Abstract
PutP and OpuE serve as proline transporters when this imino acid is used by Bacillus subtilis as a nutrient or as an osmostress protectant, respectively. The simultaneous inactivation of the PutP and OpuE systems still allows the utilization of proline as a nutrient. This growth phenotype pointed to the presence of a third proline transport system in B. subtilis. We took advantage of the sensitivity of a putP opuE double mutant to the toxic proline analog 3,4-dehydro-dl-proline (DHP) to identify this additional proline uptake system. DHP-resistant mutants were selected and found to be defective in the use of proline as a nutrient. Whole-genome resequencing of one of these strains provided the lead that the inactivation of the γ-aminobutyrate (GABA) transporter GabP was responsible for these phenotypes. DNA sequencing of the gabP gene in 14 additionally analyzed DHP-resistant strains confirmed this finding. Consistently, each of the DHP-resistant mutants was defective not only in the use of proline as a nutrient but also in the use of GABA as a nitrogen source. The same phenotype resulted from the targeted deletion of the gabP gene in a putP opuE mutant strain. Hence, the GabP carrier not only serves as an uptake system for GABA but also functions as the third proline transporter of B. subtilis. Uptake studies with radiolabeled GABA and proline confirmed this conclusion and provided information on the kinetic parameters of the GabP carrier for both of these substrates.
INTRODUCTION
The soil bacterium Bacillus subtilis lives in a taxing habitat where many microorganisms compete with each other for specific ecological microniches and nutritional resources (1, 2). Its genome sequence (3) bears the hallmarks of a bacterium that lives in association with plants and plant detritus (4, 5). It is therefore understandable that B. subtilis devotes a considerable portion of its genome coding capacity to direct the synthesis of transporters for the uptake of a wide variety of plant-derived compounds for use as nutrients (6) or as stress protectants (7–9).
l-Proline serves both as a nutrient and as a stress protectant for B. subtilis since the cell can exploit it not only as a sole carbon, nitrogen, and energy source (10–12) but also as an osmostress-relieving compound (13–16). To fuel protein synthesis, B. subtilis produces proline from the precursor glutamate (17, 18), a pathway that is present in many bacterial species (19). The anabolic proline synthesis route (ProB-ProA-ProI [ProG]) is interconnected with the osmostress-relieving production route for proline (ProJ-ProA-ProH) via the γ-glutamyl phosphate reductase (ProA) (14). The latter pathway is osmotically controlled and allows B. subtilis to produce very large amounts of the compatible solute proline (20) to fend off the detrimental effects of high salinity on cellular hydration, turgor, and physiology (13–15, 21). Proline-mediated osmoprotection can also be achieved through uptake; the osmotically inducible OpuE transporter of B. subtilis serves this function (15, 16). OpuE also operates as a recapturing device for newly synthesized proline that is released from B. subtilis grown under high-salinity conditions, probably in an effort by the cell to fine-tune turgor (22). Expression of opuE is osmotically inducible (16, 23, 24), but there is no stimulation of opuE expression in response to proline availability.
Externally provided proline not only affords osmostress protection (15, 16), but it also can serve as the sole carbon, energy, and nitrogen source for B. subtilis (10–12). Utilization of proline as a nutrient requires its capture from environmental sources, such as root exudates and organic deposits in the rhizosphere (2, 25), and relies on the PutB- and PutC-mediated catabolism to glutamate (10), a central intermediate in the interconnected carbon and nitrogen utilization systems of B. subtilis (26, 27). The PutP transporter mediates the uptake of proline for its use as a nutrient, and the induction of the expression of the catabolic putBCP operon by an external supply of the substrate proline reflects this role (10, 28, 29).
The two functionally characterized proline import systems of B. subtilis, PutP and OpuE (10, 16), are members of the solute:sodium symporter family (SSS; Transporter Classification Database [TC] accession number 2.A.21) (30) that mediate the import of their substrates tightly coupled to Na+ symport (31–33). The PutP and OpuE proteins are closely related to each other (61% amino acid sequence identity) and to the biochemically well-studied PutP proline permease from Escherichia coli and Salmonella enterica serovar Typhimurium (31, 33–37). Both the B. subtilis PutP and the OpuE l-proline transporters possess a high affinity for their substrate, with Km values being in the low μM range, and they exhibit a substantial transport capacity (10). However, their transport profiles differ significantly. The proline import activity of PutP, as reflected by the Vmax value, is upregulated when proline is present in the environment, and it is gradually inhibited when the salinity of the growth medium is increased (10). Conversely, not only can the proline import activity of OpuE withstand salt stress, but also it is actually increased in response to high osmolarity (38). Underlying these characteristics of the PutP- and OpuE-mediated proline import are influences that result from the different transcriptional profile of the putBCP and opuE genes in response to proline availability in the growth medium and its osmolarity (10, 16, 23, 24). However, posttranscriptional effects of high salinity on the transport activity of PutP and OpuE are also noticeable (10, 16).
In connection with the analysis of the utilization of proline as a nutrient by B. subtilis, Moses et al. (10) found that a putP opuE double mutant strain was still prone to growth inhibition by l-azetidine-2-carboxylic acid (AC) and 3,4-dehydro-dl-proline (DHP), toxic proline analogs (39) that can be imported into microbial cells via different types of proline transport systems (36, 40, 41). It was also noted in this study that the expression of the putBCP operon remained inducible by an external supply of proline in a putP opuE double mutant (10). These two observations therefore hint at the operation of a further proline import system in B. subtilis.
Here, we identify this third proline importer using a genetic selection for resistance to DHP and genome resequencing to provide a lead as to the identity of the mutation underlying the loss of the ability to use proline as a nutrient in the absence of functional PutP and OpuE transporters. The third proline import system of B. subtilis turned out to be GabP, the permease for γ-aminobutyrate (GABA) (42, 43). The simultaneous inactivation of the PutP, OpuE, and GabP transporters abolished the use of proline as a nutrient, but minor proline uptake activity remained and still allowed induction of putBCP expression in response to an external supply of proline.
MATERIALS AND METHODS
Chemicals.
The amino acids l-proline and GABA, the toxic proline analogues AC and DHP, the chromogenic substrate para-nitrophenyl-α-d-glucopyranoside (PNPG) for the TreA enzyme (44), the ninhydrin reagent for the quantification of proline by a colorimetric assay (45), and the antibiotics chloramphenicol, kanamycin, erythromycin, tetracycline, lincomycin, and spectinomycin were all purchased from Sigma-Aldrich (Steinheim, Germany). The antibiotic zeocin was obtained from Invitrogen (Carlsbad, CA). Radiolabeled l-[U-14C]proline (269 mCi mmol−1) and γ-[2,3-3H]aminobutyrate (35 Ci mmol−1) were purchased from PerkinElmer LAS GmbH (Rodgau, German).
Bacterial strains, media, and growth conditions.
The Escherichia coli K-12 strain DH5α (Invitrogen, Carlsbad, CA) was used for maintenance of recombinant plasmids. These strains were propagated either in Luria-Bertani (LB) liquid medium or on LB agar plates (46). For E. coli strains carrying plasmids encoding a beta-lactamase resistance gene, ampicillin was used at final concentrations of 100 μg ml−1. The genetic properties of the B. subtilis wild-type laboratory strain JH642 (kindly provided by J. Hoch, Scripps Research Institute, La Jolla, CA) and its mutant derivatives used throughout this study are summarized in Tables 1 and 3. B. subtilis strains were routinely cultivated in Spizizen's minimal medium (SMM) with 0.5% (wt/vol) glucose as the carbon source, 15 mM (NH4)2SO4 as the nitrogen source, and l-tryptophan (20 mg liter−1) and l-phenylalanine (18 mg liter−1) to satisfy the auxotrophic requirements of strain JH642 (trpC2 pheA1) and its derivatives (Table 1). A solution of trace elements (47) was added to SMM to improve the growth of B. subtilis strains; the osmolarity of the growth medium was adjusted by adding NaCl to it from a 5 M stock solution. When the potential use of l-proline or GABA as the sole carbon source was tested, glucose was replaced by the addition of 33 mM l-proline or 41 mM GABA. In growth experiments with l-proline or GABA as the sole nitrogen source, the ammonium sulfate was left out from SMM and l-proline or GABA was added to a final concentration of 30 mM. All B. subtilis cultures were inoculated from exponentially growing precultures in prewarmed (37°C) SMM to an optical density at 578 nm (OD578) of about 0.1, and the cultures were then subsequently propagated at 37°C in a shaking water bath set to 220 rpm. Growth of the strains was monitored by measuring their OD578. The antibiotics chloramphenicol (5 μg ml−1), tetracycline (10 μg ml−1), erythromycin-lincomycin (0.4 μg ml−1 and 15 μg ml−1, respectively), and spectinomycin (100 μg ml−1) were used for the selection of B. subtilis strains carrying chromosomal copies of gene disruption mutations with insertions of an antibiotic resistance cassette or of ϕ(putB′-treA)1 cat reporter fusion constructs into the nonessential chromosomal amyE locus (Table 1).
TABLE 1.
B. subtilis strains used in this study
| Straina | Relevant genotypeb | Source or reference |
|---|---|---|
| JH642 | trpC2 pheA1 | J. Hoch |
| ACB164 | Δ(ywcA::ery)1 | This study |
| ACB165 | Δ(yodF::neo)1 | This study |
| ACB199 | Δ(putP::spc)1 Δ(opuE::tet)1 Δ(ywcA::ery)1 Δ(yodF::neo)1 | This study |
| ACB214 | Δ(putP::spc)1 Δ(opuE::tet)1 Δ(ywcA::ery)1 Δ(yodF::neo)1 gabP1 | This study |
| ACB274 | Δ(gabP::zeo)1 | This study |
| ACB275 | Δ(opuE::tet)1 Δ(gabP::zeo)1 | This study |
| ACB276 | Δ(opuE::tet)1 Δ(gabP::zeo)1 Δ(treA::neo)1 [amyE::ϕ(putB′-treA)1 cat] | This study |
| ACB277 | Δ(putP::spc)1 Δ(gabP::zeo)1 | This study |
| ACB278 | Δ(putP::spc)1 Δ(gabP::zeo)1 Δ(treA::neo)1 [amyE::ϕ(putB′-treA)1 cat] | This study |
| ACB279 | Δ(putP::spc)1 Δ(opuE::tet)1 Δ(gabP::zeo)1 | This study |
| ACB288 | Δ(putP::spc)1 Δ(opuE::tet)1 Δ(treA::neo)1 [amyE::ϕ(putB′-treA)1 cat] | This study |
| ACB292 | Δ(putP::spc)1 Δ(opuE::tet)1 Δ(gabP::zeo)1 Δ(treA::neo)1 [amyE::ϕ(putB′-treA)1 cat] | This study |
| BLOB9 | Δ(opuE::tet)1 | 16 |
| JSB8ery | Δ(proHJ::ery)1 | J. Brill |
| MBB1 | Δ(treA::neo)1 | M. Brosius |
| SMB10 | Δ(treA::neo)1 [amyE::ϕ(putB′-treA)1 cat] | 10 |
| SMB11 | Δ(putP::spc)1 | 10 |
| SMB12 | Δ(putP::spc)1 Δ(opuE::tet)1 | 10 |
All strains are derivatives of the B. subtilis wild-type laboratory strain JH642 (52) and therefore carry the trpC2 and pheA1 mutations, in addition to the indicated genetic markers.
The designation amyE::ϕ(putB′-treA)1 cat indicates that the putB-treA operon reporter fusion is stably integrated into the chromosomal amyE gene as a single copy, thereby rendering the fusion strains defective in the extracellular α-amylase AmyE. The ϕ(putB′-treA)1 reporter fusion is genetically linked to a chloramphenicol resistance gene (cat).
TABLE 3.
Mutations in gabP conferring resistance to DHP in a putP opuE double mutant strain
| Straina | gabP allele | Mutation | Consequence |
|---|---|---|---|
| ACB214 | gabP1 | A1160 | Frameshift in codon 387 |
| ACB215 | gabP2 | TAT/TAA | Stop at codon 151 |
| ACB254 | gabP3 | GGT/GTT | G301V |
| ACB255 | gabP4 | TTC/TCC | F341S |
| ACB256 | gabP5 | TGG/TAG | Stop at codon 100 |
| ACB257 | gabP4 | TTC/TCC | F341S |
| ACB262 | gabP6 | GGT/AGT | G42S |
| ACB263 | gabP7 | A1156 | Frameshift in codon 386 |
| ACB264 | gabP8 | TGG/TAG | Stop at codon 118 |
| ACB265 | gabP9 | GGG/GAG | G338E |
| ACB266 | gabP10 | A985 | Frameshift in codon 329 |
| ACB267 | gabP11 | TGG/TGA | Stop at codon 241 |
| ACB268 | gabP12 | GGC/GAC | G33D |
| ACB269 | gabP13 | TAC/TAA | Stop at codon 49 |
| ACB273 | gabP14 | GGA/AGA | G414R |
All strains are derivatives of the B. subtilis wild-type laboratory strain JH642 (trpC2 pheA1), and each of them carries the Δ(putP::spc)1 and Δ(opuE::tet)1 gene disruption mutations. Strain ACB214 also possess the Δ(yodF::neo)1 and Δ(ywcA::ery)1 alleles, whereas in strain ACB215, only the ywcA gene [Δ(ywcA::ery)1] is disrupted.
The B. subtilis wild-type strain JH642 is able to use l-proline both as the sole nitrogen source and as the sole carbon source, but loss of the proline transporter PutP abrogated the use of proline as the sole carbon source when precultures grown with glucose as the carbon source were used to inoculate SMM (10). However, we observed that when strains carrying a putP gene disruption but intact putBC catabolic genes were pregrown in the presence of both glucose and l-proline, growth could occur with l-proline as the sole carbon source upon reinoculation. Accordingly, for such growth experiments, two precultures were prepared: the strains were initially grown to mid-exponential phase in SMM with glucose (28 mM) as the carbon source, and these precultures were then subsequently used to inoculate SMM that contained both glucose (14 mM) and proline (33 mM) as available carbon sources. When the second preculture had reached mid-exponential growth phase, the cells were carefully washed two times with SMM containing proline (33 mM) but no glucose. These cells were then used to inoculate the main culture that contained only proline (33 mM) as the carbon source. Cells treated in this way were able to grow even when they lacked the PutP proline import system (see Fig. S1 in the supplemental material).
Genetic constructions of bacterial strains.
The routine manipulations of plasmid DNA, the amplification of selected regions of the B. subtilis genome via PCR, the isolation of chromosomal DNA from B. subtilis, and the transformation of B. subtilis strains with plasmid or chromosomal DNA were all carried out using standard procedures (47). To construct a deletion of a chosen gene in the genome of B. subtilis, a two-step PCR-based method (48) was used, in which the 5′ and 3′ regions of the gene of interest were first amplified by PCR from chromosomal DNA and connected in a second step by long-flanking-region PCR with a DNA fragment encoding an antibiotic resistance gene. The generated PCR products were then used for the transformation of B. subtilis strain JH642, and their integration into the chromosome via homologous recombination events was selected for by the addition of the appropriate antibiotic to the agar plates. The following strains were constructed in that way, and the primer pairs used for the long-flanking-region PCR are given in parentheses: ACB164 [Δ(ywcA::ery)1] (5′-CATGTTAAAAAAGATTTGGACAAAGGTTGG-3′/5′-TTTGTCATCAATCTGAAAGAAGCCTAAATC-3′), ACB165 [Δ(yodF::neo)1] (5′-TGTATCCAAAGCTTTCCGTTAACAATTGTA-3′/5′-TGTCTATTCAGAGATGAAAGACAACAACAT-3′), and ACB274 [Δ(gabP::zeo)1] (5′-CTCTTGTTTAGCAGAAAAAATAGATTCTCC-3′/5′-TTTTAAGCGTTTTTCCTTTGGGTCATGAAT-3′). The antibiotic resistance genes used for these gene disruption constructs were derived from plasmids pDG642 (ery), pDG783 (neo) (49), and p7Z6 (zeo) (50). The presence of the correct gene disruption constructs in the newly created mutant strains was verified by PCR using primers that flank the deleted genomic region; PCR products derived from genomic DNA of the B. subtilis wild-type strain JH642 were used for comparison. To combine different gene disruption mutations (Table 1), the recipient B. subtilis strain was transformed with chromosomal DNA derived from B. subtilis donor strains carrying a gene disruption linked to an antibiotic resistance marker, and the transformants were then selected on LB agar plates containing the appropriate antibiotic.
Sensitivity of B. subtilis strains to toxic proline analogues and selection for DHP-resistant mutants.
To test the sensitivity of B. subtilis strains to the toxic proline analogues DHP and AC (10), cultures were grown in SMM in the absence and presence of 0.6 M NaCl until they reached exponential growth phase. Three hundred microliters of each culture was then plated on SMM or SMM agar plates with 0.6 M NaCl, and subsequently, a paper filter disk (diameter, 6 mm) soaked with 10 μl of a 25-mg ml−1 solution of DHP or AC was placed in the middle of each agar plate. The plates were incubated at 37°C for 24 h (SMM agar plates) or 48 h (SMM agar plates with 0.6 M NaCl), and the formation of growth inhibition zones around the filter disk was recorded by photography.
To isolate mutants resistant to DHP, strains with gene disruption mutations in putP and opuE were grown in SMM to an optical density (OD578) of 1.5, and a 300-μl aliquot was plated on SMM agar plates. A paper filter disk that was impregnated with 10 μl of a 25-mg ml−1 solution of DHP was placed in the center of each plate. After 2 days of incubation at 37°C, DHP-resistant colonies were visible in the growth inhibition zone formed around the filter disk. Fifteen independent mutants were picked and purified by streaking on SMM agar plates, and their resistance to DHP was retested with the above-described filter disk assay; all 15 strains isolated had stably lost their sensitivity to DHP.
To determine the frequency with which the DHP-resistant colonies appeared, three cultures of strain SMB12 (opuE putP) (Table 1) were grown in SMM with glucose as the carbon source and were then plated on SMM agar plates to determine the total number of viable cells and on SMM agar plates containing 50 μg ml−1 DHP (51). The plates were incubated for 2 days at 37°C; spontaneous DHP-resistant colonies arose at frequencies of about 6 × 10−6.
Genome resequencing, sequence assembly, and analysis.
To identify the spontaneous mutation(s) conferring resistance to DHP, one of the isolated DHP-resistant strains (ACB214) and its parent strain (ACB199) (Table 1) were subjected to genome resequencing. For this purpose, genomic DNA was isolated using a DNeasy blood and tissue kit from Qiagen. Genome resequencing and mapping of the DNA reads on B. subtilis reference genomes (3, 52) were done by the Göttingen Genomics Laboratory (G2L). Shotgun libraries were generated using a Nextera XT DNA sample preparation kit according to the manufacturer's instructions, and DNA sequences were obtained with a Genome Analyzer IIx instrument (Illumina). The prepared libraries were sequenced in a 112-bp single-read indexed run on 1/4 of one channel, resulting in 8.3 million and 9.2 million reads for strains ACB199 and ACB214, respectively. All shotgun reads were mapped to the genomes of B. subtilis strains JH642 (52) and 168 (3) using the GS Reference Mapper software tool, v2.8 (454 Life Sciences). Sequence differences were considered single nucleotide polymorphisms (SNPs) when the total coverage depth exceeded 25 reads and the variant frequency was >90%.
The gabP gene from the chromosomal DNA of 15 DHP-resistant mutants was amplified by PCR in two DNA segments (primer AC182_GabP_Seq1_for [5′-CACGAATTTTCGACAAACTGTATATTTATG-3′] and primer AC183_GabP_Seq1_rev [5′-GGGCTTTCTAAAATATTTGCTGAATTCC-3′]; primer AC184_GabP_Seq2_for [5′-CATCTTCTCTTTTATGGGAACTGAAATC-3′] and primer AC185_GabP_Seq2_rev [5′-GTTAATGACAATAAGGACGTGATGGTTAC-3′]), and the DNA sequence of the resulting PCR fragment was determined by Eurofins MWG Operon (Ebersberg, Germany). These DNA sequences were compared with the corresponding sequence of the genome region of the JH642 parent strain (52).
Measurements of cellular proline pools.
The intracellular proline content of B. subtilis cells was determined by a colorimetric assay that detects l-proline as a colored proline-ninhydrin complex that can be quantified by measuring the absorption of the solution at 480 nm (45). For these assays, the B. subtilis cells were grown in SMM with 0.4 M NaCl; harvesting and processing of the cells, the details of the assay conditions, and the calculation of the intracellular volume have been described previously (15, 21, 53). Consistent with previous measurements (21), the proline pool of strain JH642 was found to be 95 ± 12 mM in cells that had been grown in SMM containing 0.4 M NaCl; the concentrations of proline pools detected in the 15 tested DHP-resistant mutant strains ranged from 79 ± 4 mM to 98 ± 5 mM. Hence, none of the isolated DHP-resistant mutants overproduced proline.
TreA enzyme activity assays.
The expression of a chromosomal putB-treA reporter gene fusion was monitored by assaying the TreA [phospho-α-(1, 1)-glucosidase] reporter enzyme activity (44) using the chromogenic substrate PNPG (10). TreA enzyme activity is expressed in units per mg protein; protein concentrations were estimated from the OD578 of the B. subtilis cell culture (46).
Transport assays with radiolabeled l-proline and γ-aminobutyrate.
Uptake of γ-aminobutyric acid in B. subtilis was measured using γ-[2,3-3H]aminobutyrate (specific activity, 35 Ci mmol−1) as the substrate. The cells were grown to mid-exponential phase (OD578 = 0.3 to 0.4) in SMM without or with 0.6 M NaCl and were used immediately for the transport assay. The uptake assay was started by the addition of γ-aminobutyrate (final assay concentration, 5 μM to 1,000 μM) spiked with γ-[2,3-3H]aminobutyrate (final assay concentration, 2.8 nM) in a total reaction volume of 2 ml. This resulted in a specific activity of γ-[2,3-3H]aminobutyrate of 20 mCi mmol−1 at a final substrate concentration of 5 μM GABA and 0.1 mCi mmol−1 at a final substrate concentration of 1,000 μM GABA in the individual transport assays. Samples (0.3 ml) were taken at various time points and filtered through 0.45-μm-pore-size filters (Schleicher & Schuell GmbH, Dassel, Germany). The cells were washed with 20 ml of isotonic minimal salts solution, and the radioactivity retained on the filters was determined in a scintillation counter (PerkinElmer, Rodgau, Germany). The uptake assay conditions for measuring the import of radiolabeled l-[U-14C]proline (269 mCi mmol−1) (10) followed the above-described procedures, except that the following proline concentrations were used: for proline uptake assays involving wild-type strain JH642, 1 μM to 40 μM proline was used; in uptake assays involving strain SMB12 (putP opuE), 40 μM to 2,000 μM proline was used. The proline solution was spiked with l-[U-14C]proline (final concentration, 0.186 μM) in a 2-ml assay volume. This resulted in a specific activity of l-[U-14C]proline of 42 mCi mmol−1 at a final substrate concentration of 1 μM proline, 1.2 mCi mmol−1 at a final substrate concentration of 40 μM proline, and 0.025 mCi mmmol−1 at a final substrate concentration of 2,000 μM proline in the individual transport assays.
Database searches and alignments of amino acid sequences.
Functional annotations of proteins of B. subtilis (3) were obtained from the SubtiWiki web server (http://subtiwiki.uni-goettingen.de/wiki/index.php/Main_Page) (54). The alignment of amino acid sequences of proteins was performed with ClustalW (55), and the amino acid sequence identities were calculated by using the EMBL-EBI online tool EMBOSS Needle (http://www.ebi.ac.uk/Tools/psa/).
RESULTS
A putP opuE double mutant strain can still use proline as a nutrient.
The disruption of the PutP proline uptake system greatly impairs the growth of a B. subtilis strain with intact PutB and PutC proline catabolic enzymes in a minimal medium (SMM) with proline as the sole carbon and energy source (10). However, use of proline as a nitrogen source is still possible even in a strain with simultaneous defects in the PutP and OpuE proline transporters (10), but use of proline as a carbon source was impaired (Table 2). We then observed, however, that the preculturing of strains lacking either PutP or PutP and OpuE simultaneously in a medium containing both glucose and proline as carbon sources allowed the subsequent use of proline as the sole carbon source (see Fig. S1 in the supplemental material). The physiological mechanism underlying this difference in the use of proline as a nutrient is currently not firmly understood, but it probably reflects the different amounts of proline required to satisfy the cells' need for carbon and nitrogen under conditions where the main catabolic proline import system (PutP) is not functional. This growth pattern is definitely not the result of the accumulation of suppressor strains (53) allowing a bypass of the defect in the PutP transporter, since single colonies obtained from the proline-grown cultures showed the same growth behavior found for the originally inoculated putBC+-[Δ(putP::spc)1] strain (data not shown). In conclusion, the data documented in Table 2 and in Fig. S1 and S3 in the supplemental material demonstrate that, in addition to the so far functionally characterized PutP and OpuE transporters (10, 16), at least one further proline import system must be active in B. subtilis.
TABLE 2.
Use of proline and GABA as nutrients by B. subtilisa
| Strain | Relevant genotypeb |
Growth yield of cultures when the following nutrient was used as: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| C source |
N source |
||||||||
| PutP | OpuE | GabP | Glucose | Proline | GABA | NH4+ | Proline | GABA | |
| JH642 | + | + | + | 3.61 | 3.01 | 0.16 | 3.48 | 3.67 | 3.91 |
| ACB275 | + | − | − | 3.89 | 2.83 | 0.10 | 4.13 | 3.04 | 0.24 |
| ACB277 | − | + | − | 3.79 | 0.28 | 0.12 | 4.04 | 0.65 | 0.35 |
| SMB12 | − | − | + | 3.71 | 0.30 | 0.14 | 3.80 | 3.29 | 3.68 |
| ACB279 | − | − | − | 3.65 | 0.10 | 0.10 | 3.59 | 0.63 | 0.25 |
Cells of the B. subtilis wild-type strain JH642 and its mutant derivatives were grown in SMM with either 28 mM glucose, 33 mM proline, or 41 mM GABA as the carbon source in the presence of 30 mM ammonium as the nitrogen source. When GABA or proline was used as sole nitrogen source, it was present at a concentration of 30 mM. The cells were inoculated to an OD578 of 0.2 from precultures, and the growth yields were determined by measuring the OD578 of the cultures after 16 h of incubation at 37°C. The values given represent the means of two independent sets of experiments.
+, the presence of the wild-type gene; −, its absence.
A candidate approach to identify the third proline transporter of B. subtilis.
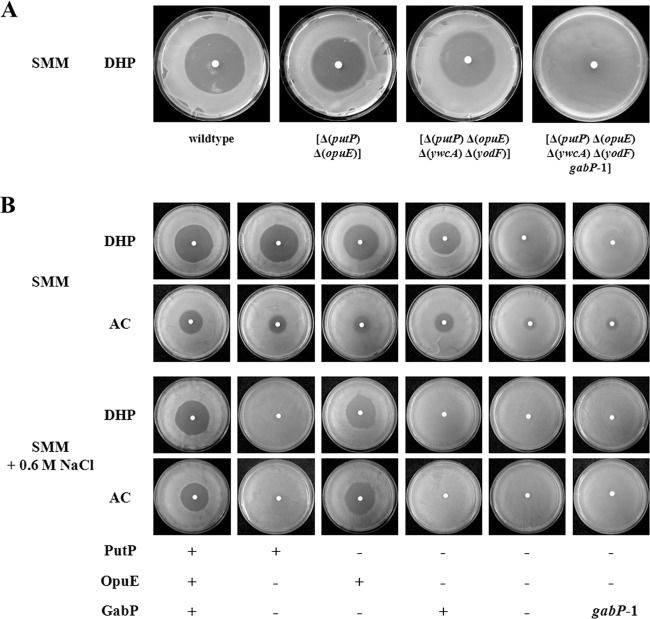
To get a possible hint at the identity of the transport system that mediates proline import in a putP opuE double mutant strain, we used the amino acid sequence of PutP as the query sequence in a BLAST search (56) of the B. subtilis genome (3). This database search identified the YwcA and YodF membrane proteins as PutP-related proteins with overall amino acid sequence identities of 24% and 21%, respectively; their amino acid sequence identities to OpuE are 22% and 22%, respectively. A domain search using the SMART database (57) recognized in the YwcA and YodF proteins a solute:sodium symporter (SSS) domain, indicating that these predicted integral membrane proteins are members of the same transporter family to which the PutP and OpuE transporters also belong (10, 16). We constructed gene disruption mutations in the ywcA and yodF genes and combined the resulting mutant alleles with gene disruption mutations in the putP and opuE genes. The resulting strains, including the putP opuE ywcA yodF quadruple mutant ACB199, all retained sensitivity toward the toxic proline analog DHP (39) (Fig. 1A) and were able to use proline as a nutrient (see Fig. S2 in the supplemental material). We therefore concluded that the YwcA and YodF proteins do not contribute significantly to proline uptake by B. subtilis.
FIG 1.
Sensitivity of the B. subtilis wild-type strain JH642 and its mutant derivatives to the toxic proline analogues AC and DHP. (A) Cells of the B. subtilis wild-type JH642 and its mutants derivatives SMB12 (putP opuE), ACB199 (putP opuE ywcA yodF), and ACB214 (putP opuE ywcA yodF gabP1) were tested for sensitivity to DHP on SMM agar plates. (B) Cells JH642 and its mutants derivatives ACB275 (opuE gabP), ACB277 (putP gabP), SMB12 (putP opuE), ACB279 (putP opuE gabP), and ACB214 (putP opuE ywcA yodF gabP1) were tested for sensitivity to DHP and AC either on SMM agar plates or on SMM agar plates that contained 0.6 M NaCl. The agar plates were incubated at 37°C for 24 h (cells grown on SMM agar plates) or 48 h (cells grown on SMM agar plates containing 0.6 M NaCl) and then recorded by photography.
A genetic approach to identify the third proline transporter of B. subtilis.
A putP opuE double mutant strain is still sensitive to the proline analogs 3,4-dehydro-dl-proline (DHP) and l-azetidine-2-carboxylic acid (AC) (Fig. 1B). These compounds can be taken up by bacteria via different types of proline import systems (36, 40, 41), mischarged onto proline-specific tRNAs, and incorporated, as a substitute for proline, into proteins that are then prone to misfolding (39). The resistance of bacteria to AC and DHP can be developed either through the overproduction of proline (58, 59), which dilutes the concentration of these toxic compounds within the overall cellular proline pool, or through the mutational inactivation of proline uptake systems (36, 40, 41), which prevents their uptake into the cell.
Mutant derivatives of the putP opuE strain SMB12 that are resistant to DHP were readily detectable in the growth-inhibition zone that developed around a filter disk soaked with this toxic proline analog. DHP-resistant mutants appeared at frequencies of about 6 × 10−6 on SMM agar plates that contained 50 μg ml−1 DHP. We isolated 15 independent spontaneously DHP-resistant mutants from the growth-inhibition zone around a DHP-soaked filter disk (10 μl of a 25-mg ml−1 solution was applied to the disk) with the aim of isolating mutant strains that were defective in proline uptake. Upon retesting, each of these mutants (e.g., ACB214) proved to be resistant to DHP at the concentration originally applied in the filter disk assay (Fig. 1A and B), and none of them overproduced proline (data not shown; see Materials and Methods for details). Hence, each of the 15 picked DHP-resistant strains exhibited the properties expected for a mutant with a defect in a proline uptake system. Indeed, when we tested these strains for the use of proline as either the sole nitrogen or carbon source, each of them was no longer able to use proline as a nutrient (see Fig. S2 and S3 in the supplemental material).
Genome resequencing provides a lead on the identity of the third proline transporter of B. subtilis.
To get a lead on the type of mutation(s) underlying the DHP resistance phenotype and the defect in proline utilization, we subjected one of the isolated mutants (strain ACB214) and its DHP-sensitive parent (strain ACB199) (Table 1) to genome resequencing using an Illumina sequencing protocol. This resulted in 9.2 million and 8.3 million reads (about 110 bp each) for strains ACB214 and ACB199, respectively, with a minimum genome coverage of 25 reads per nucleotide.
Two mutations were found in the genome sequence of DHP-resistant strain ACB214 in comparison to that of parent strain ACB199, which is a derivative of JH642 (52), a member of the domesticated 168 lineage of B. subtilis laboratory strains (3): (i) a consecutive 2-bp insertion (G·C-G·C) within codon 465 of cheA, the structural gene for the CheA signal transduction histidine kinase, a key component of the B. subtilis chemotactic system (60), and (ii) a single-base-pair insertion (a A·T base pair) within codon 387 of the gabP gene (gabP1) encoding the uptake system (GabP) for the nonproteinogenic amino acid γ-aminobutyrate (GABA) that can be used by B. subtilis as the sole nitrogen source but not as the sole carbon source (42, 43) (Table 2). Hence, the mutations found by genome resequencing of DHP-resistant strain ACB214 are predicted to cause the production of substantially truncated versions of CheA (467 amino acids) and GabP (423 amino acids), proteins that normally comprise 671 and 469 amino acids, respectively.
The occurrence of the mutation in cheA can be understood when one considers that B. subtilis displays chemotaxis not only toward l-proline but also toward toxic proline analogs (61). Inactivation of the CheA kinase would thus benefit B. subtilis cells by abrogating swimming toward higher concentrations of a poisonous compound (DHP) placed in the middle of the agar plate on a filter disk (Fig. 1A). However, the mutation in cheA does not provide a rational explanation for the inability of strain ACB214 to use proline as a nutrient (see Fig. S2 and S3 in the supplemental material). In this respect, the mutation in the gabP gene provided a very useful lead since GabP is a functionally characterized permease (42, 43); it is a member of the amino acid-polyamine-organocation (APC) superfamily of transporters (TC accession number 2.A.3) (62). Indeed, the presence of the gabP1 allele in a putP opuE strain background not only conferred DHP resistance (Fig. 1B) and abrogated utilization of proline as the sole nitrogen and carbon source (see Fig. S2 and S3 in the supplemental material) but also caused a strong defect in the use of GABA as the sole nitrogen source by B. subtilis (see Fig. S2 in the supplemental material).
If the loss of the GabP-catalyzed transporter activity was indeed causative for the development of the DHP-resistant phenotype and the concomitant inability of such strains to use proline as a nutrient in a putP opuE genetic background, then most, if not all, of the originally isolated DHP-resistant strains should carry mutations in gabP. To test this hypothesis, we sequenced the entire gabP gene from all of the remaining 14 independently isolated DHP-resistant mutant strains and found that in each of them the gabP gene had suffered a mutation (Table 3). In Fig. 2, we have projected the positions of these mutations onto a simple topological model for the GabP transporter. This model invokes the presence of 12 transmembrane-spanning regions and suggests that the N and C termini of the GabP protein face the cytoplasm (63), a topological arrangement consistently observed in several crystal structures of members of the APC superfamily (64–66). Two types of lesions were present in the gabP gene of the various DHP-resistant strains: (i) stop codons (five isolates) and frameshifts (three isolates) that are predicted to generate truncated versions of the GabP permease (Table 3) and (ii) single amino acid substitutions (seven isolates) (Table 3) that might negatively affect the transporter activity of GabP as well. Indeed, regardless of the type of mutation present in gabP, each of the 15 DHP-resistant strains tested had lost the ability to efficiently use GABA as the sole nitrogen source (see Fig. S2 in the supplemental material).
FIG 2.
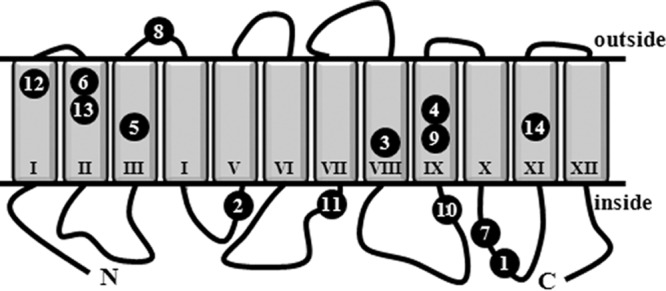
Model for the topological organization of the GabP transporter and position of mutations causing DHP resistance. The predicted 12 transmembrane domains of the GabP transporter with its amino-terminal (N) and carboxy-terminal (C) ends facing the cytoplasm are shown. The indicated numbers refer to the gabP alleles whose properties are summarized in Table 3. These gabP mutants lead to either truncated variants of the GabP permease (alleles 1, 2, 5, 7, 8, 10, 11, and 13) or single amino acid substitutions in the GabP permease (alleles 3, 4, 6, 9, 12, and 14).
With respect to the types of mutations recovered in gabP, those that lead to single amino acid substitutions in the GabP permease are the most interesting to consider further. In each of these seven GabP variants (the gabP4 allele [F341S] was isolated twice; Table 3), the mutationally altered residues are predicted to be part of the membrane-embedded core of the GabP transporter (Fig. 2). Strikingly, five of the six affected amino acids were glycine residues (G33, G42, G301, G338, G414); glycine is a type of residue that has a structural role in membrane-spanning helices, mediates helix-helix interactions in membrane proteins, and contributes to the formation of monomer-monomer interfaces (67, 68).
Targeted disruption of gabP confers DHP resistance, abolishes GABA utilization, and prevents use of proline as a nitrogen source in a putP opuE genetic background.
The data presented above provided compelling evidence for a link between the loss of the GabP transporter function and the inability of the cells to use proline as a nutrient in a putP opuE genetic background (see Fig. S2 and S3 in the supplemental material). However, it seemed remotely possible that these two phenotypes were not causally linked. To avoid any possible bias that might be associated with the originally applied selection scheme for DHP resistance, we constructed a chromosomal gabP gene disruption mutation in which an antibiotic (zeocin) resistance cassette was used to replace the entire coding region of gabP. We then introduced the Δ(gabP::zeo)1 allele in various combinations into B. subtilis strains harboring putP and/or opuE gene disruption mutations and tested the resulting comprehensive set of mutant strains for their resistance to DHP and AC (Fig. 1B) and their ability to use either proline or GABA as nutrients (Table 2).
Proline utilization occurred as long as one of the PutP, OpuE, and GabP transporters was functional; it was reduced to background levels only when all three transporters were nonoperational (Table 2). This data set thus unambiguously identifies the GabP transporter as the third proline uptake system operating in B. subtilis. Loss of the ability to use proline as a nutrient required the simultaneous inactivation of the PutP, OpuE, and GabP transporters, but use of GABA as the sole nitrogen source was abolished only when the GabP transporter was nonfunctional. The simultaneous loss of the PutP or OpuE transporters did not negatively affect GABA utilization (Table 2). This also firmly excluded the possibility that the cheA frameshift mutation present in the strain (ACB214) whose genome we initially resequenced was required in conferring resistance to toxic proline analogs (Fig. 1A and B) and causing a loss of proline utilization (see Fig. S2 in the supplemental material).
As noted previously (10), the sensitivity of B. subtilis strains to DHP and AC is influenced by the salinity of the growth medium and by which proline transport system is operational. Our isogenic set of strains expressing only one of the PutP, OpuE, and GabP proline transporters or lacking all of them allowed us to assess the individual contribution of these transporters to DHP and AC sensitivity. For these assays, we used SMM agar plates without and with additional NaCl (0.6 M). As shown in Fig. 1B, in a strain possessing only PutP, sensitivity to DHP and AC was abolished when the salinity of the growth medium was increased, whereas in a strain possessing only OpuE, not only was its sensitivity to AC maintained, but its sensitivity was actually increased. The pattern of sensitivity/resistance to DHP and AC of a strain possessing only GabP mimicked that of a strain expressing only PutP (Fig. 1B). There is a good correlation between the pattern of [14C]proline uptake in cells (cultivated in the absence or presence of increased salinity) and the extent of growth inhibition exerted by DHP and AC on agar plates lacking or containing additional amounts of NaCl (10). Based on the data shown in Fig. 1B, we surmise that the efficiency of GabP-mediated proline uptake is negatively affected in high-salinity growth medium (see below).
Kinetic parameters of proline transport via GabP relative to those of GABA transport.
The level of gabP transcription is responsive to the nitrogen source available in the growth medium, and it is subjected to genetic control by the key nitrogen regulatory protein of B. subtilis, TnrA (42, 69), and the globally acting CodY protein (42, 70). As a consequence, GabP-mediated GABA uptake is strongly stimulated when B. subtilis cells are propagated with a growth-limiting nitrogen source such as glutamate (42). We monitored the uptake of radiolabeled GABA under conditions that maximize gabP expression in cells of the wild-type strain JH642 and of a strain (SMB12) that was proficient in the GabP permease but lacked the PutP and OpuE transporters. For these experiments, the cells were grown in either SMM or SMM containing 0.6 M NaCl; both media contained glutamate as the sole nitrogen source. In the uptake assays, we used radiolabeled GABA at a final substrate concentration of 200 μM (Fig. 3A). Both strains showed the same GABA uptake rate [about 132 nmol GABA (min mg protein)−1]. This uptake rate was reduced 2.6- to 3-fold when the strains were cultivated in SMM with increased osmolarity (Fig. 3A), indicating that the activity of the GabP permease is impaired by high salinity. This property nicely explains the reduced sensitivity of a GabP+ PutP− OpuE− strain to the toxic proline analogs DHP and AC on high-salinity agar plates (Fig. 1B). The γ-[2,3-3H]aminobutyrate import activity in the GabP-deficient strain ACB279 was reduced to background levels when a substrate concentration of 200 μM was used in the uptake assay, but minor uptake activity was noticeable when the substrate concentration was increased to 700 μM (Fig. 3A). The latter finding is consistent with previous data showing the dominant role of GabP in GABA uptake and the existence of an additional, but very minor, uptake route for this compound in B. subtilis (42, 71).
FIG 3.
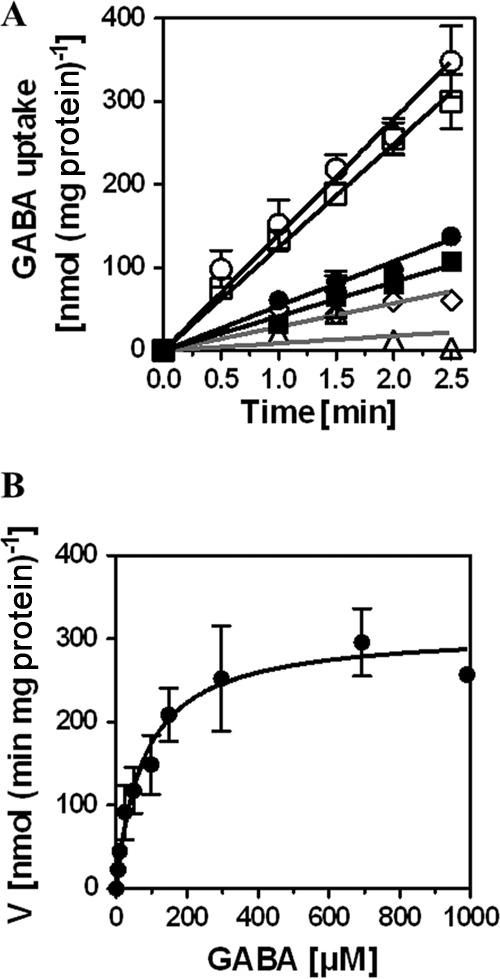
Uptake of radiolabeled GABA by B. subtilis and kinetic parameters for its GabP-mediated import. Cells were grown in SMM with glutamate as the sole nitrogen source. After the cultures reached an OD578 of 0.4, 2-ml aliquots were withdrawn to determine the uptake of γ-[2,3-3H]aminobutyrate. (A) B. subtilis wild-type JH642 (circles) and its derivative, SMB12 (GabP+ OpuE− PutP−) (squares), were grown in minimal medium either in the absence (open symbols) or in the presence (closed symbols) of 0.6 M NaCl. GABA uptake was measured at a final concentration of 200 μM. Uptake of radiolabeled GABA in the triple mutant strain ACB279 (GabP− OpuE− PutP−) was determined at final substrate concentrations of either 200 μM (triangles) or 700 μM (diamonds). Each transport assay was performed in at least two biological replicates. (B) Michaelis-Menten kinetics of GABA uptake in the GabP+ strain SMB12. Shown are the means and standard deviations of three biological replicates.
Since the details of the kinetic parameters of the GabP permease in its authentic host strain, B. subtilis, have not been reported, we determined them in cells of strain SMB12 (GabP+ PutP− OpuE−) that were grown in a minimal medium (SMM) with glucose as the carbon source and glutamate as the sole nitrogen source. γ-[2,3-3H]aminobutyrate uptake exhibited Michaelis-Menten kinetics (Fig. 3B) and a Km of 77 ± 17 μM; from the kinetic data, a Vmax of 310 ± 21 nmol (min mg protein)−1 was calculated. Hence, GabP is a transport system with a good affinity for GABA, and it exhibits a very substantial transport capacity for this nonproteinogenic amino acid. This conclusion is in full agreement with previously reported data on the stimulation of the uptake rates for GABA by B. subtilis cells grown in the presence of a poor nitrogen source and transport measurements with recombinant E. coli strains expressing the cloned B. subtilis gabP gene under the control of the lac promoter (42, 43).
To assess the ability of the GabP permease to mediate proline import, we determined under the same growth conditions that we used to measure GABA uptake the kinetic parameters for l-[14C]proline transport. For these experiments we used a strain (JH642) in which the three proline transporters PutP, OpuE, and GabP were intact and then compared its proline uptake activity with that of a strain in which only the GabP permease was functional (strain SMB12). Proline import into strain JH642 occurred with a Km of 6.8 ± 0.7 μM and a Vmax of 118 ± 4 nmol (min mg protein)−1 (Fig. 4A), demonstrating that a B. subtilis wild-type strain can readily acquire proline from scarce environmental resources, such as root exudates and plant-mediated deposits in the rhizosphere (2, 25). When proline import was measured at a final substrate concentration of 40 μM, a substrate concentration at which no proline uptake activity was previously detectable in a putP opuE double mutant strain (SMB12) grown in SMM with glucose as the carbon source and ammonium as the nitrogen source (10), l-[14C]proline import was reduced about 3-fold in strain SMB12 in comparison with that in strain JH642 (Fig. 4B). We then measured the GabP-mediated import of proline over a range of substrate concentrations, and from these data (Fig. 4C) a Km of 700 ± 330 μM was estimated. We measured the rates of l-[14C]proline uptake between 40 μM and 2 mM, but under these assay conditions, the uptake kinetics did not reach saturation. Hence, it is safe to say that GabP possesses only a rather modest affinity for proline with a Km value of about 1 mM. In comparison, the Km values of the PutP and OpuE transporters are 8 ± 2 μM and 12 ± 1 μM, respectively, in B. subtilis cells cultivated with glucose as the carbon source and ammonium as the nitrogen source in the absence of salt stress (10).
FIG 4.
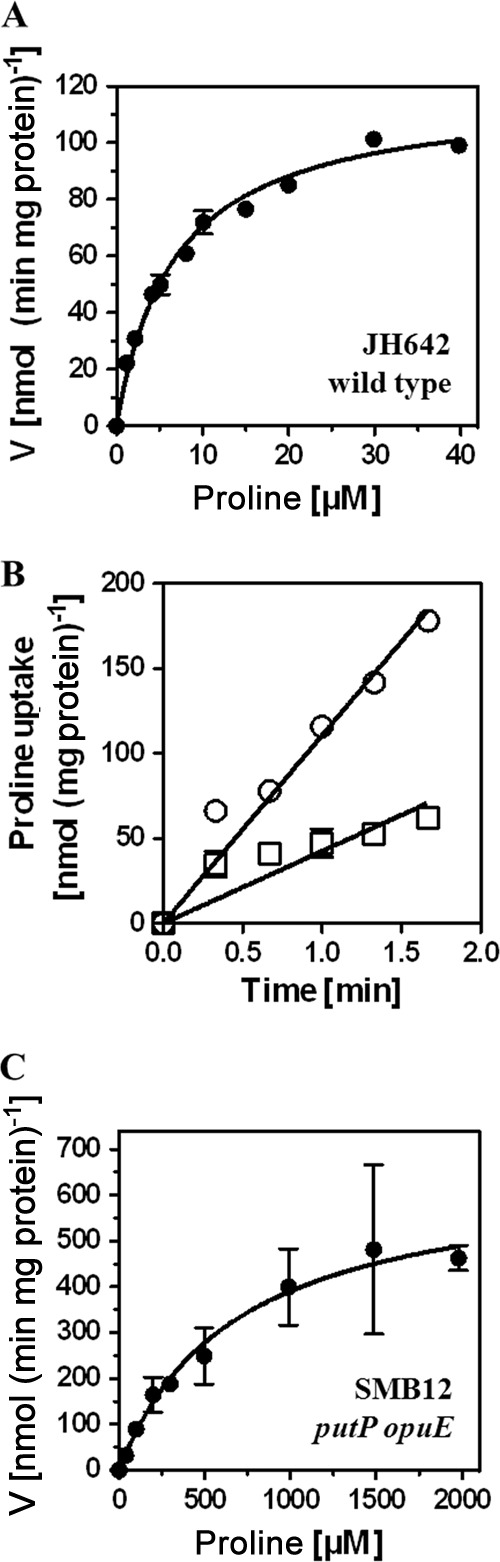
Uptake of radiolabeled proline by B. subtilis and kinetic parameters for its GabP-mediated import. Uptake of l-[U-14C]proline was determined in 2-ml aliquots of cultures that were grown in SMM to the early exponential growth phase (OD578, 0.3). (A) Michaelis-Menten kinetics of proline uptake in cultures of the wild-type strain JH642. Shown are the means and standard deviations of three biological replicates. (B) Proline uptake rates in B. subtilis wild-type JH642 (circles) and its derivative SMB12 (GabP+ OpuE− PutP−) (squares) were measured at a final proline concentration of 40 μM. Each transport assay was performed in three biological replicates. (C) Michaelis-Menten kinetics of proline uptake in cultures of the GabP+ strain SMB12 (putP opuE). Shown are the means and standard deviations of three biological replicates.
Proline can still enter the cells of a mutant strain lacking the PutP, OpuE, and GabP transporters.
We observed that a strain that did not possess the PutP, OpuE, and GabP transporters was still not completely resistant to DHP and AC. A diffuse zone of growth impairment was visible around the filter disk impregnated with DHP, and a small but distinct growth inhibition zone was noticeable around the AC-containing filter disk (Fig. 1B). Hence, it seemed possible that there is another proline uptake route present in B. subtilis beyond that afforded by the PutP, OpuE, and GabP transport systems. The weak but noticeable growth of such triple mutant strains with proline as a nutrient (Table 2; see Fig. S2 in the supplemental material) fostered this suspicion.
Enhanced transcription of putBCP expression can be triggered by very low concentrations (e.g., 25 μM) of proline in a growth medium that contains glucose as the carbon source and ammonium as the nitrogen source (10). A single-copy putB-treA operon fusion is thus a very sensitive tool to assess whether proline can enter a given B. subtilis strain. We introduced such a fusion into a set of strains that expressed only one of the PutP, OpuE, and GabP transporters and then monitored the expression of the putB-treA reporter in response to the presence of both a low (25 μM) and a high (5 mM) concentration of proline in the growth medium. We conducted these experiments with cells that were grown either in SMM or in SMM that contained 250 mM NaCl, a salt concentration that already allows good induction of opuE expression (23) but that does not strongly impair proline uptake via PutP (10) or GabP (see above). As summarized in Table 4, putB-treA gene expression was inducible by proline, regardless of which of the so far functionally characterized proline transport systems was operational. This was true even in a putP opuE gabP triple mutant strain (Table 4), demonstrating that at least one additional proline uptake system must be operational in B. subtilis. However, this transport system(s) allowed proline uptake only in quantities that were insufficient to sustain efficient growth (Table 2; see Fig. S2 and S3 in the supplemental material), and its affinity must be low, since the induction of putB-treA transcription no longer occurred in response to proline at a concentration of 25 μM in a putP opuE gabP triple mutant strain, but induction occurred at higher substrate concentrations (5 mM) (Table 4).
TABLE 4.
Induction of putB-treA expression in response to externally provided proline imported via different proline transport systemsa
| Strain | Relevant genotypeb |
TreA activity (U mg protein−1) in response to the indicated proline concn |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 M NaCl |
0.25 M NaCl |
||||||||
| PutP | OpuE | GabP | 0 M | 25 μM | 5 mM | 0 M | 25 μM | 5 mM | |
| SMB10 | + | + | + | 19 ± 1 | 50 ± 2 | 168 ± 22 | 20 ± 2 | 52 ± 4 | 290 ± 13 |
| ACB276 | + | − | − | 15 ± 2 | 47 ± 5 | 141 ± 3 | 19 ± 2 | 45 ± 3 | 154 ± 5 |
| ACB278 | − | + | − | 19 ± 4 | 41 ± 2 | 90 ± 4 | 19 ± 3 | 45 ± 5 | 212 ± 10 |
| ACB288 | − | − | + | 20 ± 5 | 28 ± 6 | 140 ± 5 | 19 ± 3 | 30 ± 4 | 157 ± 18 |
| ACB292 | − | − | − | 20 ± 1 | 20 ± 3 | 87 ± 3 | 22 ± 2 | 28 ± 1 | 101 ± 4 |
All strains carry the ϕ(putB-treA) reporter gene fusion integrated in the amyE site on the B. subtilis chromosome. Cultures were grown to early exponential growth phase (OD578, about 0.5), and proline was then added to the growth medium at a final concentration of either 25 μM or 5 mM. The cells were then propagated for another 60 min and subsequently harvested for TreA reporter enzyme assay. The values for the TreA activity given represent those for two independently grown cultures, and for each sample analyzed, the TreA activity was determined twice.
+, the presence of the wild-type gene; −, its absence.
DISCUSSION
Although the PutP and OpuE proline transporters are closely related to each other with respect to both their amino acid sequences (10, 16) and the mechanism by which they import their common substrate in cotransport with sodium ions (31, 33, 34, 37), they have clearly different cellular functions in B. subtilis. Their primary physiological roles can be discerned from the distinct transcriptional profile of their structural genes (10, 16, 23, 28, 29) and the different abilities of the PutP and OpuE proteins to withstand the inhibiting effects of high salinity on their transport activity (10). These features associate PutP with the proline catabolic system of B. subtilis (10, 28, 29) and OpuE with the cell's osmostress response systems (16, 22, 72, 73). Nevertheless, it is worth noting that our data now show not only that the OpuE transporter imports proline when it is used as an osmoprotectant (15, 16) but also that it can do so when B. subtilis uses it as a nutrient (see Fig. S1 in the supplemental material).
Here we now identify the third proline transporter operating in B. subtilis, the GabP permease. GabP has previously been physiologically and biochemically characterized as an uptake system for GABA (42, 43), a nonproteinogenic amino acid that can be found in the rhizosphere (2) primarily due to the biosynthetic activities of plants (74). The soil bacterium B. subtilis can catabolize GABA to succinate and use it as the sole nitrogen source (42, 71). We have assessed the kinetic parameters of the GabP-mediated uptake of GABA and proline under growth conditions that should maximize gabP expression (42), and these data characterize GabP as a high-affinity uptake system for GABA (Km, 77 ± 17 μM) that possesses a very substantial transport capacity [Vmax, 310 ± 21 nmol (min mg protein)−1] for this substrate (Fig. 3B). In contrast, its affinity for proline is rather low, with an estimated Km of close to 1 mM (Fig. 4C). Despite this limited affinity, enough proline can nevertheless be imported via GabP to serve as the sole nitrogen, energy, and carbon source for B. subtilis (Table 2; see Fig. S2 and S3 in the supplemental material).
The B. subtilis GabP transporter not only can import GABA (42) and proline (this study) but also can serve as an effective uptake system for β-alanine. The Km value exhibited by GabP for β-alanine (Km = 9.6 ± 5.3 μM) even exceeded that for GABA (Km = 37 ± 13.2 μM) in a recombinant E. coli strain expressing the B. subtilis gabP gene under the control of the lac promoter (43). Hence, the ligand-binding site of the GabP permease can accommodate both linear molecules (GABA and β-alanine) and also, with considerably reduced efficiency, the cyclic structure of proline. Detailed insights into the molecular determinants for the high-affinity binding of both GABA and proline by the same transporter protein were recently provided through the crystallographic analysis of a periplasmic ligand-binding protein that operates in conjunction with an ABC transport system in the plant-associated bacterium Agrobacterium tumefaciens (75).
In contrast to PutP and OpuE, which are both members of the solute:sodium symporter family (SSS; TC accession number 2.A.21) (30), GabP belongs to the amino acid-polyamine-organocation (APC) superfamily (TC accession number 2.A.3) (62). Its amino acid sequence is only distantly related to the amino acid sequences of the PutP and OpuE proteins, with sequence identities of 19% and 17%, respectively. APC-type transporters catalyze the uniport, antiport, and symport of a broad range of substrates across cell membranes (64–66). GabP is a substrate:H+-symporter (TC accession number 2.A.3.1.5), and it is categorized as a member of the amino acid transporter subfamily of the APC superfamily (30). Interestingly, this subfamily also comprises ProY, a proline transporter of S. Typhimurium, whose structural gene is normally silent in laboratory strains (76). Notable is also that one of the three GABA transporters operating in Saccharomyces cerevisiae belongs to the same transporter family and functions as a permease (PUT4) that possesses similar affinities for both GABA and proline (77). Furthermore, members of the plant AtProT family of compatible uptake systems can import not only glycine betaine but also both proline and GABA (78). Hence, uptake systems that can import both GABA and proline are not uncommon.
In addition to defining GabP as the third proline transporter of B. subtilis, our data also reveal the existence of yet another proline uptake route in this soil bacterium. Efficient use of proline as a nutrient was abrogated in strains with simultaneous defects in the PutP, OpuE, and GabP permeases (Table 2; see Fig. S2 and S3 in the supplemental material), indicating that this fourth proline import route is of limited physiological relevance as far as the use of proline as a nutrient (10) and as an osmoprotectant (15, 16) is concerned. Nevertheless, enough proline can enter the B. subtilis cell via this route to induce enhanced expression of a proline-responsive (10, 28, 29) put-treA reporter fusion (Table 4).
The gabP, putP, and opuE proline transporter genes each possess distinct regulatory profiles, but they also share common genetic determinants. Transcription of opuE is strongly induced in response to both sudden and sustained increases in the external osmolarity, a process that is mediated by SigA- and SigB-type promoters (16, 23, 24). Data derived from transcriptional profiling experiments suggest that it is also under the positive control of the central carbon metabolism regulatory protein CcpA (79). Transcription of the putBCP operon is activated by the proline-responsive PutR regulator (10, 28, 29), whereas that of the gabP gene is enhanced through the stimulating activity of TnrA (42, 69), the central regulator of nitrogen metabolism in B. subtilis (12, 26, 27). Notably, transcription of gabP is not under the control of GabR, a regulatory protein that controls the expression of the gabTD GABA catabolic operon (71, 80).
A common determinant for the transcriptional control of the putP, opuE, and gabP genes is their regulation via the globally acting CodY protein, whose DNA-binding activity is sensitively modulated through the cellular pools of branched-chain amino acids and through GTP (70, 81, 82). CodY acts as a repressor for the promoter directing transcription of the putBCP operon (28), and it also acts as a negative regulator both for the gabP transporter gene (42) and for the GABA catabolic gabTD operon (70). CodY also binds to the opuE regulatory region (70), but its mode of action for this gene still needs to be studied. One of the cues that CodY avidly responds to is the availability of amino acids in the growth medium (81, 83). Hence, the common CodY-mediated transcriptional control of putBCP, opuE, and gabP allows B. subtilis to integrate its three major proline importers into its overarching nutrient utilization systems (26), while maintaining situation-specific control over their expression through regulatory circuits tailored to the individual genes and gene clusters.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jutta Gade for expert technical assistance and Jeanette Brill and Margot Brosius (University of Marburg, Marburg, Germany) for providing bacterial strains. We are very grateful to Andrea Thürmer and Rolf Daniel from the Göttingen Genomics Laboratory for performing genome resequencing of B. subtilis strain. The kind help of Vickie Koogle in the language editing of our manuscript is very much appreciated, as are our stimulating discussions with Jörg Stülke (University of Göttingen, Göttingen, Germany), Boris Belitsky (Tufts University, Boston, MA), and Lutz Schmitt (University of Düsseldorf, Düsseldorf, Germany). E.B. greatly valued the hospitality and kind support of Tom Silhavy during a sabbatical at the Department of Molecular Biology of Princeton University (Princeton, NJ).
Financial support for this study was provided by grants from the BMBF via the Bacell-SysMo2 consortium (to E.B.), the Deutsche Forschungsgemeinschaft (DFG grant CO 1139/1-1 to F.M.C.), and the Fonds der Chemischen Industrie (to F.M.C. and E.B.). A.Z. was an associate member of the International Max Planck Graduate School for Environmental, Cellular and Molecular Microbiology (IMPRS-Mic; Marburg, Germany), and she gratefully acknowledges its financial support.
Footnotes
Published ahead of print 18 October 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01128-13.
REFERENCES
- 1.Earl AM, Losick R, Kolter R. 2008. Ecology and genomics of Bacillus subtilis. Trends Microbiol. 16:269–275. 10.1016/j.tim.2008.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moe LA. 2013. Amino acids in the rhizosphere: from plants to microbes. Am. J. Bot. 100:1692–1705. 10.3732/ajb.1300033 [DOI] [PubMed] [Google Scholar]
- 3.Barbe V, Cruveiller S, Kunst F, Lenoble P, Meurice G, Sekowska A, Vallenet D, Wang T, Moszer I, Medigue C, Danchin A. 2009. From a consortium sequence to a unified sequence: the Bacillus subtilis 168 reference genome a decade later. Microbiology 155:1758–1775. 10.1099/mic.0.027839-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belda E, Sekowska A, Le Fevre F, Morgat A, Mornico D, Ouzounis C, Vallenet D, Medigue C, Danchin A. 2013. An updated metabolic view of the Bacillus subtilis 168 genome. Microbiology 159:757–770. 10.1099/mic.0.064691-0 [DOI] [PubMed] [Google Scholar]
- 5.Beauregard PB, Chai Y, Vlamakis H, Losick R, Kolter R. 2013. Bacillus subtilis biofilm induction by plant polysaccharides. Proc. Natl. Acad. Sci. U. S. A. 110:E1621–E1630. 10.1073/pnas.1218984110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saier MH, Jr, Goldman SR, Maile RR, Moreno D, Weyler MSW, Yang N, Pauslsen IT. 2002. Overall transport capabilities of Bacillus subtilis, p 113–128 In Sonenshein AL, Hoch JA, Losick R. (ed), Bacillus subtilis and its closes relatives: from genes to cells. ASM Press, Washington, DC [Google Scholar]
- 7.Boch J, Kempf B, Bremer E. 1994. Osmoregulation in Bacillus subtilis: synthesis of the osmoprotectant glycine betaine from exogenously provided choline. J. Bacteriol. 176:5364–5371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann T, Bremer E. 2011. Protection of Bacillus subtilis against cold stress via compatible-solute acquisition. J. Bacteriol. 193:1552–1562. 10.1128/JB.01319-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holtmann G, Bremer E. 2004. Thermoprotection of Bacillus subtilis by exogenously provided glycine betaine and structurally related compatible solutes: involvement of Opu transporters. J. Bacteriol. 186:1683–1693. 10.1128/JB.186.6.1683-1693.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moses S, Sinner T, Zaprasis A, Stöveken N, Hoffmann T, Belitsky BR, Sonenshein AL, Bremer E. 2012. Proline utilization by Bacillus subtilis: uptake and catabolism. J. Bacteriol. 194:745–758. 10.1128/JB.06380-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atkinson MR, Wray LV, Jr, Fisher SH. 1990. Regulation of histidine and proline degradation enzymes by amino acid availability in Bacillus subtilis. J. Bacteriol. 172:4758–4765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher SH. 1993. Utilization of amino acids and other nitrogen-containing compounds, p 221–235 In Sonenshein AL, Hoch JA, Losick R. (ed), Bacillus subtilis and other Gram-positive bacteria. ASM Press, Washington, DC [Google Scholar]
- 13.Whatmore AM, Chudek JA, Reed RH. 1990. The effects of osmotic upshock on the intracellular solute pools of Bacillus subtilis. J. Gen. Microbiol. 136:2527–2535. 10.1099/00221287-136-12-2527 [DOI] [PubMed] [Google Scholar]
- 14.Brill J, Hoffmann T, Bleisteiner M, Bremer E. 2011. Osmotically controlled synthesis of the compatible solute proline is critical for cellular defense of Bacillus subtilis against high osmolarity. J. Bacteriol. 193:5335–5346. 10.1128/JB.05490-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaprasis A, Brill J, Thüring M, Wünsche G, Heun M, Barzantny H, Hoffmann T, Bremer E. 2013. Osmoprotection of Bacillus subtilis through import and proteolysis of proline-containing peptides. Appl. Environ. Microbiol. 79:576–587. 10.1128/AEM.01934-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Blohn C, Kempf B, Kappes RM, Bremer E. 1997. Osmostress response in Bacillus subtilis: characterization of a proline uptake system (OpuE) regulated by high osmolarity and the alternative transcription factor sigma B. Mol. Microbiol. 25:175–187. 10.1046/j.1365-2958.1997.4441809.x [DOI] [PubMed] [Google Scholar]
- 17.Belitsky BR, Brill J, Bremer E, Sonenshein AL. 2001. Multiple genes for the last step of proline biosynthesis in Bacillus subtilis. J. Bacteriol. 183:4389–4392. 10.1128/JB.183.14.4389-4392.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brill J, Hoffmann T, Putzer H, Bremer E. 2011. T-box-mediated control of the anabolic proline biosynthetic genes of Bacillus subtilis. Microbiology 157:977–987. 10.1099/mic.0.047357-0 [DOI] [PubMed] [Google Scholar]
- 19.Csonka LN, Leisinger T. 8 August 2007. Chapter 34.6.1.4, Biosynthesis of proline. In Böck A, Curtis R, III, Kaper JB, Karp PD, Neidhardt FC, Nyström T, Slauch JM, Squires CL, Ussery D. (ed), EcoSal—Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC. 10.1128/ecosalplus.3.6.1.4 [DOI] [Google Scholar]
- 20.Kempf B, Bremer E. 1998. Uptake and synthesis of compatible solutes as microbial stress responses to high osmolality environments. Arch. Microbiol. 170:319–330. 10.1007/s002030050649 [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann T, Wensing A, Brosius M, Steil L, Völker U, Bremer E. 2013. Osmotic control of opuA expression in Bacillus subtilis and its modulation in response to intracellular glycine betaine and proline pools. J. Bacteriol. 195:510–522. 10.1128/JB.01505-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann T, von Blohn C, Stanek A, Moses S, Barzantny S, Bremer E. 2012. Synthesis, release, and recapture of the compatible solute proline by osmotically stressed Bacillus subtilis cells. Appl. Environ. Microbiol. 78:5753–5762. 10.1128/AEM.01040-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiegelhalter F, Bremer E. 1998. Osmoregulation of the opuE proline transport gene from Bacillus subtilis: contributions of the sigma A- and sigma B-dependent stress-responsive promoters. Mol. Microbiol. 29:285–296. 10.1046/j.1365-2958.1998.00929.x [DOI] [PubMed] [Google Scholar]
- 24.Young JW, Locke JC, Elowitz MB. 2013. Rate of environmental change determines stress response specificity. Proc. Natl. Acad. Sci. U. S. A. 110:4140–4145. 10.1073/pnas.1213060110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vilchez S, Molina L, Ramos C, Ramos JL. 2000. Proline catabolism by Pseudomonas putida: cloning, characterization, and expression of the put genes in the presence of root exudates. J. Bacteriol. 182:91–99. 10.1128/JB.182.1.91-99.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonenshein AL. 2007. Control of key metabolic intersections in Bacillus subtilis. Nat. Rev. Microbiol. 5:917–927. 10.1038/nrmicro1772 [DOI] [PubMed] [Google Scholar]
- 27.Gunka K, Commichau FM. 2012. Control of glutamate homeostasis in Bacillus subtilis: a complex interplay between ammonium assimilation, glutamate biosynthesis and degradation. Mol. Microbiol. 85:213–224. 10.1111/j.1365-2958.2012.08105.x [DOI] [PubMed] [Google Scholar]
- 28.Belitsky BR. 2011. Indirect repression by Bacillus subtilis CodY via displacement of the activator of the proline utilization operon. J. Mol. Biol. 413:321–336. 10.1016/j.jmb.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang SC, Lin TH, Shaw GC. 2011. PrcR, a PucR-type transcriptional activator, is essential for proline utilization and mediates proline-responsive expression of the proline utilization operon putBCP in Bacillus subtilis. Microbiology 157:3370–3377. 10.1099/mic.0.054197-0 [DOI] [PubMed] [Google Scholar]
- 30.Saier MH, Jr, Yen MR, Noto K, Tamang DG, Elkan C. 2009. The transporter classification database: recent advances. Nucleic Acids Res. 37:D274–D278. 10.1093/nar/gkn862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung H, Hilger D, Raba M. 2012. The Na+/l-proline transporter PutP. Front. Biosci. 17:745–759. 10.2741/3955 [DOI] [PubMed] [Google Scholar]
- 32.Raba M, Baumgartner T, Hilger D, Klempahn K, Hartel T, Jung K, Jung H. 2008. Function of transmembrane domain IX in the Na+/proline transporter PutP. J. Mol. Biol. 382:884–893. 10.1016/j.jmb.2008.07.070 [DOI] [PubMed] [Google Scholar]
- 33.Mazier S, Quick M, Shi L. 2011. Conserved tyrosine in the first transmembrane segment of solute:sodium symporters is involved in Na+-coupled substrate co-transport. J. Biol. Chem. 286:29347–29355. 10.1074/jbc.M111.263327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olkhova E, Raba M, Bracher S, Hilger D, Jung H. 2011. Homology model of the Na+/proline transporter PutP of Escherichia coli and its functional implications. J. Mol. Biol. 406:59–74. 10.1016/j.jmb.2010.11.045 [DOI] [PubMed] [Google Scholar]
- 35.Wood JM. 1988. Proline porters effect the utilization of proline as nutrient or osmoprotectant for bacteria. J. Membr. Biol. 106:183–202. 10.1007/BF01872157 [DOI] [PubMed] [Google Scholar]
- 36.Csonka LN. 1982. A third l-proline permease in Salmonella typhimurium which functions in media of elevated osmotic strength. J. Bacteriol. 151:1433–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cairney J, Higgins CF, Booth IR. 1984. Proline uptake through the major transport system of Salmonella typhimurium is coupled to sodium ions. J. Bacteriol. 160:22–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hahne H, Mäder U, Otto A, Bonn F, Steil L, Bremer E, Hecker M, Becher D. 2010. A comprehensive proteomics and transcriptomics analysis of Bacillus subtilis salt stress adaptation. J. Bacteriol. 192:870–882. 10.1128/JB.01106-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bach TM, Takagi H. 2013. Properties, metabolisms, and applications of l-proline analogues. Appl. Microbiol. Biotechnol. 97:6623–6634. 10.1007/s00253-013-5022-7 [DOI] [PubMed] [Google Scholar]
- 40.Wood JM. 1981. Genetics of l-proline utilization in Escherichia coli. J. Bacteriol. 146:895–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.May G, Faatz E, Villarejo M, Bremer E. 1986. Binding protein dependent transport of glycine betaine and its osmotic regulation in Escherichia coli K12. Mol. Gen. Genet. 205:225–233. 10.1007/BF00430432 [DOI] [PubMed] [Google Scholar]
- 42.Ferson AE, Wray LV, Jr, Fisher SH. 1996. Expression of the Bacillus subtilis gabP gene is regulated independently in response to nitrogen and amino acid availability. Mol. Microbiol. 22:693–701. 10.1046/j.1365-2958.1996.d01-1720.x [DOI] [PubMed] [Google Scholar]
- 43.Brechtel CE, King SC. 1998. 4-Aminobutyrate (GABA) transporters from the amine-polyamine-choline superfamily: substrate specificity and ligand recognition profile of the 4-aminobutyrate permease from Bacillus subtilis. Biochem. J. 333:565–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gotsche S, Dahl MK. 1995. Purification and characterization of the phospho-alpha-(1,1)-glucosidase (TreA) of Bacillus subtilis 168. J. Bacteriol. 177:2721–2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bates SL, Waldren RP, Teare ID. 1973. Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. 10.1007/BF00018060 [DOI] [Google Scholar]
- 46.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 47.Harwood CR, Archibald AR. 1990. Growth, maintenance and general techniques, p 1–26 In Harwood CR, Cutting SM. (ed), Molecular biological methods for Bacillus. John Wiley & Sons, Chichester, United Kingdom [Google Scholar]
- 48.Kuwayama H, Obara S, Morio T, Katoh M, Urushihara H, Tanaka Y. 2002. PCR-mediated generation of a gene disruption construct without the use of DNA ligase and plasmid vectors. Nucleic Acids Res. 30:E2. 10.1093/nar/30.2.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guerout-Fleury AM, Shazand K, Frandsen N, Stragier P. 1995. Antibiotic-resistance cassettes for Bacillus subtilis. Gene 167:335–336. 10.1016/0378-1119(95)00652-4 [DOI] [PubMed] [Google Scholar]
- 50.Yan X, Yu HJ, Hong Q, Li SP. 2008. Cre/lox system and PCR-based genome engineering in Bacillus subtilis. Appl. Environ. Microbiol. 74:5556–5562. 10.1128/AEM.01156-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stalmach ME, Grothe S, Wood JM. 1983. Two proline porters in Escherichia coli K-12. J. Bacteriol. 156:481–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Srivatsan A, Han Y, Peng J, Tehranchi AK, Gibbs R, Wang JD, Chen R. 2008. High-precision, whole-genome sequencing of laboratory strains facilitates genetic studies. PLoS Genet. 4:e1000139. 10.1371/journal.pgen.1000139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zaprasis A, Hoffmann T, Wunsche G, Florez LA, Stülke J, Bremer E. 19 June 2013. Mutational activation of the RocR activator and of a cryptic rocDEF promoter bypass loss of the initial steps of proline biosynthesis in Bacillus subtilis. Environ. Microbiol. [Epub ahead of print.]. 10.1111/1462-2920.12193 [DOI] [PubMed] [Google Scholar]
- 54.Mäder U, Schmeisky AG, Florez LA, Stülke J. 2012. SubtiWiki—a comprehensive community resource for the model organism Bacillus subtilis. Nucleic Acids Res. 40:D1278–D1287. 10.1093/nar/gkr923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson JD, Plewniak F, Thierry JC, Poch O. 2000. DbClustal: rapid and reliable global multiple alignments of protein sequences detected by database searches. Nucleic Acids Res. 28:2919–2926. 10.1093/nar/28.15.2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410 [DOI] [PubMed] [Google Scholar]
- 57.Letunic I, Doerks T, Bork P. 2012. SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res. 40:D302–D305. 10.1093/nar/gkr931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Csonka LN. 1981. Proline over-production results in enhanced osmotolerance in Salmonella typhimurium. Mol. Gen. Genet. 182:82–86. 10.1007/BF00422771 [DOI] [PubMed] [Google Scholar]
- 59.Smith CJ, Deutch AH, Rushlow KE. 1984. Purification and characteristics of a gamma-glutamyl kinase involved in Escherichia coli proline biosynthesis. J. Bacteriol. 157:545–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bischoff DS, Ordal GW. 1992. Bacillus subtilis chemotaxis: a deviation from the Escherichia coli paradigm. Mol. Microbiol. 6:23–28. 10.1111/j.1365-2958.1992.tb00833.x [DOI] [PubMed] [Google Scholar]
- 61.Ordal GW, Villani DP, Nicholas RA, Hamel FG. 1978. Independence of proline chemotaxis and transport in Bacillus subtilis. J. Biol. Chem. 253:4916–4919 [PubMed] [Google Scholar]
- 62.Wong FH, Chen JS, Reddy V, Day JL, Shlykov MA, Wakabayashi ST, Saier MH., Jr 2012. The amino acid-polyamine-organocation superfamily. J. Mol. Microbiol. Biotechnol. 22:105–113. 10.1159/000338542 [DOI] [PubMed] [Google Scholar]
- 63.Hu LA, King SC. 1998. Membrane topology of the Escherichia coli gamma-aminobutyrate transporter: implications on the topography and mechanism of prokaryotic and eukaryotic transporters from the APC superfamily. Biochem. J. 336:69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shaffer PL, Goehring A, Shankaranarayanan A, Gouaux E. 2009. Structure and mechanism of a Na+-independent amino acid transporter. Science 325:1010–1014. 10.1126/science.1176088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao X, Lu F, Zhou L, Dang S, Sun L, Li X, Wang J, Shi Y. 2009. Structure and mechanism of an amino acid antiporter. Science 324:1565–1568. 10.1126/science.1173654 [DOI] [PubMed] [Google Scholar]
- 66.Ma D, Lu P, Yan C, Fan C, Yin P, Wang J, Shi Y. 2012. Structure and mechanism of a glutamate-GABA antiporter. Nature 483:632–636. 10.1038/nature10917 [DOI] [PubMed] [Google Scholar]
- 67.Javadpour MM, Eilers M, Groesbeek M, Smith SO. 1999. Helix packing in polytopic membrane proteins: role of glycine in transmembrane helix association. Biophys. J. 77:1609–1618. 10.1016/S0006-3495(99)77009-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dong H, Sharma M, Zhou HX, Cross TA. 2012. Glycines: role in alpha-helical membrane protein structures and a potential indicator of native conformation. Biochemistry 51:4779–4789. 10.1021/bi300090x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wray LV, Jr, Zalieckas JM, Ferson AE, Fisher SH. 1998. Mutational analysis of the TnrA-binding sites in the Bacillus subtilis nrgAB and gabP promoter regions. J. Bacteriol. 180:2943–2949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Belitsky BR, Sonenshein AL. 2013. Genome-wide identification of Bacillus subtilis CodY-binding sites at single-nucleotide resolution. Proc. Natl. Acad. Sci. U. S. A. 110:7026–7031. 10.1073/pnas.1300428110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Belitsky BR, Sonenshein AL. 2002. GabR, a member of a novel protein family, regulates the utilization of gamma-aminobutyrate in Bacillus subtilis. Mol. Microbiol. 45:569–583. 10.1046/j.1365-2958.2002.03036.x [DOI] [PubMed] [Google Scholar]
- 72.Bremer E. 2002. Adaptation to changing osmolarity, p 385–391 In Sonenshein AL, Hoch JA, Losick R. (ed), Bacillus subtilis and its closes relatives: from genes to cells. ASM Press, Washington, DC [Google Scholar]
- 73.Bremer E, Krämer R. 2000. Coping with osmotic challenges: osmoregulation through accumulation and release of compatible solutes, p 79–97 In Storz G, Hengge-Aronis R. (ed), Bacterial stress responses. ASM Press, Washington, DC [Google Scholar]
- 74.Shelp BJ, Mullen RT, Waller JC. 2012. Compartmentation of GABA metabolism raises intriguing questions. Trends Plant Sci. 17:57–59. 10.1016/j.tplants.2011.12.006 [DOI] [PubMed] [Google Scholar]
- 75.Planamente S, Vigouroux A, Mondy S, Nicaise M, Faure D, Morera S. 2010. A conserved mechanism of GABA binding and antagonism is revealed by structure-function analysis of the periplasmic binding protein Atu2422 in Agrobacterium tumefaciens. J. Biol. Chem. 285:30294–30303. 10.1074/jbc.M110.140715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liao MK, Gort S, Maloy S. 1997. A cryptic proline permease in Salmonella typhimurium. Microbiology 143:2903–2911. 10.1099/00221287-143-9-2903 [DOI] [PubMed] [Google Scholar]
- 77.Jauniaux JC, Vandenbol M, Vissers S, Broman K, Grenson M. 1987. Nitrogen catabolite regulation of proline permease in Saccharomyces cerevisiae. Cloning of the PUT4 gene and study of PUT4 RNA levels in wild-type and mutant strains. Eur. J. Biochem. 164:601–606 [DOI] [PubMed] [Google Scholar]
- 78.Grallath S, Weimar T, Meyer A, Gumy C, Suter-Grotemeyer M, Neuhaus JM, Rentsch D. 2005. The AtProT family. Compatible solute transporters with similar substrate specificity but differential expression patterns. Plant Physiol. 137:117–126. 10.1104/pp.104.055079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lulko AT, Buist G, Kok J, Kuipers OP. 2007. Transcriptome analysis of temporal regulation of carbon metabolism by CcpA in Bacillus subtilis reveals additional target genes. J. Mol. Microbiol. Biotechnol. 12:82–95. 10.1159/000096463 [DOI] [PubMed] [Google Scholar]
- 80.Belitsky BR. 2004. Bacillus subtilis GabR, a protein with DNA-binding and aminotransferase domains, is a PLP-dependent transcriptional regulator. J. Mol. Biol. 340:655–664. 10.1016/j.jmb.2004.05.020 [DOI] [PubMed] [Google Scholar]
- 81.Shivers RP, Sonenshein AL. 2004. Activation of the Bacillus subtilis global regulator CodY by direct interaction with branched-chain amino acids. Mol. Microbiol. 53:599–611. 10.1111/j.1365-2958.2004.04135.x [DOI] [PubMed] [Google Scholar]
- 82.Levdikov VM, Blagova E, Colledge VL, Lebedev AA, Williamson DC, Sonenshein AL, Wilkinson AJ. 2009. Structural rearrangement accompanying ligand binding in the GAF domain of CodY from Bacillus subtilis. J. Mol. Biol. 390:1007–1018. 10.1016/j.jmb.2009.05.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Molle V, Nakaura Y, Shivers RP, Yamaguchi H, Losick R, Fujita Y, Sonenshein AL. 2003. Additional targets of the Bacillus subtilis global regulator CodY identified by chromatin immunoprecipitation and genome-wide transcript analysis. J. Bacteriol. 185:1911–1922. 10.1128/JB.185.6.1911-1922.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.