studies (12, 13). C. elegans has been developed as a model to assess virulence of many human, animal, and plant microbial pathogens (14) and the toxicity of different compounds (11). The effects observed in C. elegans are similar to those observed in mammalian models (15). The crustacean Artemia spp. have also, in the recent years, gained importance as a model organism in ecotoxicology (16) since it adapts easily to changes in nutrients, salinity, temperature, and oxygen, is easy to culture, is resistant to manipulation, and has a short life cycle (17). Furthermore, Artemia spp. are used as live feed for cultured fish and shellfish in aquaculture, and this gives it additional value as a toxicity model in aquaculture systems, e.g., for testing probiotic cultures.
The purpose of the present study was to determine whether marine bacteria that produce antibiotic and/or antivirulence compounds had any potential toxic side effects in eukaryotic organisms. The toxicity of all live bacterial cultures was analyzed by using both Artemia sp. and C. elegans as eukaryotic models but, since the majority of the compounds were not commercially available and could be extracted only in small amounts, the toxicity of the pure compounds was only tested on Artemia sp.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains analyzed in the present study are listed in Table 1. Vibrio coralliilyticus DSM19607 (21) and Pseudoalteromonas tunicate D2 (22) were used as positive killing controls in Artemia sp. and C. elegans assays, respectively, and Escherichia coli OP50 (Caenorhabditis Genetics Center, University of Minnesota, Minneapolis, MN) was used as a negative killing control in C. elegans assays. Vibrio anguillarum 90-11-287 (23) was used as the target strain in a bioassay for testing the antagonism of the extracted secondary metabolites (24).
TABLE 1.
Bacterial strains and antibacterial compounds analyzed in this study
| Group | Species | Strain | Bioactive compound | Reference(s) |
|---|---|---|---|---|
| Actinobacteria class | Arthrobacter davidanieli | WX-11 | Arthrobacilin | 6 |
| Pseudoalteromonas genus | Pseudoalteromonas luteoviolacea | S4054 | Indolmycin | 3, 18 |
| S4047-1 | Indolmycin | |||
| S4060 | Pentabromopseudilin | |||
| S2607 | Pentabromopseudilin | |||
| Pseudoalteromonas piscicida | S2049 | Unknown | ||
| Pseudoalteromonas rubra | S2471 | Prodigiosin | ||
| Roseobacter clade | Phaeobacter inhibens (formerly Phaeobacter gallaeciensis) | DSM17395 | Tropodithietic acid | 19 |
| Ruegeria mobilis | F1926 | Tropodithietic acid | 3 | |
| Photobacteriaceae family | Photobacterium halotolerans | S2753 | Holomycin | 3,5 |
| Vibrionaceae family | Vibrio coralliilyticus | S2052 | Andrimid | 3, 5 |
| Vibrio nigripulchritudo | S2604 | Nigribactin | 3, 20 |
All marine strains were grown in marine broth (MB; Difco 2216) or marine agar (MA; Difco 2216). Escherichia coli OP50 was grown in Luria-Bertani (LB) broth (Miller Difco) and LB agar (Miller Difco). In the toxicity assay using C. elegans, bacterial strains were grown on Väätänen nine salts solution (VNSS) (25). All marine strains were incubated at 25°C for 24 to 48 h and stagnant conditions, and E. coli OP50 was incubated at 37°C and 250 rpm for 24 h. The bacterial strains were preserved as frozen stocks prepared in skim milk freeze medium (26) and stored at −80°C.
Pure antibacterial compounds.
Indolmycin and violacein were purchased from Bioaustralis (Australia) and Sigma (USA), respectively, and tropodithietic acid (TDA) was purchased from BioViotica (Germany). Andrimid and holomycin were purified as previously described (5), and pentabromopseudilin was extracted from a 3-day-old P. luteoviolacea S4060 culture grown in 6 liters of marine minimal medium (27) with 0.4% mannose and 0.3% Casamino Acids as a carbon source, followed by incubation at 25°C and 100 rpm. Briefly, 12 g of sterile Diaion HP-20 resin (Supelco)/liter was added to the outgrown culture. After 24 h, the resin was filtered off, and the bacterial cells were pelleted. The resin was extracted with 1 liter of methanol, while the pellet was extracted with 1 liter of methanol (MeOH)-ethyl acetate (EtOAc) (30/60 [vol/vol], 110 rpm overnight). The extracts were dried on a rotatory evaporator, redissolved in MeOH, and pooled. Violacein was quantitatively removed from the extract by cation-exchange. The extract was absorbed onto a 10-g Sepra SCX (Phenomenex) and dry-loaded onto a 50-g SNAP column (Biotage) with 40 g of pure SCX resin at the bottom. An Isolera flash purification system (Biotage) was used to fractionate the extracts with a mixed pH and solvent gradient (35 ml/min). The fractionation started with 100% water with 1% formic acid (FA; pH 2, 5 min) increasing to 100% MeOH with 1% FA over 10 min (pH 2), switched directly to MeOH with 2% ammonium hydroxide (pH 11, 20 min), and finally washed with MeOH (pH 7, 5 min). Fractions were automatically collected using UV detection (200 and 400 nm). Liquid chromatography-ultraviolet/electrospray ionization mass spectrometry (LC-UV/EI-MS) operated in negative ionization mode was used to determine the fractions containing the pentabromopseudilin as previously described (28). In addition, a bioassay against V. anguillarum was performed as previously described (24) to validate the antibacterial activity of the fractions. Pentabromopseudilin containing fractions (eluting with pH 2) were pooled and further purified by normal-phase chromatography. The active fractions were dry-loaded onto 2 g of Isolute diol (Biotage) in a 25-g SNAP column with 20 g of pure resin in the base. Fractions were collected by using solvents ranging from heptane, dichlormethane (DCM), and EtOAc to pure MeOH (15 ml/min). Pentabromopseudilin was recovered from the fractions eluting with heptane to DCM, together with an unidentified analogue (Mr = 496.7068).
Effect of live bacterial cultures on Artemia nauplii.
The Artemia cysts (INVE Aquaculture, Salt Lake City, UT) were disinfected and decapsulated by sodium hypochlorite (NaOCl) treatment (29). In brief, 0.1-g portions of Artemia cysts were treated with 1.5 ml of 0.5% NaOCl (vol/vol) and vortexed for 3 min. The cysts were filtered on a 10-μm-pore-size NY10 filter (Millipore) and washed three times with 25 ml of sterile deionized water. The cysts were transferred to a sterile 50-ml Falcon tube by washing the filter in 20 ml of sterile 3% (wt/vol) Sigma sea salts (SSS; Sigma-Aldrich), and the cysts hatched after being exposed to light for 24 h while shaking at 50 to 60 rpm and 25°C. Then, 20-ml portions of disinfected Artemia nauplius solution were transferred to 250-ml bottles containing 78 ml of 3% SSS.
Bacterial cultures were grown for 2 days in MB, and the concentration was estimated by plating 10-fold dilutions of the cultures on MA plates that were incubated at 25°C for 48 h. All bacterial cultures were added to the Artemia cysts at a final concentration of ∼107 CFU/ml. The Artemia cultures exposed to the bacteria were incubated at 25°C and 90 to 100 rpm. MB was used as a negative control, and V. coralliilyticus DSM19607 was used as a positive control for infection (21). Live nauplii were counted daily in a home-made methacrylate counting chamber using a stereomicroscope (SZ40; Olympus). For each time point, three samples were counted after 0, 4, 24, and 48 h. The bacterial density was determined by plating of 10-fold serial dilutions on MA plates. Two independent biological replicates were carried out.
Effect of live bacterial cultures on C. elegans.
C. elegans strain Bristol N2 (wild type) was maintained on nematode growth medium (NGM) plates seeded with E. coli OP50 as a food source (30), followed by incubation at room temperature (22°C). The synchronization protocol for C. elegans was adapted from Strange et al. (31). In brief, adult nematodes from NGM agar plates were washed with sterile deionized water and transferred to a 50-ml sterile tube. The tube was stored on ice to allow the nematodes to settle down. Settled nematodes were transferred to a fresh tube containing 15 ml of sterile deionized water and washed two times more or until the supernatant was clear of bacteria. Then, the nematodes were lysed by incubation with 3 ml of 10 to 15% NaOCl and 2 ml of 2.5 M NaOH for 5 min. The lysis reaction was stopped by adding 10 ml of egg-buffer (47.2 ml of 2.5 M NaCl, 16 ml of 3 M KCl, 2 ml of 1 M MgCl2, 2 ml of 1 M CaCl2, 20 ml of 0.5 HEPES, and 930 ml of deionized water) and centrifugation at 275 × g for 3 min. The pellet was redissolved in 10 ml of fresh egg-buffer and again centrifuged. This step was repeated twice to remove all NaOCl and NaOH. Washed eggs were transferred to NGM plates without E. coli OP50 and allowed to hatch overnight. The next day, 500 μl of E. coli OP50 overnight culture was added, and the nematodes were incubated for 2 to 3 days at room temperature (22°C) to achieve the L4 stage.
Portions (50 μl) of a 2-day-old bacterial culture grown in MB were spread on VNSS agar, and the plates were incubated at 25°C for 2 days. Pseudoalteromonas tunicata D2 was used as a positive killing control (22). The negative control was E. coli OP50 from a 24-h LB liquid culture. Synchronized L4 stage nematodes were purified from E. coli OP50 as described, and approximately 30 to 40 synchronized and washed nematodes were transferred to each plate after pregrowth of the bacterial cultures. The plates were incubated at 25°C. The number of living nematodes was counted under a stereomicroscope (SZ61; Olympus) every day over a period of 4 days (96 h). The assay was carried out in duplicate.
Effect of pure compounds on Artemia nauplii.
Since several of the pure antibacterial compounds were only available in very limited amounts, the Artemia assay was scaled down to 96-well plates (Nunc, catalog no. 167008). Then, 10 to 15 disinfected Artemia nauplii per 100 μl of 3% SSS was transferred to each well, and the pure compounds were added to achieve a final volume of 200 μl in each well. The MIC of each compound against S. aureus was estimated based on literature searches (see Table S1 in the supplemental material) and 2× MIC, 1× MIC, 0.5× MIC, and 0.05× MIC were tested in the Artemia assay. TDA was tested at 1× MIC, 0.5× MIC, 0.25× MIC, and 0.025× MIC. All compounds were dissolved in acetonitrile except TDA, which was dissolved in dimethyl sulfoxide (DMSO). Solvent controls were included in every test using the appropriate concentrations. Live nauplii were counted at 0, 4, 24, and 48 h under an inverted microscope (IX51; Olympus). At each time point, two counts were made, and the assay was carried out in two independent trials.
Statistical analysis.
A one-way analysis of variance and Tukey test (level of significance = 0.05) were used to determine the significance of the effect of bacterial cultures and pure compounds on the survival of Artemia nauplii and C. elegans.
RESULTS
Effect of live bacterial cultures on Artemia nauplii.
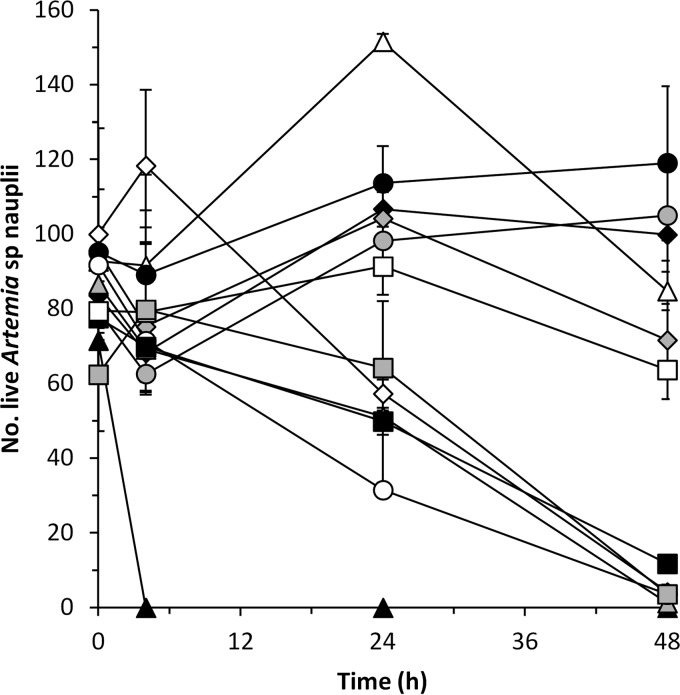
The positive control V. coralliilyticus DSM19607 reduced survival of Artemia nauplii by 43 and 96% after 24 and 48 h in coculture, respectively. Similarly, the survival of the nauplii was significantly affected (P < 0.05) by P. rubra S2471, P. piscicida S2049, P. halotolerans S2753, and V. coralliilyticus S2052, which reduced the viability of nauplii by 35 to 65% after 24 h and by 85 to 99% after 48 h in coculture. P. luteoviolacea S4060 killed all nauplii after 4 h of incubation. The presence of A. davidanieli WX-11, P. inhibens DSM 17395, P. luteoviolacea S4054, R. mobilis F1926, and V. nigripulchritudo S2604 had no effect on nauplius survival, which was not different (P > 0.05) from that in the MB control (Fig. 1).
FIG 1.
Influence of A. davidanieli WX-11 (△), P. luteoviolacea S4054 (gray diamond), P. luteoviolacea S4060 (▲), P. rubra S2471 (gray triangle), P. piscicida S2049 (○), P. inhibens DSM17395 (gray circle), R. mobilis F1926 (●), V. coralliilyticus S2052 (■), V. nigripulchritudo S2604 (□), and P. halotolerans S2753 ( ) on the survival of Artemia nauplii. MB (◆) was used as a killing negative control, and V. coralliilyticus DSM19607 (◊) was used as a killing positive control. The results are means and standard deviations of two independent assays.
) on the survival of Artemia nauplii. MB (◆) was used as a killing negative control, and V. coralliilyticus DSM19607 (◊) was used as a killing positive control. The results are means and standard deviations of two independent assays.
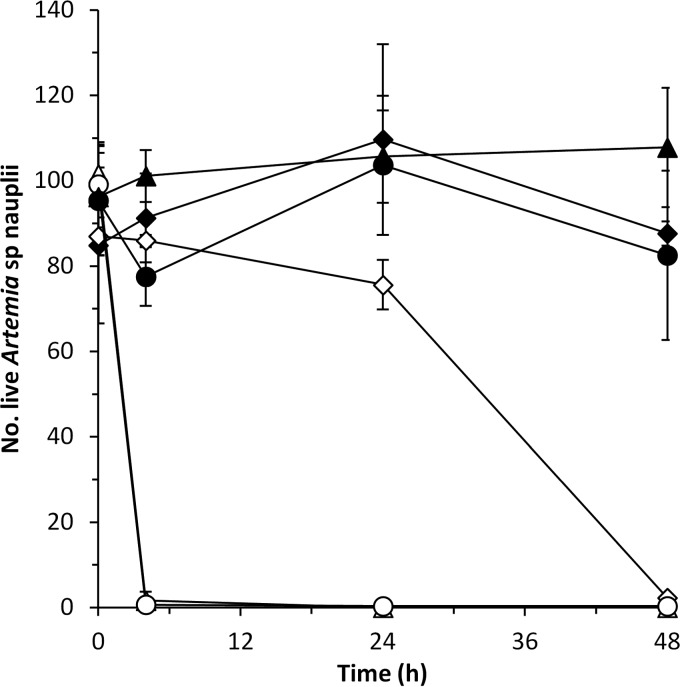
P. luteoviolacea S4054 produces violacein and indolmycin, whereas P. luteoviolacea S4060 produces violacein and pentabromopseudilin (18). To check whether the different effect observed by these two strains in Artemia nauplii could be related to their different chemical profiles, we also analyzed the effect of P. luteoviolacea strains S4047-1 and S2607, which produce, respectively, indolmycin and pentabromopseudilin, together with violacein (18). The two strains producing pentabromopseudilin killed all of the Artemia nauplii after 4 h of incubation, whereas those producing indolmycin had no effect on nauplius survival and did not differ from the MB control (P > 0.05) (Fig. 2).
FIG 2.
Artemia survival when cocultured with P. luteoviolacea S4060 (△) and S2607 (○), which produce violacein and pentabromopseudilin, and with S4054 (▲) and S4047-1 (●), which produce violacein and indolmycin. MB (◆) was used as a killing negative control, and V. coralliilyticus DSM19607 (◊) was used as a killing positive control. The results are means and standard deviations of two independent assays.
Effect of live bacterial cultures on C. elegans.
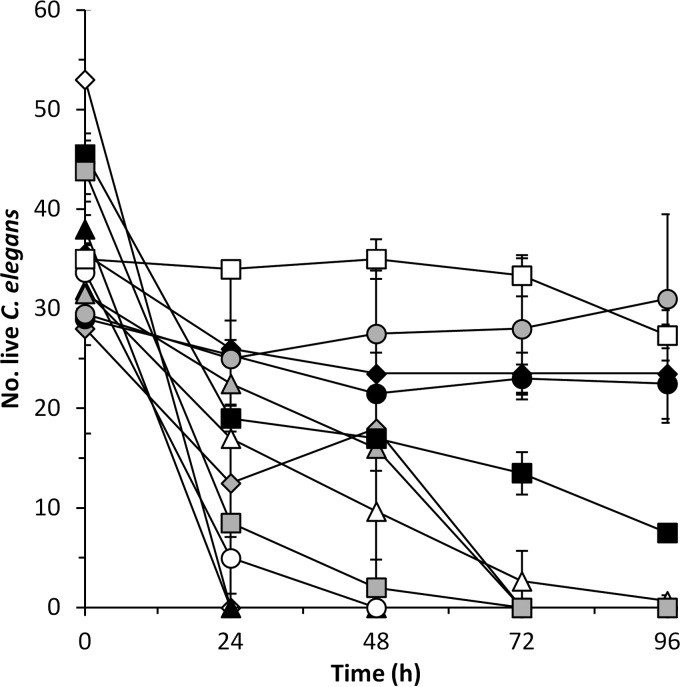
After 24 h of incubation, the positive control P. tunicata D2 and P. luteoviolacea S4060 killed all of the nematodes. The presence of P. rubra S2471 and P. piscicida S2049 reduced C. elegans survival by 29 and 85%, respectively, after 24 h and killed all nematodes after 72 h in coculture (Fig. 3). A. davidanieli WX-11, P. luteoviolacea S4054, and P. halotolerans S2753 reduced C. elegans numbers by 92 to 100% after 72 h of incubation (Fig. 3). The survival of C. elegans was also reduced in the presence of V. coralliilyticus S2052, but it was only significantly different (P < 0.05) from the E. coli OP50 negative control after 72 h of incubation. No negative effect was observed in the presence of P. inhibens DSM17395, R. mobilis F1926, or V. nigripulchritudo S2604, where nematode survival was not different (P > 0.05) from the E. coli OP50 negative control (Fig. 3).
FIG 3.
Influence of A. davidanieli WX-11 (△), P. luteoviolacea S4054 (gray diamond), P. luteoviolacea S4060 (▲), P. rubra S2471 (gray triangle), P. piscicida S2049 (○), P. inhibens DSM17395 (gray circle), R. mobilis F1926 (●), V. coralliilyticus S2052 (■), V. nigripulchritudo S2604 (□), and P. halotolerans S2753 ( ) on the survival of C. elegans. E. coli OP50 grown in VNSS medium (◆) was used as a killing negative control, and P. tunicata D2 (◊) was used as a killing positive control. The results are means and standard deviations of two independent assays.
) on the survival of C. elegans. E. coli OP50 grown in VNSS medium (◆) was used as a killing negative control, and P. tunicata D2 (◊) was used as a killing positive control. The results are means and standard deviations of two independent assays.
Effect of pure compounds on Artemia nauplii.
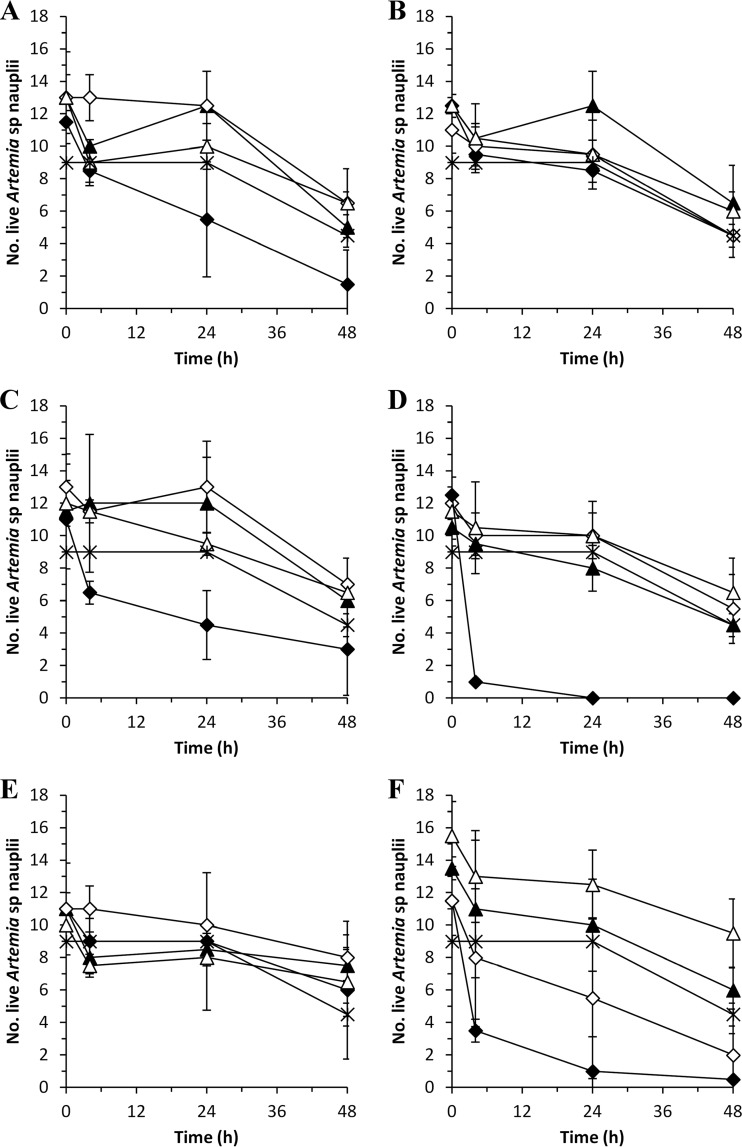
Target-guided purification of pentabromopseudilin resulted in a semipure fraction with two closely eluting compounds. One peak (Mr = 547.6143) was identified as pentabromopseudilin. The other peak (Mr = 496.7068) is potentially a new analogue due to a similar isotope pattern and it did not match any compound in AntiBase 2010 (32) or any known analogue. This semipure fraction of pentabromopseudilin was used and named the pentabromopseudilin mix.
Acetonitrile and DMSO were used as solvents for preparation of solutions of the different compounds. Acetonitrile killed almost all Artemia nauplii at concentrations between 25 and 4%, which are equivalent to that in the solutions with 2× MIC and 1× MIC of andrimid, holomycin, indolmycin, pentabromopseudilin mix, and violacein (Table 2). A similar effect was observed for DMSO at the concentrations present in TDA solutions with 1× MIC (7.5%) and 0.5× MIC (3.75%) values (Table 2). Therefore, we analyzed the effect of the different compounds based on 0.25× MIC and 0.025× MIC for TDA and 0.5× MIC and 0.05× MIC for the other compounds. The pentabromopseudilin mix killed all nauplii at a final concentration of 10 μM (0.5× MIC) but did not have any lethal effect at 1 μM (0.05× MIC) (Fig. 4) and was not different (P > 0.05) from the MB control. Andrimid, holomycin, indolmycin, TDA, and violacein did not have any significant effect on nauplius survival in the tested concentrations compared to the MB control (P > 0.05).
TABLE 2.
Effect of acetonitrile and DMSO on the survival of Artemia naupliia
| Solvent | Concn (%) | Mean survival (%) ± SD |
|
|---|---|---|---|
| 4 h | 24 h | ||
| Acetonitrile | 4 | 0 ± 0 | 0 ± 0 |
| 2.5 | 88 ± 8 | 100 ± 0 | |
| 1 | 83 ± 8 | 83 ± 8 | |
| 0.625 | 84 ± 19 | 81 ± 13 | |
| 0.5 | 100 ± 0 | 92 ± 15 | |
| 0.25 | 96 ± 8 | 79 ± 8 | |
| 0.2 | 84 ± 16 | 76 ± 16 | |
| 0.1 | 91 ± 9 | 87 ± 9 | |
| DMSO | 7.5 | 0 ± 0 | 0 ± 0 |
| 3.75 | 65 ± 26 | 17 ± 2 | |
| 1.88 | 100 ± 0 | 91 ± 0 | |
| 0.21 | 75 ± 10 | 80 ± 0 | |
| Control | 3 | 100 ± 0 | 100 ± 0 |
The results are means of two independent experiments. Artemia nauplii were cultured in 3% Sigma Sea Salts.
FIG 4.
Survival of Artemia nauplii in the presence of andrimid (A), holomycin (B), indolmycin (C), pentabromopseudilin mix (D), TDA (E), and violacein (F) at the concentrations needed for 0.5× MIC (◆) and 0.05× MIC (▲), or at 0.25× MIC (◆) and 0.025× MIC (▲) in the case of TDA, against S. aureus. MB (✳) was used as a negative control. The effect of acetonitrile and DMSO concentrations used for the preparation of 0.5× MIC or 0.25× MIC (◊) and 0.05× MIC or 0.025× MIC (△) solutions is also represented. The results are means and standard deviations of two independent assays.
DISCUSSION
Marine microorganisms can produce potent bioactive compounds and, although some of these bioactive substances have been known for decades, little is known about their adverse effect on eukaryotic organisms, and only a few have been recently approved (4). In the present study, Artemia sp. and C. elegans were used as eukaryotic models to provide a first assessment of potential toxicity of bacterial bioactive compounds and the producing bacteria themselves. We have shown that some antibiotic-producing marine bacteria belonging to the Actinobacteria class, the Pseudoalteromonas genus, and the Photobacteriaceae and Vibrionaceae families have a lethal effect on one or both target organisms and hence should be used with great caution. In contrast, other bacteria, e.g., from the Roseobacter clade, had no adverse effect.
The actinobacterium A. davidanieli WX-11 had no effect on Artemia sp. but killed almost all nematodes after 72 h of incubation. Arthrobacilins A, B, and C have been detected in WX-11 (6) and, although they have antibacterial activity, it has been suggested that the antibacterial activity of WX-11 could also be related to other compounds, since arthrobacilins were also detected in extracts without antibacterial activity (6). Arthrobacilins have a cytotoxic effect against human cancer cells (33) and the closer relation of C. elegans to humans than to Artemia sp. could explain their different sensitivities to WX-11. The potential mode of action of arthrobacilin is not known and, hence, the specific mechanism of toxicity is difficult to explain. A. davidanieli is used as a live vaccine for disease control in salmonids, although the effect is probably due to the stimulation of the immune system rather than to antibacterial activity (34).
The P. luteoviolacea strains differed in their effect on survival of Artemia nauplii, and this could be explained by the different chemical profiles in the strains. Strains S4054 and S4047-1 produce violacein and indolmycin (18) and had no effect on Artemia nauplii, whereas strains S2607 and S4060 produce violacein and pentabromopseudilin (18) and killed all nauplii after 4 h in coculture. These results already indicated that violacein was not the compound responsible of the toxicity observed, and this was confirmed by testing the pure compounds. Violacein and indolmycin did not have any significant effect on the survival of Artemia nauplii, whereas the pentabromopseudilin mix killed all nauplii after 24 h of incubation. Both nauplii and nematodes were rapidly paralyzed in the presence of the pentabromopseudilin-producing strains, and this also indicates that pentabromopseudilin was the main toxic compound. Indeed, it has been demonstrated that pentabromopseudilin inhibits the myosin ATPase and motor activity in several myosins (35). At present, no toxic effect of indolmycin on eukaryotic organisms has been reported. In the present study, strain S4054 that produces violacein and indolmycin did not affect Artemia nauplii but had a negative effect on C. elegans. This could be explained by the different culture methods used, VNSS plates for the nematodes and 3% SSS in the Artemia assay, which could have led to variations in the production of the compounds. In addition, Artemia spp. have been shown to be less sensitive in ecotoxicology tests than other organisms, such as algae, insects, and other crustaceans (18), and having an exoskeleton might also prevent the uptake of toxins.
P. piscicida and P. rubra killed both Artemia sp. and C. elegans. A lethal effect of P. piscicida on eukaryotic organisms, such as algae and crustaceans, has previously been reported (36). P. piscicida S2049 produces several bromoalterochromides (37), which have inhibitory properties against Bacillus subtilis (38) and a toxic effect on eukaryotic organisms, such as sea urchins (37). The production of bromoalterochromides by S2049 could explain the toxic effect we observed in the tested eukaryotic organisms. P. rubra strains produce the pigment prodigiosin (18), which can antagonize bacteria (39) and have a negative effect on eukaryotic organisms, including algae (40), and parasites (41) corresponding to the effects observed in the present study.
V. corallilyticus S2052 had a lethal effect on Artemia sp. after 24 h in coculture, and this is in agreement with previous studies (56). It also affected the survival of C. elegans; however, the effects were not seen until after 96 h of incubation. Strain S2052 produces the antibiotic andrimid (5); however, andrimid in itself did not adversely affect Artemia nauplii. Andrimid targets the multisubunit acetyl coenzyme A carboxylase, which catalyzes the first step in fatty acid biosynthesis and is essential for bacterial growth (42). Eukaryotes carry out these reactions by a large multifunctional protein (43), and it is therefore not surprising that andrimid did not significantly affect Artemia sp. and C. elegans survival. S2052 is able to colonize and use the chitin from the exoskeleton of Artemia sp. as a nutrient (56), and, since respiration in Artemia spp. occurs through the surfaces of the legs, where the exoskeleton is thinner, it is possible that S2052 colonization interferes with Artemia oxygen uptake, compromising the survival of the nauplii and explaining the differences observed between Artemia sp. and C. elegans.
P. halotolerans S2753 killed both Artemia sp. and C. elegans. Biofilms of P. halotolerans can have an inhibitory effect in the settlement of the barnacle Amphibalanus improvisus in macroalgae, although the mechanism involved in such inhibition is not known (10). Similarly to andrimid, holomycin, which is the main antibacterial compound produced by S2753, did not affect Artemia survival. The activity of holomycin is related to the inhibition of RNA polymerase and, since prokaryotic and eukaryotic RNA polymerases are different, it was expected that holomycin did not affect the survival of Artemia sp. Similarly, Oliva et al. (44) showed that the yeasts Saccharomyces cerevisiae and Candida kefyr are not affected by holomycin. P. halotolerans produces two depsipeptides called solonamide A and B, which have a chemical structure similar to unnarmicins (45). Unnarmicins have been identified in a Photobacterium strain (46) and inhibit fungal ATP binding cassette transporters (ABC), which are involved in drug resistance (47). C. elegans possess several ABC transporters involved in drug resistance (48), and solonamides could thus potentially inhibit one or several of these proteins, making the nematodes sensitive to holomycin or other secondary metabolites produced by S2753. Similarly to V. corallilyticus, P. halotolerans is able to use chitin (49) and form biofilms (10), and it is therefore possible that the toxic effect observed in Artemia sp. was caused by degradation of the exoskeleton in the nauplii.
V. nigripulchritudo S2604, P. inhibens DSMZ19395, and R. mobilis F1926 had no effect on the survival of Artemia sp. and C. elegans. The antibacterial activity of V. nigripulchritudo is linked to the production of the siderophore nigribactin (20). Siderophores are iron-chelating compounds, which have been related to virulence in bacterial pathogens. In contrast to our results, the link between siderophores and virulence in several human pathogens has been demonstrated by using C. elegans as a model organism (50). Similarly, several strains of V. nigripulchritudo have been identified as the cause of high mortalities in shrimp farms (51); however, different strains of V. nigripulchritudo have different virulence patterns, and it is not known what determines the pathogenicity in this species (51). Therefore, it is possible that S2604 is a nonpathogenic strain or that the culture conditions used in the present study were not appropriate for virulence expression.
P. inhibens DSMZ17395 and R. mobilis F1926 are innocuous for fish larvae (7), algae, and live prey (52). Their antibacterial activity is caused by production of TDA, which is a broad-spectrum antibiotic able to inhibit fish and shellfish pathogens (53) and is the main compound responsible for antagonism in laboratory broth cultures (54), in live feed, and in fish larval systems (52).
Pseudoalteromonas, Roseobacter, and Vibrionaceae bacteria have been suggested as probiotics in aquaculture (7–9). However, our results suggest that several Pseudoalteromonas and Vibrionaceae strains may not be suitable, since they can be toxic to Artemia sp. or potentially toxic (e.g., V. nigripulchritudo). Nevertheless, their secondary metabolites provide them with the capability to regulate bacterial virulence expression (20) and kill bacteria (18), algae causing harmful algal blooms (HAB) (40), parasites (41), or tumor cells (55). These bacteria could therefore have value, e.g., as control agents of biofouling and HAB in the marine environment, as producers of cancer treatment compounds, or as antibacterial or antiparasitic agents. In contrast, the lack of toxicity of the live cultures of the TDA-producing Roseobacter species reaffirms their suitability as probiotics in aquaculture. Furthermore, bacterial resistance against TDA is hard to develop (53), and this, together with the innocuousness of pure TDA in the tested eukaryotic organisms, also raises the interesting possibility of applications in the control of human infections.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Programme Commission on Health, Food, and Welfare under the Danish Council for Strategic Research. A.K.N. received a grant from the European Erasmus program, and M.J.P.-G. was financed by the Technical University of Denmark Ørsted postdoctoral program.
We thank Claus Asperud Reesbøll for fabrication of the methacrylate counting chambers.
Footnotes
Published ahead of print 18 October 2013
The present study was carried out as part of the Galathea 3 expedition under the auspices of the Danish Expedition Foundation. This is Galathea 3 contribution no. P100.
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02717-13.
REFERENCES
- 1.ECDC/EMEA 2009. The bacterial challenge: time to react. ECDC/EMEA, Stockholm, Sweden. 10.2900/2518 [DOI] [Google Scholar]
- 2.Molinski TF, Dalisay DS, Lieyens SL, Saludes J. 2009. Drug development from marine natural products. Nat. Rev. 8:69–85. 10.1038/nrd2487 [DOI] [PubMed] [Google Scholar]
- 3.Gram L, Melchiorsen M, Bruhn JB. 2010. Antibacterial activity of marine culturable bacteria collected from a global sampling of ocean surface waters and surface swabs of marine organisms. Mar. Biotechnol. 12:439–451. 10.1007/s10126-009-9233-y [DOI] [PubMed] [Google Scholar]
- 4.Imhoff JF, Labes A, Wiese J. 2011. Bio-mining the microbial treasures of the ocean: new natural products. Biotechnol. Adv. 29:468–482. 10.1016/j.biotechadv.2011.03.001 [DOI] [PubMed] [Google Scholar]
- 5.Wietz M, Månsson M, Gotfredsen CH, Larsen TO, Gram L. 2010. Antibacterial compounds from marine Vibrionaceae isolated on a global expedition. Mar. Drugs 8:2946–2960. 10.3390/md8122946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wietz M, Månsson M, Bowman J, Blom N, Ng Y, Gram L. 2012. Wide distribution of closely related, antibiotic producing Arthrobacter strains throughout the Arctic Ocean. Appl. Environ. Microbiol. 78:2039–2042. 10.1128/AEM.07096-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Alvise PW, Lillebø S, Wergeland HI, Gram L, Bergh Ø 2013. Protection of cod larvae from vibriosis by Phaeobacter spp.: a comparison of strains and introduction times. Aquaculture 384–387:82–86. 10.1016/j.aquaculture.2012.12.013 [DOI] [Google Scholar]
- 8.Goulden EF, Hall MR, Pereg LL, Baillie BK, Høj L. 2013. Probiont niche specialization contributes to additive protection against Vibrio owensii in spiny lobster larvae. Environ. Microbiol. Rep. 5:39–48. 10.1111/1758-2229.12007 [DOI] [PubMed] [Google Scholar]
- 9.Prol-García MJ, Pintado J. 2013. Effectiveness of probiotic Phaeobacter bacteria grown in biofilters against Vibrio anguillarum infections in the rearing of turbot (Psetta maxima) larvae. Mar. Biotechnol. 15:726–738. 10.1007/s10126-013-9521-4 [DOI] [PubMed] [Google Scholar]
- 10.Nasrolahi A, Stratil SB, Jacob KJ, Wahl M. 2012. A protective coat of microorganisms on macroalgae: inhibitory effects of bacterial biofilms and epibiotic microbial assemblages on barnacle attachment. FEMS Microbiol. Ecol. 81:583–595. 10.1111/j.1574-6941.2012.01384.x [DOI] [PubMed] [Google Scholar]
- 11.Ballestriero F, Thomas T, Burke C, Egan S, Kjelleberg S. 2010. Identification of compounds with bioactivity against the nematode Caenorhabditis elegans by a screen based on the functional genomics of the marine bacterium Pseudoalteromonas tunicate D2. Appl. Environ. Microbiol. 76:5710–5717. 10.1128/AEM.00695-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hilbi H, Weber SS, Ragaz C, Nyfeler Y, Urwyler S. 2007. Environmental predators as models for bacterial pathogenesis. Environ. Microbiol. 9:563–575. 10.1111/j.1462-2920.2007.01238.x [DOI] [PubMed] [Google Scholar]
- 13.Sese BT, Grant A, Reid BJ. 2009. Toxicity of polycyclic aromatic hydrocarbons to the nematode Caenorhabditis elegans. J. Toxicol. Environ. Health 72:1168–1180. 10.1080/15287390903091814 [DOI] [PubMed] [Google Scholar]
- 14.Sifri CD, Begun J, Ausubel FM. 2005. The worm has turned-microbial virulence in Caenorhabditis elegans. Trends Microbiol. 13:119–127. 10.1016/j.tim.2005.01.003 [DOI] [PubMed] [Google Scholar]
- 15.Sprando RL, Olejnik N, Cinar HN, Ferguson M. 2009. A method to rank order water soluble compounds according to their toxicity using Caenorhabditis elegans, a complex object parametric analyzer and sorter, and axenic liquid media. Food Chem. Toxicol. 47:722–728. 10.1016/j.fct.2009.01.007 [DOI] [PubMed] [Google Scholar]
- 16.Kalčíková G, Zagorc-Končan J, Gotvajn AŽ. 2012. Artemia salina acute immobilization test: a possible tool for aquatic ecotoxicity assessment. Water Sci. Technol. 66:903–908. 10.2166/wst.2012.271 [DOI] [PubMed] [Google Scholar]
- 17.Nunes BS, Carvalho FD, Guilhermino LM, Stappen GV. 2006. Use of the genus Artemia in ecotoxicity testing. Environ. Pollut. 144:453–462. 10.1016/j.envpol.2005.12.037 [DOI] [PubMed] [Google Scholar]
- 18.Vynne NG, Månsson M, Nielsen KF, Gram L. 2011. Bioactivity, chemical profiling, and 16S rRNA-based phylogeny of Pseudoalteromonas strains collected on a global research cruise. Mar. Biotechnol. 13:1062–1073. 10.1007/s10126-011-9369-4 [DOI] [PubMed] [Google Scholar]
- 19.Buddruhs N, Pradella S, Göker M, Paüker O, Pukall R, Spröer C, Schumann P, Petersen J, Brinkhoff T. Molecular and phenotypic analyses reveal the non-identity of the Phaeobacter gallaeciensis type strain deposits CIP 105210T and DSM 17395. Int. J. Syst. Evol. Microbiol., in press [DOI] [PubMed] [Google Scholar]
- 20.Nielsen A, Månsson M, Wietz M, Varming AN, Phipps RK, Larsen TO, Gram L, Ingmer H. 2012. Nigribactin, a novel siderophore from Vibrio nigripulchritudo, modulates Staphylococcus aureus virulence gene expression. Mar. Drugs. 10:2584–2595. 10.3390/md10112584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austin B, Dawn A, Sutherland R, Thompson F, Swings J. 2005. Pathogenicity of vibrios to rainbow trout (Oncorhynchus mykiss, Walbaum) and Artemia nauplii. Environ. Microbiol. 7:1488–1495. 10.1111/j.1462-2920.2005.00847.x [DOI] [PubMed] [Google Scholar]
- 22.Holström C, Egan S, Franks A, McCloy S, Kjelleberg S. 2002. Antifouling activities expressed by marine surface associated Pseudoalteromonas species. FEMS Microbiol. Ecol. 41:47–58. 10.1111/j.1574-6941.2002.tb00965.x [DOI] [PubMed] [Google Scholar]
- 23.Skov MN, Pedersen K, Larsen JL. 1995. Comparison of pulsed-field gel electrophoresis, ribotyping, and plasmid profiling for typing of Vibrio anguillarum serovar O1. Appl. Environ. Microbiol. 61:1540–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hjelm M, Bergh Ø, Riaza A, Nielsen J, Melchiorsen J, Jensen S, Duncan H, Ahrens P, Birkbeck H, Gram L. 2004. Selection and identification of autochthonous potential probiotic bacteria from turbot larvae (Scolphthalmus maximus) rearing units. Syst. Appl. Microbiol. 27:360–371. 10.1078/0723-2020-00256 [DOI] [PubMed] [Google Scholar]
- 25.Marden P, Tunlid A, Malmcronafriberg K, Odham G, Kjelleberg S. 1985. Physiological and morphological-changes during short-term starvation of marine bacterial isolates. Arch. Microbiol. 142:326–332. 10.1007/BF00491898 [DOI] [Google Scholar]
- 26.Gibson LF, Khoury JT. 1986. Storage and survival of bacteria by ultra-freeze. Lett. Appl. Microbiol. 3:127–129. 10.1111/j.1472-765X.1986.tb01565.x [DOI] [Google Scholar]
- 27.Ostling J, Goodman A, Kjelleberg S. 1991. Behaviour of Incp-1 plasmids and a minimum transposon in a marine Vibrio sp.: isolation of starvation inducible lac operon fusions. FEMS Microbiol. Ecol. 86:83–94. 10.1111/j.1574-6968.1991.tb04797.x [DOI] [Google Scholar]
- 28.Månsson M, Nielsen KF, Kjaerulff L, Gotfredsen CH, Wietz M, Ingmer H, Gram L, Larsen TO. 2011. Inhibition of virulence gene expression in Staphylococcus aureus by novel depsipeptides from a marine Photobacterium. Mar. Drugs. 9:2537–2552. 10.3390/md9122537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sorgeloos P, Bossuyt E, Lavina E, Baezamesa M, Persoone G. 1977. Decapsulation of Artemia cysts: simple technique for improvement of use of brine shrimp in aquaculture. Aquaculture 12:311–315. 10.1016/0044-8486(77)90209-5 [DOI] [Google Scholar]
- 30.Brenner S. 1974. Genetics of Caenorhabditis elegans. Genetics 77:71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strange K, Christensen M, Morrison R. 2007. Primary culture of Caenorhabditis elegans developing embryo cells for electrophysiological, cell biological and molecular studies. Nat. Protoc. 2:1003–1012. 10.1038/nprot.2007.143 [DOI] [PubMed] [Google Scholar]
- 32.Laatsch H. 2010. AntiBase 2010. Wiley-VCH, Weinheim, Germany: http://www.wiley-vch.de/stmdata/antibase.php [Google Scholar]
- 33.Ohtsuka T, Itezono Y, Nakayama N, Kurano M, Nakada N, Tanaka H, Sawairi S, Yokose K, Seto H. 1992. Structural elucidation of Arthrobacilin-A, Arthrobacilin-B and Arthrobacilin-C, structurally unique secondary metabolisms of a microorganism. Tetrahedron Lett. 33:2705–2708. 10.1016/S0040-4039(00)79062-9 [DOI] [Google Scholar]
- 34.Salonius K, Siderakis C, MacKinnon AM, Griffiths SG. 2005. Use of Arthrobacter davidanieli as a live vaccine against Renibacterium salmoninarum and Piscirickettsia salmonis in salmonids. Prog. Fish Vaccinol. 121:189–197 [PubMed] [Google Scholar]
- 35.Fedorov R, Bohl M, Tsiavaliaris G, Hartmann FK, Taft MH, Baruch P, Brenner B, Martin R, Knolker HJ, Gutzeit HO, Manstein DJ. 2009. The mechanism of pentabromopseudilin inhibition of myosin motor activity. Nat. Struct. Mol. Biol. 16:80–88. 10.1038/nsmb.1542 [DOI] [PubMed] [Google Scholar]
- 36.Talpur AD, Memon AJ, Khan MI, Ikhwanuddin M, Daniel MMD, Abol-Munafi AB. 2011. Pathogenicity and antibiotic sensitivity of pathogenic flora associated with the gut of blue swimming crab, Portunus pelagicus (Linnaeus, 1985). JAVA 10:2106–2119. 10.3923/javaa.2011.2106.2119 [DOI] [Google Scholar]
- 37.Speitling M, Smetanina OF, Kuznetsova TA, Laatsch H. 2007. Bromoalterochromines A and A′ unprecedented chromopeptides from a marine Pseudoalteromonas maricaloris strain KMM636T. J. Antibiot. 60:36–42. 10.1038/ja.2007.5 [DOI] [PubMed] [Google Scholar]
- 38.Ngyen DD, Wu C, Moree WJ, Lamsa A, Medema MH, Zhao X, Gavilan R, Aparicio M, Atencio L, Jackson C, Ballesteros J, Sánchez J, Watrous JD, Phelan VV, van de Wiel C, Kersten RD, Mehnaz S, De Mot R, Shank EA, Charusanti P, Nagarajan H, Duggan BM, Moore BS, Bandeira N, Palsson BŒ, Pogliano K, Guiérrez M, Dorrestein PC. 2013. MS/MS networking guided analysis of molecule and gene cluster families. Proc. Natl. Acad. Sci. U. S. A. 110:2611–2620. 10.1073/pnas.1300057110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samrot AV, Chandana K, Senthilkumar P, Narendra KG. 2011. Optimization of prodigiosin production by Serratia marcescens SU-10 and evaluation of its bioactivity. Int. Res. J. Biotechnol. 2:128–133 [Google Scholar]
- 40.Dockyu K, Kim JF, Yim JH, Kwon S, Lee CW. 2008. Red to red: the marine bacterium Hahella chejuensis and its product prodigiosin for mitigation of harmful algal blooms. J. Microbiol. Biotechnol. 18:1621–1629 [PubMed] [Google Scholar]
- 41.Pappireddy K, Smilkstein M, Kelly JX, Shweta Salem SM, Alhamadsheh M, Haynes SW, Challis GL, Reynolds KA. 2011. Antimalarial activity of natural and synthetic prodiginines. J. Med. Chem. 54:5296–5306. 10.1021/jm200543y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pohlman J, Lampe T, Shimada M, Nell PG, Pernerstorfer J, Svenstrup N, Brunner NA, Schiffer G, Freiberg C. 2005. Pyrrolidinedione derivatives as antibacterial agents with a novel mode of action. Bioorg. Med. Chem. Lett. 15:1189–1192. 10.1016/j.bmcl.2004.12.002 [DOI] [PubMed] [Google Scholar]
- 43.Chirala SS, Huang WY, Jayakumar A, Sakai K, Wakil SJ. 1997. Animal fatty acid synthase: functional mapping and cloning and expression of the domain I constituent activities. Proc. Natl. Acad. Sci. U. S. A. 94:5588–5593. 10.1073/pnas.94.11.5588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oliva B, O'Neill A, Wilson JM, O'Halon PJ, Chopra I. 2001. Antimicrobial properties and mode of action of the pyrrothine holomycin. Antimicrob. Agents Chemother. 45:532–539. 10.1128/AAC.45.2.532-539.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Månsson M, Gram L, Larsen TO. 2011. Production of bioactive secondary metabolites by marine Vibrionaceae. Mar. Drugs 9:1440–1468. 10.3390/md9091440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oku N, Kawabata K, Adachi K, Katsuta A, Shizuri Y. 2008. Unnarmicins A and C, new antibacterial depsipeptides produced by marine bacterium Photobacterium sp. MBIC06485. J. Antibiot. 61:11–17. 10.1038/ja.2008.103 [DOI] [PubMed] [Google Scholar]
- 47.Tanabe K, Lamping E, Adachi K, Takano Y, Kawabata K, Shizuri Y, Niimi M, Uehara Y. 2007. Inhibitions of fungal ABC transporters by unnarmicin A and unnarmicin C, novel cyclic peptides from marine bacterium. Biochem. Biophys. Res. Commun. 364:990–995. 10.1016/j.bbrc.2007.10.110 [DOI] [PubMed] [Google Scholar]
- 48.Sheps JA, Ralph S, Zhao Z, Baillie DL, Ling V. 2004. The ABC transporter gene family of Caenorhabditis elegans has implications for the evolutionary dynamics of multidrug resistance in eukaryotes. Genome Biol. 5:R15 http://genomebiology.com/2004/5/3/R15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wietz M. 2011. Global patterns of marine bacterioplankton diversity and characterization of bioactive Vibrionaceae isolates. Ph.D. thesis Technical University of Denmark, Lyngby, Denmark [Google Scholar]
- 50.Kurz CL, Chayvet S, Andres E, Arouze M, Vallet I, Michel GPF, Uh M, Celli J, Filloux A, de Bentzmann S, Steinmetz I, Hoffmann JA, Finlay BB, Gorvel JP, Ferrrandon D, Ewbank JJ. 2003. Virulence factors of the human opportunistic pathogen Serratia marcescens identified by in vivo screening. EMBO J. 22:1451–1460. 10.1093/emboj/cdg159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Labreuche Y, Pallandre L, Ansquer D, Herlin J, Wapotro B, Le Roux F. 2012. Pathotyping of Vibrio isolates by multiplex PCR reveals a risk of virulent strain spreading in New Caledonian shrimp farms. Microb. Ecol. 63:127–138. 10.1007/s00248-011-9951-3 [DOI] [PubMed] [Google Scholar]
- 52.D'Alvise PW, Lillebœ S, Prol-Garcia MJ, Wergeland HI, Nielsen KF, Bergh Ø, Gram L. 2012. Phaeobacter gallaeciensis reduces Vibrio anguillarum in cultures of microalgae and rotifers, and prevents vibriosis in cod larvae. PLoS One 7:e43996. 10.1371/journal.pone.0043996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Porsby CH, Webber MA, Nielsen KF, Piddock LJV, Gram L. 2011. Resistance and tolerance to tropodithietic acid, an antimicrobial in aquaculture, is hard to select. Antimicrob. Agents Chemother. 55:1332–1337. 10.1128/AAC.01222-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D'Alvise P, Melchiorsen J, Porsby CH, Nielsen KF, Gram L. 2010. Inactivation of Vibrio anguillarum by attached and planktonic Roseobacter. Appl. Environ. Microbiol. 76:2366–2370. 10.1128/AEM.02717-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, Nakajima A, Hosokawa K, Soliev AB, Osaka I, Arakawa R, Enomoto K. 2012. Cytotoxic prodigiosin family pigments from Pseudoalteromonas sp. 1020R isolated from the Pacific Coast of Japan. Biosci. Biotechnol. Biochem. 76:1229–1232 [DOI] [PubMed] [Google Scholar]
- 56.Wietz M, Månsson M, Gram L. 2011. Chitin stimulates production of the antibiotic andrimid in a Vibrio coralliilyticus strain. Environ. Microbiol. Rep. 3:559–564. 10.1111/j.1758-2229.2011.00259.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.