Abstract
The nucleotide (p)ppGpp inhibits GTP biosynthesis in the Gram-positive bacterium Bacillus subtilis. Here we examined how this regulation allows cells to grow in the absence of amino acids. We showed that B. subtilis cells lacking (p)ppGpp, due to either deletions or point mutations in all three (p)ppGpp synthetase genes, yjbM, ywaC, and relA, strongly require supplementation of leucine, isoleucine, valine, methionine, and threonine and modestly require three additional amino acids. This polyauxotrophy is rescued by reducing GTP levels. Reduction of GTP levels activates transcription of genes responsible for the biosynthesis of the five strongly required amino acids by inactivating the transcription factor CodY, which represses the ybgE, ilvD, ilvBHC-leuABCD, ilvA, ywaA, and hom-thrCB operons, and by a CodY-independent activation of transcription of the ilvA, ywaA, hom-thrCB, and metE operons. Interestingly, providing the eight required amino acids does not allow for colony formation of (p)ppGpp0 cells when transitioning from amino acid-replete medium to amino acid-limiting medium, and we found that this is due to an additional role that (p)ppGpp plays in protecting cells during nutrient downshifts. We conclude that (p)ppGpp allows adaptation to amino acid limitation by a combined effect of preventing death during metabolic transitions and sustaining growth by activating amino acid biosynthesis. This ability of (p)ppGpp to integrate a general stress response with a targeted reprogramming of gene regulation allows appropriate adaptation and is likely conserved among diverse bacteria.
INTRODUCTION
Bacterial cells employ an extensive network of regulatory mechanisms to tune cellular metabolism and gene expression in response to a wide variety of nutritional conditions. The general stress response mechanisms are integrated with specific regulatory programs controlling the expression of biosynthesis genes, and the proper coupling of these processes ensures viability and allows optimal growth under shifting nutrient conditions.
In bacteria, the stringent response, mediated by the nucleotide guanosine (penta)tetraphosphate, or (p)ppGpp, alerts cells to conditions of nutrient limitation and is also involved in regulation of amino acid biosynthesis (1). Escherichia coli cells that cannot produce (p)ppGpp [referred to as (p)ppGpp0] require ∼11 amino acids for growth, but the identities of the required amino acids vary depending on the strain background (2, 3). In E. coli, (p)ppGpp upregulates several amino acid biosynthesis genes directly by binding to RNA polymerase (RNAP) or indirectly via passive redistribution of RNAPs that are released by (p)ppGpp-mediated inhibition of transcription of stable RNA and other genes (4–7). However, transcriptome analyses showed that upon isoleucine starvation, amino acid biosynthesis genes are not induced en masse (8). Additionally, supplementing the medium with the auxotrophic requirements alone does not allow efficient colony formation of (p)ppGpp0 cells, an unsolved mystery (2).
In other bacteria, (p)ppGpp is also required for viability in the absence of amino acids. In the Gram-positive bacterium Bacillus subtilis, (p)ppGpp is synthesized by three enzymes: RelA, YjbM, and YwaC (9–11). Deleting all three (p)ppGpp synthetase genes results in cells that do not form colonies on minimal medium containing glucose but form colonies efficiently when all 20 amino acids are added, confirming that these cells are auxotrophic for certain amino acids (12). While deletion of relA alone was shown to render B. subtilis cell growth strongly dependent on externally supplemented valine and weakly dependent on leucine, isoleucine, and methionine (9), the precise auxotrophic requirements of (p)ppGpp0 cells have not been characterized.
(p)ppGpp0 cells have high levels of GTP, and the inability of (p)ppGpp0 cells to form colonies on minimal medium can be suppressed by mutations in guaA, guaB, and gmk, which encode enzymes in the GTP biosynthesis pathway, and by mutations in codY, which encodes the global transcriptional regulator CodY (12). High levels of GTP activate CodY (13) to repress multiple genes in the biosynthetic pathways for the branched-chain amino acids (BCAA) (isoleucine, leucine, and valine) (14, 15), threonine, and arginine (16). In addition, GTP and ATP levels are often inversely correlated, and increased levels of ATP stimulate transcription of the BCAA biosynthetic genes ilvB and ywaA, both of which initiate with ATP (17, 18). However, the relative contributions of these mechanisms to (p)ppGpp-dependent prototrophy and how the lack of cellular (p)ppGpp can be circumvented by mutations in codY and GTP biosynthesis genes are not understood.
To answer these questions, we documented the precise amino acid requirements in B. subtilis (p)ppGpp0 cells and identified broader amino acid requirements than previously observed. Strong requirements for BCAAs, methionine, and threonine and modest requirements for 3 additional amino acids were found. We then systematically characterized the effects of (p)ppGpp, CodY, and GTP levels on global transcription profiles to reveal their relative contributions to the amino acid auxotrophy of (p)ppGpp0 cells. Finally, we found an apparent residual amino acid requirement when all eight defined auxotrophic requirements are supplemented, and this was due to a failure of (p)ppGpp0 cells to survive a sudden nutrient downshift.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
All B. subtilis strains are derivatives of SMY (19) and are listed in Table 1. For liquid growth, cells were grown in S7 defined synthetic medium (20) containing 5 mM potassium phosphate (pH 7.0), 10 mM (NH4)2SO4, 2 mM MgCl2, 0.7 mM CaCl2, 50 mM MnCl2, 5 mM FeCl3, 1 mM ZnCl2, 2 mM thiamine, and 50 mM 3-(N-morpholino)propanesulfonic acid (MOPS), supplemented with 0.1% (wt/vol) glutamate, 1% (wt/vol) glucose, 0.5% (wt/vol) Casamino Acids (Bacto Casamino Acids, BD 223050), and 0.02 mg/ml tryptophan (which is not present in Casamino Acids). Cells were grown at 37°C with vigorous shaking (250 rpm). For growth on solid medium, cells were plated on 1.5% (wt/vol) agar with Spizizen minimal salts (21), 1% (wt/vol) glucose, and 0.1% (wt/vol) glutamate. Where indicated, defined solid medium was supplemented with 0.05 mg/ml of each amino acid, with the exception of 0.5 mg/ml aspartic acid and glutamic acid, 0.02 mg/ml tryptophan and tyrosine, and 0.04 mg/ml cysteine (22).
TABLE 1.
Strains and plasmids
| Strain or plasmid | Genotype | Reference |
|---|---|---|
| Strains | ||
| SMY | Prototroph | 19 |
| JDW1021 | SMY ΔywaC ΔyjbM ΔcodY ΔrelA::mls | 12 |
| JDW1332 | SMY ΔyjbM::spc ΔywaC::kan ΔrelA::mls | 12 |
| JDW1391 | SMY ΔywaC::kan ΔyjbM::spc ΔrelA::cm guaB::Pspac-guaB erm | 12 |
| JDW1392 | SMY ilvBp4 ilvBpΔT2 ΔyjbM::spc ΔywaC::kan ΔrelA::mls | This work |
| JDW1430 | SMY ΔywaC ΔyjbM | 12 |
| JDW1464 | SMY relAD264G ywaCD87G yjbMD72G | This work |
| JDW1824 | SMY ilvBp4 ilvBpΔT2 ybgEpΔCBS3 ilvDpΔCBS ΔyjbM::spc ΔywaC::kan ΔrelA::mls | This work |
| JDW1898 | SMY ΔywaC::kan ΔyjbM::spc | 12 |
| SRB107 | SMY ilvBp4 ilvBpΔT2 | 25 |
| SRB194 | SMY ilvBp4 ilvBpΔT2 ybgEpΔCBS3 ilvDpΔCBS | 25 |
| Plasmids | ||
| pJW204 | pGEM/ΔywaC::kan amp cat | 12 |
| pJW239 | pEX44/ΔyjbM amp cat | 12 |
| pJW265 | pEX44/ΔyjbM::spc amp cat | 12 |
| pJW299 | pEX44/I-SceI site amp cat | 12 |
| pJW300 | pJW239/ΔyjbM I-SceI site amp cat | 12 |
| pJW305 | pMUTIN4/Pspac-guaB erm | 12 |
| pJW306 | pJW299/ΔywaC I-SceI site amp cat | 12 |
| pJW368 | pJW299/relA I-SceI site amp cat | This work |
| pJW369 | pJW299/ywaC I-SceI site amp cat | This work |
| pJW370 | pJW299/yjbM I-SceI site amp cat | This work |
| pJW371 | pJW299/relAD264G I-SceI site amp cat | This work |
| pJW372 | pJW299/ywaCD87G I-SceI site amp cat | This work |
| pJW373 | pJW299/yjbMD72G I-SceI site amp cat | This work |
| pJW418 | pJW299/ΔcodY I-SceI site amp cat | 12 |
| pSS4332 | oriU Pamy-I-SceI kan | 23 |
Strain construction.
Strain JDW1464 (relAD264G ywaCD87G yjbMD72G) was created by substituting a conserved aspartic acid residue in each of the three (p)ppGpp synthetases, corresponding to D264 in RelA, D87 in YwaC, and D72 in YjbM, with glycine (Fig. 1C). To change these residues, regions of relA, ywaC, or yjbM were first cloned into the EcoRI site of pJW299 (12) to create pJW368, pJW369, and pJW370, respectively. Site-directed mutagenesis was then performed with the QuikChange site-directed mutagenesis kit to create pJW371, pJW372, and pJW373, respectively. The following mutagenic primers were used (capital letters denote sites of mutation): for relA, oJW984 (ggacagccaacaaaCcgtaaatttcattga) and oJW985 (tcaatgaaatttacgGtttgttggctgtcc); for ywaC, oJW992 (gcacgccggcaatgCcgtgaatatgctcctt) and oJW993 (aaggagcatattcacgGcattgccggcgtgc); for yjbM, oJW988 (ctaaggccagcaatgCcctgcatggtttcaa) and oJW989 (ttgaaaccatgcaggGcattgctggccttag).
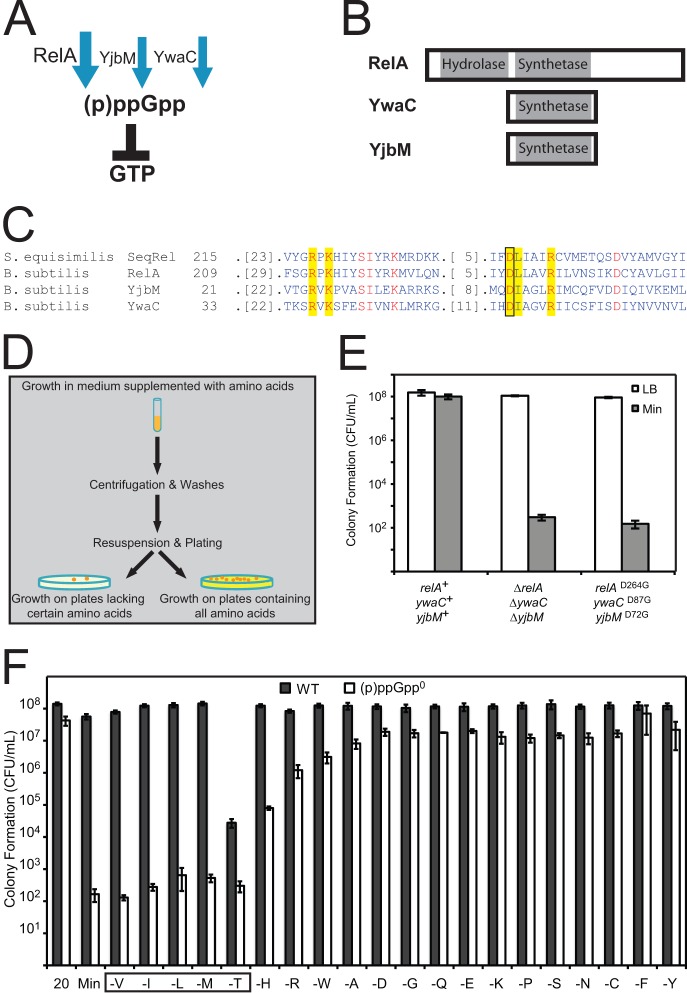
FIG 1.
(p)ppGpp synthesis is required for amino acid prototrophy. (A) In B. subtilis, the enzymes RelA, YwaC, and YjbM synthesize (p)ppGpp. The bifunctional enzyme RelA also hydrolyzes (p)ppGpp. (B) Conserved N-terminal (p)ppGpp synthetase and hydrolase domains of YwaC, YjbM, and RelA. (C) Sequence alignment showing the conserved residues (shown in red), active site residues (highlighted in yellow), and aspartic acid active site residue (boxed) that when mutated in the RelA protein of S. equisimilis completely abolishes (p)ppGpp synthetase activity without affecting hydrolase activity. The respective conserved aspartic acid residues (RelAD264, YwaCD87, and YjbMD72), aligned using the Conserved Domain Database (44), were mutated in each (p)ppGpp synthetase of B. subtilis in order to specifically abolish (p)ppGpp synthetase activity without affecting other potential functions of these proteins. (D) Schematic of the plating efficiency assay for determining amino acid auxotrophy. (E) Wild-type (relA+ ywaC+ yjbM+), synthetase deletion (ΔrelA ΔywaC ΔyjbM), and synthetase point mutant (relAD264G ywaCD87G yjbMD72G) cells were plated on LB and minimal medium (Min) plates, and colonies were counted the next day. “CFU/ml” indicates CFU per ml of culture, normalized to an OD600 of 1. (F) (p)ppGpp0 cells display strong requirements for valine, leucine, isoleucine, threonine, and methionine (boxed); (p)ppGpp0 cells also display weaker requirements for arginine, histidine, and tryptophan. Cells were grown in medium supplemented with Casamino Acids, washed, and then plated on defined medium plates supplemented with all 20 amino acids (20) or supplemented with only 19 amino acids (denoted as “-X” to indicate the amino acid that has not been supplemented in the indicated plates). Colonies were counted the next day. “CFU/ml” indicates CFU per ml of culture, normalized to an OD600 of 1.
The mutations were introduced into SMY sequentially (ywaC, followed by yjbM, followed by relA). At each step, plasmid pJW371 (or pJW372 or pJW373) was integrated into the chromosome by transformation (selected for chloramphenicol resistance), followed by subsequent transformation with pSS4332 (expressing I-SceI endonuclease; a kind gift from Scott Stibitz) to introduce a double-strand break that promotes recombination and loss of the integrated plasmid (23). Mutant alleles were confirmed by sequencing.
To reduce expression of guaB, pJW305 was created by inserting a 5′ fragment of guaB, from 22 bp upstream of ATG to 500 bp into the coding region (includes the ribosome-binding site but not the promoter) between the HindIII and EcoRI sites of pMUTIN4 (24). JDW1391 [guaBdown (p)ppGpp0] was created by integrating pJW305 into the chromosome of a ΔywaC::kan ΔyjbM::spc strain, placing guaB under tight Pspac-dependent (isopropyl-β-d-thiogalactopyranoside [IPTG]-inducible) control. The guaBdown allele was confirmed by sequencing. The relA allele was introduced as the last step in strain construction. To deplete guaB, guaBdown cells were grown in medium lacking IPTG.
JDW1392 [ilvBup (p)ppGpp0] was created by sequentially transforming deletions of ywaC, yjbM, and relA into SRB107 (ilvBp4 ilvBpΔT2) (25). JDW1824 [BCAAup (p)ppGpp0] was created by sequentially transforming deletions of ywaC, yjbM, and relA into SRB194 (ilvBp4 ilvBpΔT2 ybgEpΔCBS3 ilvDpΔCBS) (25).
Measurement of intracellular nucleotides by TLC.
Nucleotides were extracted, analyzed by thin-layer chromatography (TLC), and measured using a PhosphorImager, as described previously (26). Cells were treated for 20 min with arginine hydroxamate (RHX) (0.5 mg/ml) to mimic amino acid starvation by depleting charged arginine-tRNAs or with guanosine (Guo) (1 mM) to increase intracellular GTP pools via the salvage pathway (12).
Microarray-based gene expression profiling.
Wild-type, (p)ppGpp0, ΔcodY (p)ppGpp0, and guaBdown (p)ppGpp0 cells were grown to an optical density at 600 nm (OD600) of ∼0.3 and treated for 20 min with RHX (0.5 mg/ml) to induce the stringent response or guanosine (1 mM) to increase GTP levels. Samples were collected by rapidly mixing cultures with equal volumes of methanol at −20°C. Cells were then pelleted by centrifugation at 4,000 × g for 5 min. RNA was isolated using the Qiagen RNeasy kit. The procedures for reverse transcription, labeling, hybridization, and analysis were performed as described previously (27). Transcript levels were normalized to a common reference mixture, and data were filtered to include only significantly affected genes (1,524 genes). For identification of significantly affected genes, significance analysis of microarrays (SAM analysis) (28) was performed as multiclass among all samples (i.e., 12 classes, 3 replicates each); significantly affected genes were selected using a q value cutoff of <0.01. Transcript levels of the 1,524 significantly altered genes were hierarchically clustered (centered, average linkage) using the software program Cluster (29, 30) and plotted using the program TreeView (31).
RESULTS
Loss of (p)ppGpp synthetase activity in B. subtilis results in failure to grow on minimal medium.
In B. subtilis, (p)ppGpp is synthesized by three enzymes: a large bifunctional enzyme, RelA (9), and two small enzymes, YwaC and YjbM (10, 11) (Fig. 1A and B). We have previously shown that deletion of all three genes (ΔrelA ΔywaC ΔyjbM) from the prototrophic B. subtilis strain SMY results in failure to form colonies on minimal medium (12). To confirm that the phenotypes we observed are due to loss of (p)ppGpp synthesis activities rather than other potential functions of RelA, YwaC, or YjbM, we inactivated the synthetase activity of each enzyme by mutating a conserved active site residue required for (p)ppGpp synthesis based on homology to the RelA enzyme from Streptococcus equisimilis (SeqRel) (Fig. 1C) (32). The mutation of this conserved residue in all three (p)ppGpp synthetases of B. subtilis resulted in the relAD264G ywaCD87G yjbMD72G strain, which is unable to synthesize (p)ppGpp although the encoded proteins are expected to retain their three-dimensional structure. Using a plating assay (Fig. 1D) in which cells were grown in liquid medium supplemented with Casamino Acids and then plated comparatively on minimal and rich solid media to count the numbers of CFU, we confirmed that loss of (p)ppGpp synthesis, via either deletions (ΔrelA ΔywaC ΔyjbM) or point mutations (relAD264G ywaCD87G yjbMD72G), renders cells incapable of forming colonies on minimal medium efficiently (except for spontaneous suppressor mutants, which form at a frequency of ∼10−5) (Fig. 1E). Therefore, the synthesis of (p)ppGpp, rather than other protein functions, allows cells to grow on minimal medium.
B. subtilis (p)ppGpp0 cells are auxotrophic for multiple amino acids in addition to BCAA.
The inability of (p)ppGpp0 cells to form colonies on minimal medium indicates that they are auxotrophs. To determine the precise requirements of (p)ppGpp0 cells, we examined their ability to form colonies on 20 amino acid dropout plates, each lacking one amino acid, similar to experiments performed in E. coli (2). Wild-type SMY forms colonies on any group of 19 amino acids with the same efficiency as on all 20 amino acids, with the exception of a moderate reduction of plating efficiency on plates lacking threonine (Fig. 1F). The partial requirement for threonine may be due to feedback inhibition of Hom (homoserine dehydrogenase) by isoleucine and methionine, both downstream products of threonine (33). In contrast to wild-type cells, (p)ppGpp0 cells exhibited strong requirements for BCAA, methionine, and threonine and moderate requirements for histidine, arginine, and tryptophan (Fig. 1F). These requirements are far more stringent than those of ΔrelA cells, which have only a strong requirement for valine and modest requirements for leucine, isoleucine, and methionine (9).
We previously performed a genetic screen revealing that the requirement for exogenous amino acids in (p)ppGpp0 cells can be relieved by loss-of-function mutations in the GTP biosynthesis genes guaA, guaB, and gmk and in the transcriptional regulator codY (Fig. 2A) (12), suggesting that CodY and/or GTP are responsible for the auxotrophy. However, further analysis of these strains was limited by the tryptophan and methionine auxotrophies of the background strain (YB886) due to mutations in the biosynthesis genes trpC and metB (34). To comprehensively test the effects of CodY and GTP on (p)ppGpp0 strain auxotrophies, we used the prototrophic SMY strain (19). We introduced into SMY a codY deletion along with yjbM, ywaC, and relA deletions to eliminate CodY activity in (p)ppGpp0 cells. Deletion of codY partially suppressed the plating efficiency defect of (p)ppGpp0 cells on minimal medium (Fig. 2B, Min). Plating on amino acid dropout plates revealed that deletion of codY relieved the strong requirements for valine, isoleucine, leucine, methionine, and threonine and the moderate requirement for arginine, although it only partially relieved the requirements for histidine and tryptophan (Fig. 2B). We also reduced expression of guaB, encoding the IMP dehydrogenase, which synthesizes XMP from IMP, in (p)ppGpp0 SMY cells (Fig. 2A). Because guaB was reported to be an essential gene (35), we placed it under an IPTG-inducible promoter at its endogenous locus. Reduction in guaB expression (guaBdown), when IPTG was removed from the medium, relieved the requirement for all 8 amino acids of (p)ppGpp0 cells in the SMY background (Fig. 2B), indicating that reduction of GTP biosynthesis suppresses all auxotrophies of (p)ppGpp0 cells.
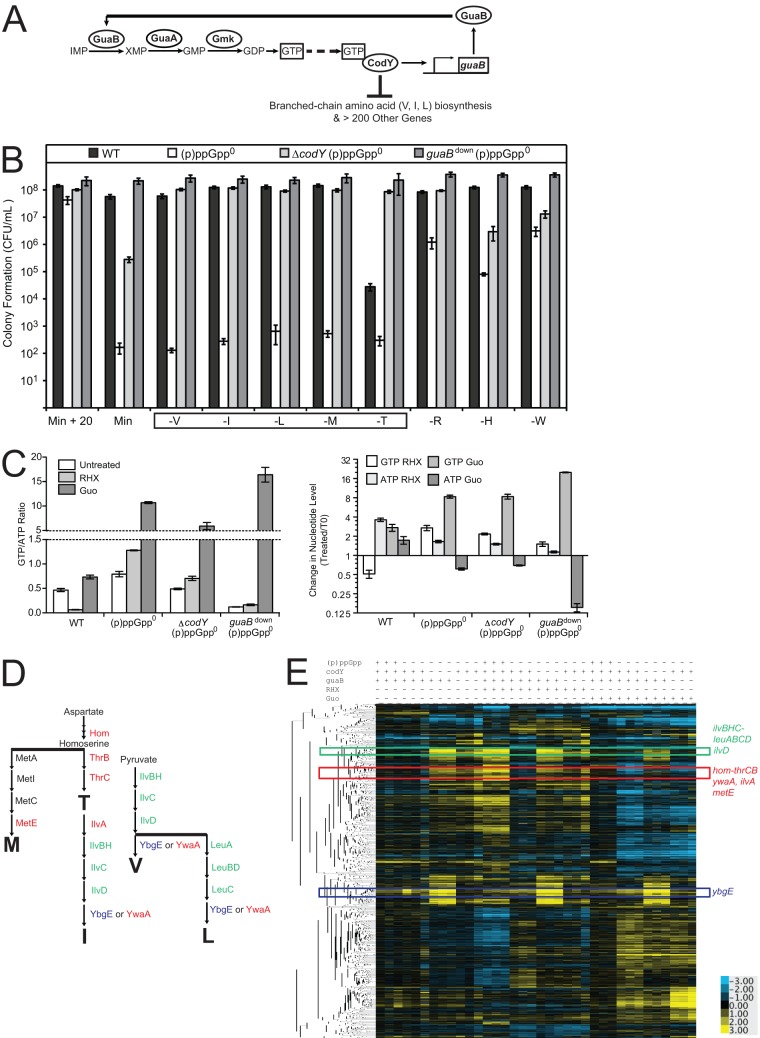
FIG 2.
Effects of (p)ppGpp, guaB, and codY on global transcription profiles of B. subtilis cells. (A) Schematic of the pathways affected by the (p)ppGpp0 suppressor mutations. GuaB, GuaA, and Gmk are enzymes in the GTP biosynthesis pathway. GTP activates CodY. CodY activates transcription of guaB and represses numerous other genes, including those involved in biosynthesis of BCAA: valine, isoleucine, and leucine. (B) Colony formation of WT, (p)ppGpp0, ΔcodY (p)ppGpp0, and guaBdown (p)ppGpp0 cells on plates supplemented with all 20 amino acids or plates lacking valine (V), isoleucine (I), leucine (L), methionine (M), threonine (T), arginine (R), histidine (H), or tryptophan (W). Cells were plated as for Fig. 1F. The box indicates strong amino acid requirements. (C) GTP/ATP ratios or GTP and ATP levels relative to those for untreated cells at t = 0 of wild-type, (p)ppGpp0, ΔcodY (p)ppGpp0, and guaBdown (p)ppGpp0 cells. 32P-labeled cells were treated with 0.5 mg/ml RHX or 1 mM Guo for 20 min. Error bars represent standard errors of the means for three independent biological replicates. (D) Schematic of the amino acid biosynthesis pathways that lead to the production of the amino acids that are strongly required by (p)ppGpp0 cells: threonine, methionine, isoleucine, valine, and leucine. (E) Hierarchical clustering analysis of transcript levels obtained from microarrays. Rows correspond to the genes; columns correspond to samples with strains and treatments labeled above the heat map for WT [(p)ppGpp +], (p)ppGpp0 [(p)ppGpp −], ΔcodY (p)ppGpp0 (codY −), and guaBdown (p)ppGpp0 (guaB −) cells that are either untreated, treated with RHX or treated with Guo. Three independent experimental replicates are shown. Transcript levels (log2) are indicated by color such that high levels are yellow and low levels are blue. Genes were hierarchically clustered using the software program Cluster and plotted with TreeView software. Colored boxes correspond to distinct clusters containing the genes involved in synthesis of the amino acids that are strongly required by (p)ppGpp0 cells, which are indicated with the corresponding colors in panel C. For an expanded view, see Fig. S1B in the supplemental material.
Effects of guaB and codY on global transcription profiles in (p)ppGpp0 cells.
To understand whether these strong auxotrophic requirements and their suppressions are due to alteration of gene expression by CodY and/or GTP, we examined gene expression profiles of wild-type, (p)ppGpp0, guaBdown (p)ppGpp0, and ΔcodY (p)ppGpp0 strains (see Fig. S1A in the supplemental material) during exponential growth upon addition of arginine hydroxamate (RHX) to induce (p)ppGpp in wild-type cells and upon addition of guanosine, which massively elevates GTP levels in all three (p)ppGpp-deficient strains (Fig. 2C) (12). We found that these strains displayed profound differences in gene expression upon various treatments, with 1,524 genes significantly altered (q < 0.01) (see Table S1); biological replicates behaved consistently with each other (see Fig. S1B). We observed some expected changes, including a general decrease in expression of de novo purine biosynthesis genes (the purEKBCSQLFMNHD operon) in response to guanosine treatment in all cells (see Fig. S2A), likely due to negative feedback regulation of the de novo biosynthesis pathway (36). We also observed a decrease in expression of ribosomal protein genes upon accumulation of (p)ppGpp (see Fig. S2B and Table S1), as was observed before (37). In addition, we observed prominent changes in the CodY regulon that are highly consistent with a previous analysis in (p)ppGpp+ cells (see Table S2) (14). Of particular interest to our study are genes involved in synthesizing amino acids that are strongly required by (p)ppGpp0 cells (Val, Ilv, Leu, Met, and Thr) (Fig. 2D). Upon RHX treatment, these genes were strongly induced in wild-type cells but failed to be induced in (p)ppGpp0 cells. In addition, these genes are located among three distinct clusters on the hierarchical tree of the microarray data (Fig. 2E), and their transcription is strongly affected by mutations in codY or guaB or both. Combining gene expression profiling with auxotrophic analysis, we found that the regulation of these genes contributes to the strong amino acid requirements.
Hyperrepression of ilvBHC-leuABCD, ilvD, and ybgE by CodY in (p)ppGpp0 cells.
We first examined two clusters on the hierachical tree (Fig. 2E, green and blue) that include BCAA biosynthetic genes known to be strongly and directly repressed by CodY (14, 15): the ilvB (ilvBHC-leuABCD) and ilvD operons (green) and ybgE (blue) (Fig. 3A). CodY is activated by GTP (13, 38, 39), and therefore reduction of intracellular GTP, due to either regulation by (p)ppGpp or reduced expression of guaB, should inactivate CodY-mediated repression of these genes. Examination of expression profiles of these CodY-regulated BCAA biosynthesis genes verified that they are indeed derepressed in wild-type cells upon (p)ppGpp induction by RHX and in ΔcodY (p)ppGpp0 and guaBdown (p)ppGpp0 cells (Fig. 3B to F). In contrast, in (p)ppGpp0 cells, their expression is strongly inhibited, suggesting that their direct repression by CodY underlies the failure to synthesize BCAA in (p)ppGpp0 cells.
FIG 3.
Transcript levels of ilvBHC-leuABCD, ilvD, and ybgE in (p)ppGpp0 cells and the effects of CodY and GTP. (A) Schematic of the BCAA (isoleucine, valine, and leucine) biosynthesis pathways. Genes in two major clusters in the hierarchical tree of the microarray data in Fig. 2D are colored accordingly: the ilvBHC-leuABCD and ilvD operons are in the green cluster; the ybgE operon clusters separately in blue. (B) A cluster in which genes in the ilvB and ilvD operons (green) are located. Transcript levels (log2) are indicated by color such that high levels are yellow and low levels are blue. (C to F) Transcript levels of ilvB (C), leuC (D), ilvD (E), or ybgE (F) in wild-type, (p)ppGpp0, ΔcodY (p)ppGpp0, and guaBdown (p)ppGpp0 cells both before and after RHX or Guo treatment. Transcript levels were normalized to a common reference, and log2 ratios are plotted. Similar trends were obtained for other genes in the ilvB operon: ilvH, ilvC, leuA, leuB, and leuD. For this and all subsequent figures, the averages of three biological replicates and the standard errors of the means are presented for each strain and treatment.
Direct repression by CodY is not the only factor contributing to the regulation of ilvB and ilvD. An additional CodY-independent effect of ATP and GTP concentrations on transcription was observed previously. Upon amino acid starvation, (p)ppGpp accumulation results in a decrease in the intracellular GTP concentration and a reciprocal increase in the ATP concentration; this increase kinetically stimulates the transcription initiation rates of genes whose initiating nucleotide is ATP, including the ilvB operon, and inhibits transcription initiation of genes whose initiating nucleotide is GTP, including the rrn operons (17, 18, 40, 41). This mechanism may contribute to the increased transcription of ilvB observed in wild-type and guaBdown (p)ppGpp0 cells, and, although less obviously, this mechanism could also contribute to the increased transcription of ilvB seen in ΔcodY (p)ppGpp0 cells because deletion of codY lowers the GTP levels of (p)ppGpp0 cells (Fig. 2C), since CodY positively regulates guaB expression (14). To conclusively demonstrate this mechanism, we tested whether altering GTP and ATP levels can affect transcription of the ilvB operon in the absence of both CodY and (p)ppGpp. We treated ΔcodY (p)ppGpp0 cells with guanosine, which increased GTP levels by ∼8-fold and reduced ATP levels by ∼30% (Fig. 2C). We found that genes in the ilvB operon were reduced by ∼4-fold in ΔcodY (p)ppGpp0 cells (e.g., Fig. 3C and D), demonstrating an unambiguous CodY-independent response.
Interestingly, ilvD, located in the same cluster as members of the ilvB operon on the hierarchical map of the microarrays (Fig. 3B), was similarly affected, since its expression was reduced by ∼6-fold upon guanosine addition even in the absence of CodY (Fig. 3E). In contrast, expression of ybgE, which is not located close to ilvB and ilvD in the hierarchical tree of the microarrays (Fig. 2D; see also Fig. S3 in the supplemental material), was not affected by guanosine addition in the absence of CodY (Fig. 3F). In summary, the ilvB and ilvD operons but not ybgE are downregulated by GTP accumulation and/or ATP depletion via both CodY-dependent and CodY-independent mechanisms.
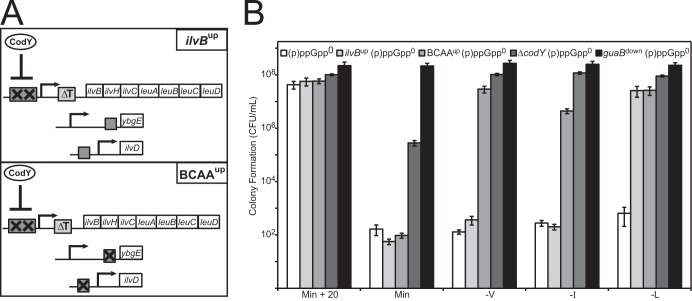
To address whether the BCAA requirements of (p)ppGpp0 cells are predominantly through direct CodY-mediated repression of ilvBHC-leuABCD, ilvD, and ybgE, we reduced CodY-mediated repression of these BCAA genes in (p)ppGpp0 cells without inactivating CodY itself. The ilvBup mutant strain harbors a deletion of the leucine-sensing T box and two point mutations in the high-affinity CodY binding site (Fig. 4A), effectively reducing CodY-dependent regulation of the ilvBHC-leuABCD operon and increasing its basal expression level. The BCAAup mutant strain contains the alleles above as well as deletions of CodY-binding sites at ilvD and ybgE (Fig. 4A) (25). We observed that ilvBup (p)ppGpp0 cells were relieved of the requirement for leucine, while BCAAup (p)ppGpp0 cells were relieved of the requirements for leucine, valine, and to a lesser extent isoleucine (Fig. 4B). These data demonstrate that CodY-mediated repression of ilvBHC-leuABCD and ilvD plays a major role in causing (p)ppGpp0 cells to be BCAA auxotrophs.
FIG 4.
Hyperrepression by CodY causes the branched-chain amino acid requirements of (p)ppGpp0 cells. (A) Alteration of CodY-binding sites in the ilvBup and BCAAup mutants. In the ilvBup mutant, two point mutations (X) in the high-affinity CodY-binding site (rectangle) at the promoter of the ilvBHC-leuABCD operon prevent CodY from binding to the promoter. An additional deletion of the leucine-dependent terminator (T box) increases the basal expression of the ilvB operon (rectangle labeled ΔT). In the BCAAup mutant, in addition to the ilvB promoter mutations, CodY binding sites (rectangles) in the promoters of ybgE and ilvD are deleted (X) to prevent CodY from repressing transcription of the ilvBHC-leuABCD, ilvD, and ybgE operons. (B) Colony formation of (p)ppGpp0 (white), ilvBup (p)ppGpp0 (light gray), BCAAup (p)ppGpp0 (medium gray), ΔcodY (p)ppGpp0 (dark gray), and guaBdown (p)ppGpp0 (black) cells on dropout plates. Cells were plated as for Fig. 1F.
Relieving CodY-mediated repression of these genes did not completely relieve the requirements for valine, leucine, and isoleucine to the same extent as deletion of codY. Assuming that the BCAAup mutant is fully CodY derepressed for the ilvB, ilvD, and ybgE operons (we cannot rule out the possibility of residual repression by CodY), the further relief of the BCAA requirements observed in ΔcodY (p)ppGpp0 cells suggests a contribution of CodY-independent BCAA gene regulation to the auxotrophy. Thus, the additional CodY-independent effect of nucleotide levels on transcription of the ilvB and ilvD operons and/or regulation of biosynthesis genes that are not affected by the mutations in the BCAAup strain (i.e., ilvA, ywaA, and hom-thrCB) may also play a role in completely relieving the BCAA requirements.
Contribution of CodY and GTP levels to repression of ilvA, ywaA, and hom-thrCB.
An additional group of genes involved in biosynthesis of BCAA and threonine, ywaA, ilvA, and hom-thrCB (Fig. 5A and B), exhibit expression patterns distinct from those of ilv-leu (red, Fig. 2D). Threonine synthesis requires hom-thrCB, while isoleucine requires both threonine and ilvA for its synthesis (Fig. 5A). Their transcript levels were strongly upregulated in wild-type cells following amino acid starvation and in ΔcodY (p)ppGpp0 and guaBdown (p)ppGpp0 cells and failed to be upregulated in (p)ppGpp0 cells (Fig. 5C to E). Thus, their misregulation may also contribute to the BCAA and threonine auxotrophies.
FIG 5.
Transcript levels of ilvA, ywaA, hom-thrCB, and metE in (p)ppGpp0 cells and the effects of CodY and GTP. (A) Schematic of the amino acid biosynthesis pathways that lead to the production of those amino acids that are strongly required by (p)ppGpp0 cells: threonine, methionine, isoleucine, valine, and leucine. Proteins involved in the biosynthesis of these amino acids whose genes localized together in clustering analysis of transcriptional profiles, separately from the ilvB, ilvD, and ybgE operons, are highlighted in red. (B) A cluster of genes obtained from Fig. 2D (red), where the ilvA, ywaA, hom-thrCB, and metE operons are located. Transcript levels (log2) are indicated by color such that high levels are yellow and low levels are blue. (C to F) Transcript levels of ilvA (C), ywaA (D), hom (E), or metE (F) both before and after RHX or Guo treatment in wild-type, (p)ppGpp0, ΔcodY (p)ppGpp0, and guaBdown (p)ppGpp0 cells. Similar results were obtained for the other genes in the hom operon: thrC and thrB.
ywaA, ilvA, and hom-thrCB have recently been revealed as in vitro targets of CodY (16). In addition, their transcript levels decrease in ΔcodY (p)ppGpp0 cells upon guanosine treatment, indicating that GTP and/or ATP levels can also affect their transcription independently of CodY (Fig. 5C to E). Although ywaA, ilvA, and hom-thrCB are regulated by GTP levels via both CodY-dependent and CodY-independent mechanisms similarly to findings for ilvB and ilvD, the magnitudes of their CodY-dependent and CodY-independent regulatory components are different, with ywaA, ilvA, and hom-thrCB displaying a stronger transcriptional response to guanosine treatment in the absence of (p)ppGpp and CodY.
Repression of methionine biosynthesis genes in (p)ppGpp0 cells.
(p)ppGpp0 cells also displayed a strong requirement for methionine. We observed that one of the genes required for methionine biosynthesis, metE, clustered near ywaA, ilvA, and hom-thrCB (Fig. 5B). Specifically, transcript levels of metE were upregulated by ∼10- to 30-fold upon the stringent response in wild-type cells but not in (p)ppGpp0 cells (Fig. 5F). Such strong changes in metE transcription, as well as changes in hom (producing homoserine, a precursor for methionine biosynthesis), are likely sufficient to account for the observed methionine auxotrophy in the absence of (p)ppGpp. The metE operon is not known to be a direct target of CodY, and transcript levels of metE decrease by ∼4-fold in (p)ppGpp0 ΔcodY cells following guanosine treatment (Fig. 5F), indicating that GTP and/or ATP levels indeed affect transcription of metE independently of CodY.
We also examined transcript levels of other genes involved in methionine biosynthesis: metA and metIC (yjcIJ) (see Fig. S4 in the supplemental material). metA expression was not significantly affected by (p)ppGpp during starvation. metIC was upregulated in wild-type cells during the stringent response, and this upregulation was abolished in (p)ppGpp0 cells. Upregulation of metIC was not restored in either the ΔcodY or guaBdown (p)ppGpp0 strains (see Fig. S4), suggesting that regulation of metIC does not account for the relief of the methionine auxotrophy of (p)ppGpp0 cells by these mutations.
Failure to survive nutrient downshift contributes to an apparent complex auxotrophy in (p)ppGpp0 cells.
We have identified strong requirements for five amino acids in (p)ppGpp0 cells and revealed that most of the genes specifically responsible for their synthesis are strongly misregulated in (p)ppGpp0 cells by elevated GTP levels via a combination of CodY-dependent and CodY-independent effects. We also identified three additional amino acids that are modestly required in (p)ppGpp0 cells. In theory, if the failure of (p)ppGpp0 cells to grow on minimal medium stemmed from their inability to produce the eight required amino acids, then supplementing these amino acids in the medium should restore colony formation. To test this prediction, we performed the standard plating assays in which cells were grown in liquid medium supplemented with Casamino Acids and then plated on medium supplemented with all 8 amino acids required by (p)ppGpp0 cells: valine, leucine, isoleucine, methionine, threonine, arginine, histidine, and tryptophan (Fig. 6A). However, we found that, similar to results observed for E. coli ppGpp0 cells (2), the (p)ppGpp0 strain of B. subtilis cannot efficiently form colonies on plates supplemented with all auxotrophic requirements (Fig. 6B). This suggests that either (p)ppGpp0 cells have additional auxotrophic requirements or, alternatively, another factor in combination with repression of amino acid biosynthesis genes prevents colony formation of (p)ppGpp0 cells.
FIG 6.
Supplementing required amino acids allows for colony formation of (p)ppGpp0 cells only in the absence of nutrient downshift. (A) Standard plating efficiency assay for determining amino acid auxotrophy involves nutrient downshift (Step 1). Cells were grown in liquid medium supplemented with Casamino Acids and then plated on defined medium supplemented with the auxotrophic requirements of (p)ppGpp0 cells: valine, isoleucine, leucine, methionine, threonine, histidine, arginine, and tryptophan (8 aa). Cells were also plated on medium supplemented with all 20 amino acids (20 aa) and on medium without amino acid supplementation (Min) for comparison. (B) Colony formation (CFU/ml) results from Step 1. (C) To determine whether supplementing the required amino acids allows colony formation of (p)ppGpp0 cells in the absence of nutrient downshift (Step 2), cells that formed colonies on medium supplemented with 8 aa were suspended in buffer and plated directly on medium supplemented with the auxotrophic requirements (8 aa). Cells were also plated on medium supplemented with all 20 amino acids (20 aa) for comparison and on minimal medium without amino acid supplementation (Min) to test for suppressor mutants. (D) Colony formation (CFU/ml) results from Step 2.
We reasoned that the apparent inability of (p)ppGpp0 cells to form colonies on medium supplemented with the 8 auxotrophic requirements may stem from failure to withstand a nutrient shift from amino acid-replete liquid medium to only 8 amino acids on solid medium. We have previously observed rapid death of (p)ppGpp0 cells upon RHX treatment (12), and we reasoned that death could occur in (p)ppGpp0 cells during nutrient downshift in our plating assay. A prediction from this hypothesis is that when not subjected to nutritional downshift, (p)ppGpp0 cells should form colonies efficiently on medium supplemented with the 8 required amino acids. We tested this prediction by taking colonies on plates with 8 amino acids that survived the initial downshift (from step 1, Fig. 6A), suspending them in Spizizen buffer, and comparatively plating them on media with 20 amino acids, 8 amino acids, and no amino acids (step 2, Fig. 6C). We verified that the majority of colonies we picked do in fact form colonies efficiently on plates with 8 amino acids but do not form colonies on minimal medium, indicating that they are not suppressor mutants (Fig. 6D). These data confirmed that (p)ppGpp0 cells are indeed only auxotrophic for 8 amino acids, supporting our hypothesis that the inability to withstand nutrient downshift is what prevents efficient colony formation of (p)ppGpp0 cells on medium containing exclusively the auxotrophic requirements.
DISCUSSION
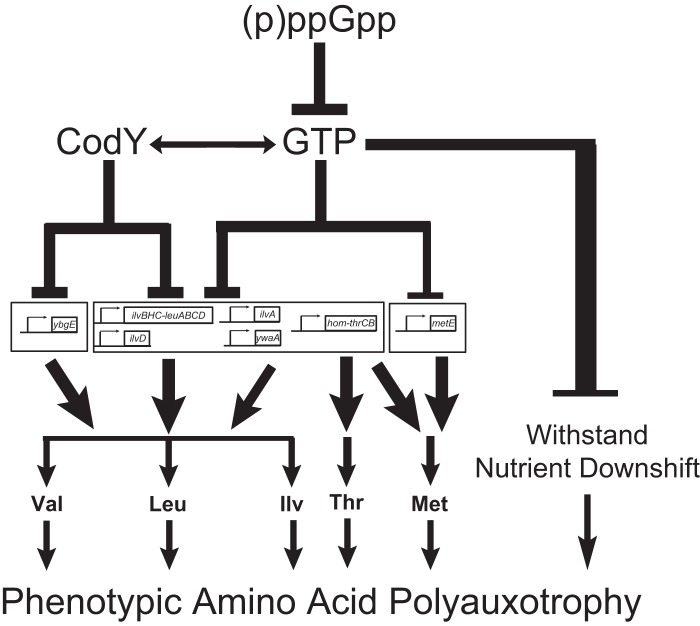
In this study, we document that the inability to produce (p)ppGpp in B. subtilis results in high GTP levels and consequent strong requirements for BCAA, methionine, and threonine and moderate requirements for arginine, histidine, and tryptophan. The BCAA auxotrophy is due primarily to direct repression of transcription of the BCAA biosynthesis genes ilvBHC-leuABCD and ilvD by GTP-mediated activation of CodY. The threonine auxotrophy is likely due to a combined direct effect of elevated GTP levels and GTP-mediated activation of CodY on transcription of the hom-thrCB operon. The methionine auxotrophy is likely due to both the effects on transcription of hom and also GTP-mediated downregulation of transcription of metE, potentially via initiating nucleotide concentrations. Reducing GTP levels allows cells to adapt to amino acid limitation both by activating transcription of biosynthesis genes and by protecting (p)ppGpp0 cells against death during nutrient shifts that prevent efficient colony formation on plates supplemented with the auxotrophic requirements. We conclude that (p)ppGpp regulates GTP levels to allow resistance to amino acid limitation by two distinct physiological roles: first by preventing cell death during nutrient downshift and then by transcriptionally enabling amino acid biosynthesis (Fig. 7).
FIG 7.
(p)ppGpp decreases GTP levels to upregulate amino acid biosynthesis and protect cellular viability during nutritional downshifts. (p)ppGpp allows growth on minimal medium by decreasing GTP levels. Decreased GTP levels allow increased transcription of various amino acid biosynthesis genes, through both CodY-dependent and CodY-independent mechanisms. Specifically, transcription of ybgE is controlled by CodY, transcription of the ilvB and ilvD operons is controlled primarily by CodY but is also affected by GTP levels, and transcription of ywaA and ilvA is likely directly regulated by CodY but also controlled by GTP levels. In the absence of (p)ppGpp-mediated regulation of GTP levels, hyperactivity of CodY is primarily responsible for causing the auxotrophies for valine, leucine, and isoleucine. Transcription of the hom-thrCB operon is likely directly regulated by CodY but is also strongly affected by GTP levels. Transcription of metE is also strongly affected by GTP levels. Thus, the inability of (p)ppGpp0 cells to properly regulate GTP levels and thus transcription of these operons likely leads to the auxotrophies for threonine and methionine. Additionally, even when all of the amino acid requirements of (p)ppGpp0 cells are met, these cells still cannot form colonies following a downshift in amino acid availability. Hence, (p)ppGpp-mediated regulation of GTP levels also allows cells to survive nutrient downshifts. This complex regulatory cascade, initiated by the inhibition of GTP biosynthesis by (p)ppGpp, allows cells to rapidly respond to changing nutrient conditions and sustain growth afterwards.
Overlapping and differential transcriptional regulation of amino acid biosynthesis genes by CodY and GTP levels accounts for strong auxotrophies of (p)ppGpp0 cells.
The strong requirements for BCAA, threonine, and methionine in (p)ppGpp0 cells can be explained by the effect of GTP levels on transcription of amino acid biosynthesis genes, via both GTP-dependent activation of the transcriptional regulator CodY and a CodY-independent effect. CodY-dependent repression of ybgE, ilvBHC-leuABCD, ilvD, ilvA, ywaA, and hom-thrCB was previously characterized (14–16). CodY-independent regulation was previously characterized for ilvB and ywaA; transcription of these genes initiates with ATP, and their transcription increases in wild-type cells during amino acid starvation in response to elevated ATP levels (17, 18). Beyond ilvB and ywaA, we identified additional targets that are similarly regulated by nucleotide levels in a CodY-independent manner, including the BCAA biosynthesis genes ilvA and ilvD and the threonine and methionine biosynthesis genes hom-thrCB and metE, as evidenced by their strong downregulation (>4-fold) in (p)ppGpp0 ΔcodY cells upon guanosine addition. Based on the locations of the conserved −10 and −35 regions of the hom-thrCB promoter, we predict that the transcription start site (TSS) of this operon is likely to be an A (42). The TSS of metE could not be predicted. It is possible that these genes are activated by increased ATP levels similarly to ilvB and ywaA (17, 18, 41). Additionally, they may also be regulated by GTP levels via an indirect and negative mechanism; i.e., decreased GTP levels reduce transcription of genes initiating with GTP, such as rrn operons (40), that engage the majority of cellular RNAP, thus increasing the availability of free RNAP for transcription of all other genes not initiating with GTP via passive redistribution. A similar situation takes place in E. coli, in which ppGpp accumulation not only directly activates transcription of amino acid biosynthesis genes (5) but also indirectly activates their transcription by inhibiting rRNA transcription, leading to passive redistribution of RNA polymerase to amino acid promoters (6). The difference is that in B. subtilis, (p)ppGpp does not directly regulate transcription by binding to RNAP but indirectly regulates transcription by modulating GTP levels. Future in vitro and in vivo mechanistic analyses are required to test these models.
Our results provide a rough estimation of the relative contributions of these gene regulatory mechanisms to the amino acid auxotrophies of (p)ppGpp0 cells (Fig. 7). We find that hyperrepression of the ilvB operon by CodY is responsible for the leucine requirement [Fig. 4B, ilvBup (p)ppGpp0 cells] and that CodY-mediated repression of the ilvB, ybgE, and ilvD operons is collectively responsible for the isoleucine and valine requirements [Fig. 4B, BCAAup (p)ppGpp0 cells], while regulation of hom-thrCB, ilvA, and ywaA expression likely plays an additional role in the isoleucine requirement. The threonine and methionine requirements are likely due to effects of GTP and ATP levels on transcription of hom-thrCB and metE. However, demonstrating their contribution to threonine and methionine auxotrophies would require characterizing and manipulating their promoters, particularly their transcription start sites.
A note of caution is that the regulation of the genes characterized here is within the context of (p)ppGpp0 cells, whose GTP and ATP levels (especially upon guanosine addition) may lie beyond the range of concentrations typically found in wild-type cells. Due to the different physiological ranges of nucleotide levels in the microarray samples compared to findings for cells in the auxotrophy experiments, these two concepts are not necessarily equivalent, i.e., a larger variation in the microarrays may not necessarily mean a bigger physiological contribution to the auxotrophy on plates. For example, the metIC operon is affected by changes in GTP levels in a CodY-independent manner during RHX and decoyinine treatments (41), and indeed we verified that metIC is induced upon RHX treatment (see Fig. S4 in the supplemental material). However, in ΔcodY (p)ppGpp0 cells, addition of guanosine did not result in strong downregulation of this operon. This suggests that metIC may be responsive to low GTP levels (during RHX or decoyinine treatment) but not responsive to high GTP levels (during guanosine treatment). These data suggest that different operons may be tunable within different dynamic ranges of nucleotide levels. In wild-type cells, the expression of different amino acid biosynthesis genes is modulated in a complex fashion by the continuum of physiological activities of CodY, GTP, and ATP.
Moderate arginine, histidine, and tryptophan auxotrophies.
(p)ppGpp0 cells exhibit moderate requirements for arginine, histidine, and tryptophan. Both the arginine biosynthesis genes argG and argJ have recently been revealed as targets of CodY in vivo (S. R. Brinsmade and A. L. Sonenshein, unpublished), and the argC operon containing argJ has also been identified as a direct target of CodY in vitro (16). We observed moderate but significant upregulation of argJ in wild-type cells upon amino acid starvation (∼6-fold) that depends on (p)ppGpp (see Fig. S5 in the supplemental material). We also observed a mild derepression of argJ upon deletion of codY in (p)ppGpp0 cells in our microarray analysis. The CodY-dependent expression changes in argJ and argG observed here (when glutamate is provided as a nitrogen source) are not as strong as results with ammonium as the nitrogen source (SRB and ALS, unpublished), likely because the argJ and argG operons are strongly repressed by CodY only when glutamate, a precursor of arginine biosynthesis, is not available. Nonetheless, the observation of a CodY-dependent change in argJ transcription, combined with the fact that deletion of CodY rescues the arginine auxotrophy, suggests that CodY-mediated repression of arginine biosynthesis genes causes the partial arginine requirement of (p)ppGpp0 cells.
In contrast to the arginine auxotrophy, the moderate auxotrophic requirements for histidine and tryptophan are not completely rescued by codY deletion and are unlikely to be caused by altered transcription of their biosynthesis genes. Interestingly, the synthesis of these amino acids relies heavily on the precursor of purine biosynthesis, phosphoribosyl pyrophosphate (PRPP). Perhaps PRPP is exhausted in (p)ppGpp0 cells due to overproduction of GTP, and thus histidine and tryptophan production is compromised, whereas inhibition of de novo GTP biosynthesis by (p)ppGpp could free up PRPP for synthesis of these amino acids. Alternatively, these apparent amino acid requirements may stem from the effects of nutrient downshift and not actually constitute bona fide amino acid auxotrophies.
Amino acid biosynthesis versus adjusting to nutrient downshift: dual protection by (p)ppGpp.
Our results suggest that the inability of (p)ppGpp0 cells to form colonies does not stem solely from the inability to properly upregulate transcription of amino acid biosynthesis genes. (p)ppGpp accumulation not only affects the transcription of BCAA, threonine, and methionine biosynthesis genes but also protects cells from death during nutrient downshift (Fig. 6 and 7). This explains why supplementing all of the amino acid requirements of (p)ppGpp0 cells does not completely allow colony formation: it relieves the auxotrophic requirements but does not protect cells from nutrient downshift from growth in 20 amino acids to that in merely 8 amino acids (Fig. 6B). When not subjected to nutrient downshift, (p)ppGpp0 cells are capable of forming colonies on medium supplemented with the required amino acids (Fig. 6D). Since nutrient downshift is often an intermediate step in auxotrophic analysis, (p)ppGpp allows cells to form colonies on amino acid-limiting medium by acting on two distinct successive processes: surviving nutrient downshift and allowing prototrophy. The failure to survive nutrient downshift when GTP levels are dysregulated in the absence of (p)ppGpp could be due to transcriptional misregulation of genes other than the amino acid biosynthesis genes, e.g., failure to downregulate rRNA and ribosomal protein synthesis at high GTP levels (40), or due to GTP-related metabolic dysregulation via an unknown cause, e.g., posttranscriptional or allosteric regulations.
Similarly to what we found in B. subtilis, failure to survive nutrient downshift could also account for the observed inconsistency of polyauxotrophy in E. coli (p)ppGpp0 cells (2). Additionally, in E. coli the DksA transcription factor also performs dual functions during amino acid starvation: upregulating transcription of amino acid biosynthesis genes (5) and protecting cells from transcription-replication conflict (43). It will be interesting to examine whether components of the stringent response also protect E. coli and other bacteria by the sequential effect of ensuring survival during the change of conditions and then engaging the cell's biosynthetic machinery to adapt to the new situation.
Supplementary Material
ACKNOWLEDGMENTS
We thank Scott Stibitz for the kind gift of strains and Kathy Krasny for critical reading of the manuscript.
This work was supported by NIH grants GM084003 to J.D.W. and GM042219 to A.L.S.
Footnotes
Published ahead of print 25 October 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00918-13.
REFERENCES
- 1.Potrykus K, Cashel M. 2008. (p) ppGpp: still magical? Annu. Rev. Microbiol. 62:35–51. 10.1146/annurev.micro.62.081307.162903 [DOI] [PubMed] [Google Scholar]
- 2.Xiao H, Kalman M, Ikehara K, Zemel S, Glaser G, Cashel M. 1991. Residual guanosine 3′,5′-bispyrophosphate synthetic activity of relA null mutants can be eliminated by spoT null mutations. J. Biol. Chem. 266:5980–5990 [PubMed] [Google Scholar]
- 3.Murphy H, Cashel M. 2003. Isolation of RNA polymerase suppressors of a (p)ppGpp deficiency. Methods Enzymol. 371:596–601. 10.1016/S0076-6879(03)71044-1 [DOI] [PubMed] [Google Scholar]
- 4.Paul BJ, Barker MM, Ross W, Schneider DA, Webb C, Foster JW, Gourse RL. 2004. DksA: a critical component of the transcription initiation machinery that potentiates the regulation of rRNA promoters by ppGpp and the initiating NTP. Cell 118:311–322. 10.1016/j.cell.2004.07.009 [DOI] [PubMed] [Google Scholar]
- 5.Paul BJ, Berkmen MB, Gourse RL. 2005. DksA potentiates direct activation of amino acid promoters by ppGpp. Proc. Natl. Acad. Sci. U. S. A. 102:7823–7828. 10.1073/pnas.0501170102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker MM, Gaal T, Gourse RL. 2001. Mechanism of regulation of transcription initiation by ppGpp. II. Models for positive control based on properties of RNAP mutants and competition for RNAP. J. Mol. Biol. 305:689–702. 10.1006/jmbi.2000.4328 [DOI] [PubMed] [Google Scholar]
- 7.Haugen SP, Berkmen MB, Ross W, Gaal T, Ward C, Gourse RL. 2006. rRNA promoter regulation by nonoptimal binding of sigma region 1.2: an additional recognition element for RNA polymerase. Cell 125:1069–1082. 10.1016/j.cell.2006.04.034 [DOI] [PubMed] [Google Scholar]
- 8.Traxler MF, Summers SM, Nguyen HT, Zacharia VM, Hightower GA, Smith JT, Conway T. 2008. The global, ppGpp-mediated stringent response to amino acid starvation in Escherichia coli. Mol. Microbiol. 68:1128–1148. 10.1111/j.1365-2958.2008.06229.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wendrich TM, Marahiel MA. 1997. Cloning and characterization of a relA/spoT homologue from Bacillus subtilis. Mol. Microbiol. 26:65–79. 10.1046/j.1365-2958.1997.5511919.x [DOI] [PubMed] [Google Scholar]
- 10.Srivatsan A, Han Y, Peng J, Tehranchi AK, Gibbs R, Wang JD, Chen R. 2008. High-precision, whole-genome sequencing of laboratory strains facilitates genetic studies. PLoS Genet. 4:e1000139. 10.1371/journal.pgen.1000139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nanamiya H, Kasai K, Nozawa A, Yun CS, Narisawa T, Murakami K, Natori Y, Kawamura F, Tozawa Y. 2008. Identification and functional analysis of novel (p) ppGpp synthetase genes in Bacillus subtilis. Mol. Microbiol. 67:291–304. 10.1111/j.1365-2958.2007.06018.x [DOI] [PubMed] [Google Scholar]
- 12.Kriel A, Bittner AN, Kim SH, Liu K, Tehranchi AK, Zou WY, Rendon S, Chen R, Tu BP, Wang JD. 2012. Direct regulation of GTP homeostasis by (p)ppGpp: a critical component of viability and stress resistance. Mol. Cell 48:231–241. 10.1016/j.molcel.2012.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brinsmade SR, Sonenshein AL. 2011. Dissecting complex metabolic integration provides direct genetic evidence for CodY activation by guanine nucleotides. J. Bacteriol. 193:5637–5648. 10.1128/JB.05510-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molle V, Nakaura Y, Shivers RP, Yamaguchi H, Losick R, Fujita Y, Sonenshein AL. 2003. Additional targets of the Bacillus subtilis global regulator CodY identified by chromatin immunoprecipitation and genome-wide transcript analysis. J. Bacteriol. 185:1911–1922. 10.1128/JB.185.6.1911-1922.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tojo S, Satomura T, Morisaki K, Deutscher J, Hirooka K, Fujita Y. 2005. Elaborate transcription regulation of the Bacillus subtilis ilv-leu operon involved in the biosynthesis of branched-chain amino acids through global regulators of CcpA, CodY and TnrA. Mol. Microbiol. 56:1560–1573. 10.1111/j.1365-2958.2005.04635.x [DOI] [PubMed] [Google Scholar]
- 16.Belitsky BR, Sonenshein AL. 2013. Genome-wide identification of Bacillus subtilis CodY-binding sites at single-nucleotide resolution. Proc. Natl. Acad. Sci. U. S. A. 110:7026–7031. 10.1073/pnas.1300428110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tojo S, Satomura T, Kumamoto K, Hirooka K, Fujita Y. 2008. Molecular mechanisms underlying the positive stringent response of the Bacillus subtilis ilv-leu operon, involved in the biosynthesis of branched-chain amino acids. J. Bacteriol. 190:6134–6147. 10.1128/JB.00606-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krasny L, Tiserova H, Jonak J, Rejman D, Sanderova H. 2008. The identity of the transcription +1 position is crucial for changes in gene expression in response to amino acid starvation in Bacillus subtilis. Mol. Microbiol. 69:42–54. 10.1111/j.1365-2958.2008.06256.x [DOI] [PubMed] [Google Scholar]
- 19.Schaeffer P, Millet J, Aubert JP. 1965. Catabolic repression of bacterial sporulation. Proc. Natl. Acad. Sci. U. S. A. 54:704–711. 10.1073/pnas.54.3.704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasantha N, Freese E. 1980. Enzyme changes during Bacillus subtilis sporulation caused by deprivation of guanine nucleotides. J. Bacteriol. 144:1119–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spizizen J. 1958. Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleate. Proc. Natl. Acad. Sci. U. S. A. 44:1072–1078. 10.1073/pnas.44.10.1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harwood CR, Cutting SM. 1990. Molecular biological methods for Bacillus. John Wiley & Sons, Inc., New York, NY [Google Scholar]
- 23.Janes BK, Stibitz S. 2006. Routine markerless gene replacement in Bacillus anthracis. Infect. Immun. 74:1949–1953. 10.1128/IAI.74.3.1949-1953.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vagner V, Dervyn E, Ehrlich SD. 1998. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology 144:3097–3104. 10.1099/00221287-144-11-3097 [DOI] [PubMed] [Google Scholar]
- 25.Brinsmade SR, Kleijn RJ, Sauer U, Sonenshein AL. 2010. Regulation of CodY activity through modulation of intracellular branched-chain amino acid pools. J. Bacteriol. 192:6357–6368. 10.1128/JB.00937-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang JD, Sanders GM, Grossman AD. 2007. Nutritional control of elongation of DNA replication by (p)ppGpp. Cell 128:865–875. 10.1016/j.cell.2006.12.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Britton RA, Eichenberger P, Gonzalez-Pastor JE, Fawcett P, Monson R, Losick R, Grossman AD. 2002. Genome-wide analysis of the stationary-phase sigma factor (sigma-H) regulon of Bacillus subtilis. J. Bacteriol. 184:4881–4890. 10.1128/JB.184.17.4881-4890.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tusher VG, Tibshirani R, Chu G. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. U. S. A. 98:5116–5121. 10.1073/pnas.091062498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Hoon MJ, Imoto S, Nolan J, Miyano S. 2004. Open source clustering software. Bioinformatics 20:1453–1454. 10.1093/bioinformatics/bth078 [DOI] [PubMed] [Google Scholar]
- 30.Eisen MB, Spellman PT, Brown PO, Botstein D. 1998. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. U. S. A. 95:14863–14868. 10.1073/pnas.95.25.14863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saldanha AJ. 2004. Java Treeview—extensible visualization of microarray data. Bioinformatics 20:3246–3248. 10.1093/bioinformatics/bth349 [DOI] [PubMed] [Google Scholar]
- 32.Hogg T, Mechold U, Malke H, Cashel M, Hilgenfeld R. 2004. Conformational antagonism between opposing active sites in a bifunctional RelA/SpoT homolog modulates (p)ppGpp metabolism during the stringent response. Cell 117:57–68 (Erratum, 117:415.) 10.1016/S0092-8674(04)00260-0 [DOI] [PubMed] [Google Scholar]
- 33.Yeggy JP, Stahly DP. 1980. Sporulation and regulation of homoserine dehydrogenase in Bacillus subtilis. Can. J. Microbiol. 26:1386–1391. 10.1139/m80-231 [DOI] [PubMed] [Google Scholar]
- 34.Yasbin RE, Fields PI, Andersen BJ. 1980. Properties of Bacillus subtilis 168 derivatives freed of their natural prophages. Gene 12:155–159. 10.1016/0378-1119(80)90026-8 [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi K, Ehrlich SD, Albertini A, Amati G, Andersen KK, Arnaud M, Asai K, Ashikaga S, Aymerich S, Bessieres P, Boland F, Brignell SC, Bron S, Bunai K, Chapuis J, Christiansen LC, Danchin A, Debarbouille M, Dervyn E, Deuerling E, Devine K, Devine SK, Dreesen O, Errington J, Fillinger S, Foster SJ, Fujita Y, Galizzi A, Gardan R, Eschevins C, Fukushima T, Haga K, Harwood CR, Hecker M, Hosoya D, Hullo MF, Kakeshita H, Karamata D, Kasahara Y, Kawamura F, Koga K, Koski P, Kuwana R, Imamura D, Ishimaru M, Ishikawa S, Ishio I, Le Coq D, Masson A, Mauel C, Meima R, Mellado RP, et al. 2003. Essential Bacillus subtilis genes. Proc. Natl. Acad. Sci. U. S. A. 100:4678–4683. 10.1073/pnas.0730515100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebbole DJ, Zalkin H. 1987. Cloning and characterization of a 12-gene cluster from Bacillus subtilis encoding nine enzymes for de novo purine nucleotide synthesis. J. Biol. Chem. 262:8274–8287 [PubMed] [Google Scholar]
- 37.Eymann C, Homuth G, Scharf C, Hecker M. 2002. Bacillus subtilis functional genomics: global characterization of the stringent response by proteome and transcriptome analysis. J. Bacteriol. 184:2500–2520. 10.1128/JB.184.9.2500-2520.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Handke LD, Shivers RP, Sonenshein AL. 2008. Interaction of Bacillus subtilis CodY with GTP. J. Bacteriol. 190:798–806. 10.1128/JB.01115-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ratnayake-Lecamwasam M, Serror P, Wong KW, Sonenshein AL. 2001. Bacillus subtilis CodY represses early-stationary-phase genes by sensing GTP levels. Genes Dev. 15:1093–1103. 10.1101/gad.874201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krasny L, Gourse RL. 2004. An alternative strategy for bacterial ribosome synthesis: Bacillus subtilis rRNA transcription regulation. EMBO J. 23:4473–4483. 10.1038/sj.emboj.7600423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tojo S, Kumamoto K, Hirooka K, Fujita Y. 2010. Heavy involvement of stringent transcription control depending on the adenine or guanine species of the transcription initiation site in glucose and pyruvate metabolism in Bacillus subtilis. J. Bacteriol. 192:1573–1585. 10.1128/JB.01394-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Helmann JD. 1995. Compilation and analysis of Bacillus subtilis sigma A-dependent promoter sequences: evidence for extended contact between RNA polymerase and upstream promoter DNA. Nucleic Acids Res. 23:2351–2360. 10.1093/nar/23.13.2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tehranchi AK, Blankschien MD, Zhang Y, Halliday JA, Srivatsan A, Peng J, Herman C, Wang JD. 2010. The transcription factor DksA prevents conflicts between DNA replication and transcription machinery. Cell 141:595–605. 10.1016/j.cell.2010.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marchler-Bauer A, Lu S, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, Fong JH, Geer LY, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Jackson JD, Ke Z, Lanczycki CJ, Lu F, Marchler GH, Mullokandov M, Omelchenko MV, Robertson CL, Song JS, Thanki N, Yamashita RA, Zhang D, Zhang N, Zheng C, Bryant SH. 2011. CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 39:D225–D229. 10.1093/nar/gkq1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.