Abstract
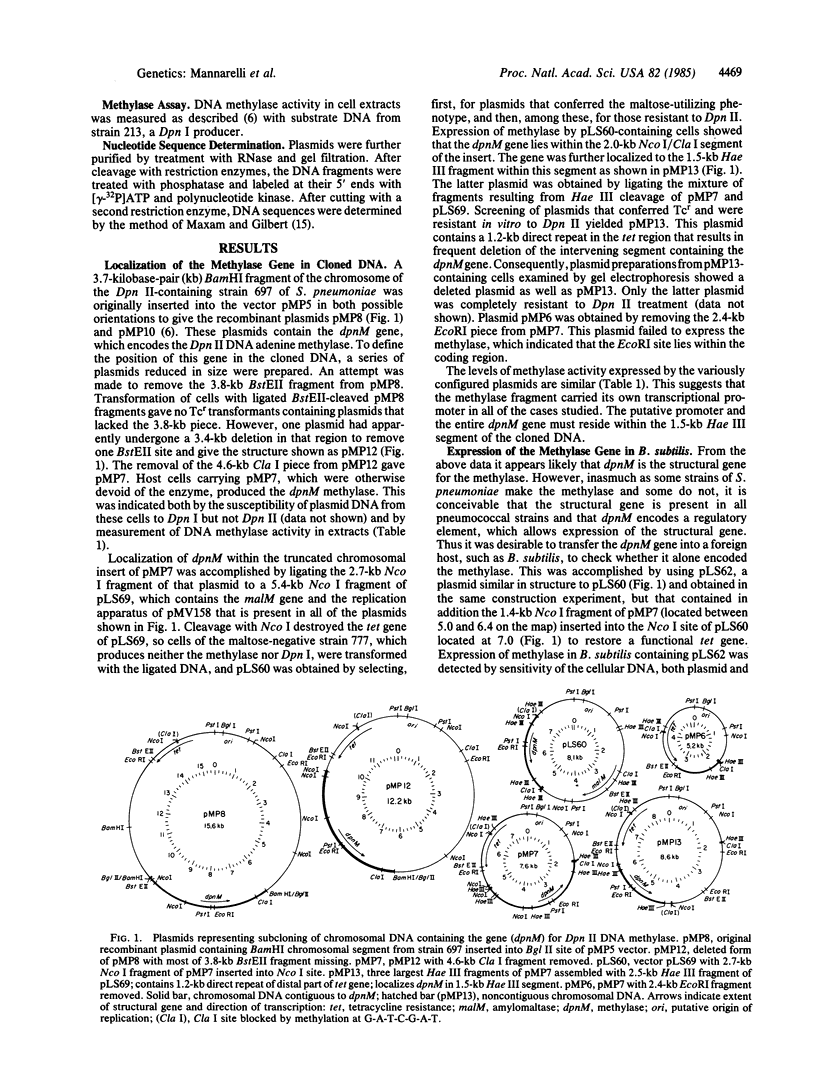
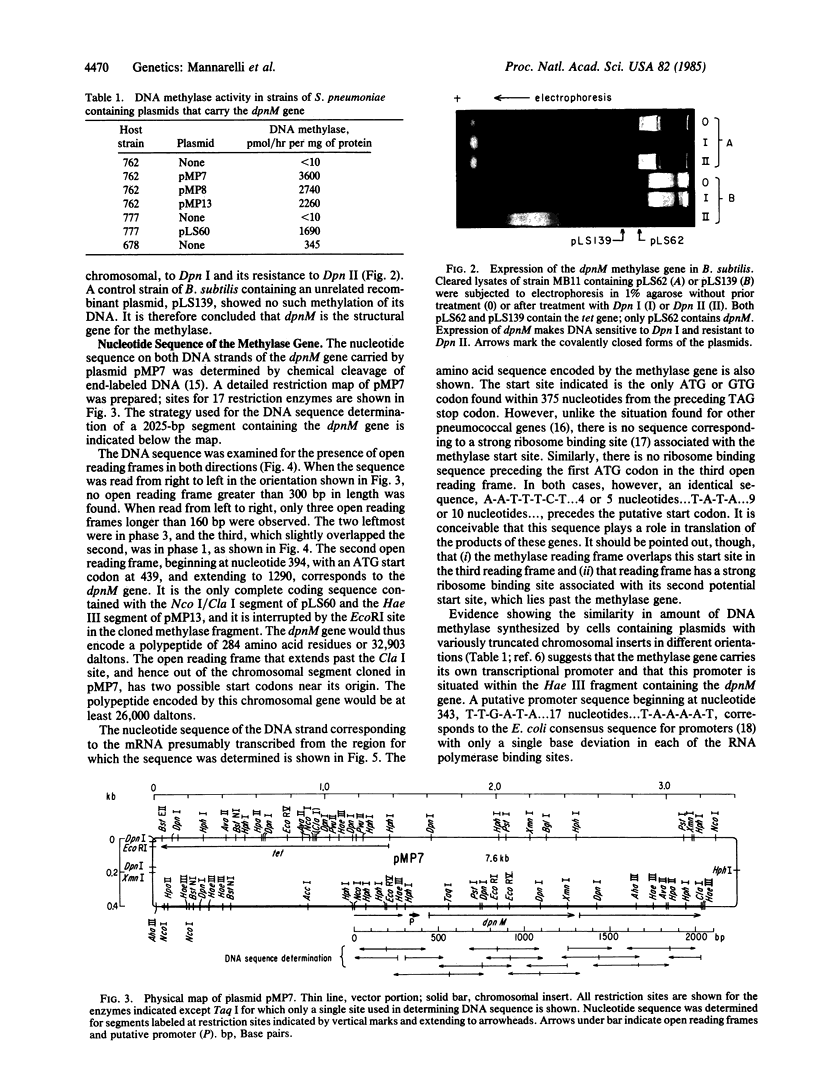
The structural gene (dpnM) for the Dpn II DNA methylase of Streptococcus pneumoniae, which is part of the Dpn II restriction system and methylates adenine in the sequence 5'-G-A-T-C-3', was identified by subcloning fragments of a chromosomal segment from a Dpn II-producing strain in an S. pneumoniae host/vector cloning system and demonstrating function of the gene also in Bacillus subtilis. Determination of the nucleotide sequence of the gene and adjacent DNA indicates that it encodes a polypeptide of 32,903 daltons. A putative promoter for transcription of the gene lies within a hundred nucleotides of the polypeptide start codon. Comparison of the coding sequence to that of the dam gene of Escherichia coli, which encodes a similar methylase, revealed 30% of the amino acid residues in the two enzymes to be identical. This homology presumably reflects a common origin of the two genes prior to the divergence of Gram-positive and Gram-negative bacteria. It is suggested that the restriction function of the gene is primitive, and that the homologous restriction system in E. coli has evolved to play an accessory role in heteroduplex DNA base mismatch repair.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernheimer H. P. Lysogenic pneumococci and their bacteriophages. J Bacteriol. 1979 May;138(2):618–624. doi: 10.1128/jb.138.2.618-624.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J. E., Blumenthal R. M., Gingeras T. R. The isolation and characterization of the Escherichia coli DNA adenine methylase (dam) gene. Nucleic Acids Res. 1983 Feb 11;11(3):837–851. doi: 10.1093/nar/11.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Espinosa M., Lopez P., Perez-Ureña M. T., Lacks S. A. Interspecific plasmid transfer between Streptococcus pneumoniae and Bacillus subtilis. Mol Gen Genet. 1982;188(2):195–201. doi: 10.1007/BF00332675. [DOI] [PubMed] [Google Scholar]
- Glickman B. W., Radman M. Escherichia coli mutator mutants deficient in methylation-instructed DNA mismatch correction. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1063–1067. doi: 10.1073/pnas.77.2.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattman S. DNA methyltransferase-dependent transcription of the phage Mu mom gene. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5518–5521. doi: 10.1073/pnas.79.18.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Morisato D., Roberts D., Bender J. Mechanism and regulation of Tn10 transposition. Cold Spring Harb Symp Quant Biol. 1984;49:235–244. doi: 10.1101/sqb.1984.049.01.027. [DOI] [PubMed] [Google Scholar]
- Lacks S. A., Dunn J. J., Greenberg B. Identification of base mismatches recognized by the heteroduplex-DNA-repair system of Streptococcus pneumoniae. Cell. 1982 Dec;31(2 Pt 1):327–336. doi: 10.1016/0092-8674(82)90126-x. [DOI] [PubMed] [Google Scholar]
- Lacks S. A. Purification and properties of the complementary endonucleases DpnI and DpnII. Methods Enzymol. 1980;65(1):138–146. doi: 10.1016/s0076-6879(80)65019-8. [DOI] [PubMed] [Google Scholar]
- Lacks S. A., Springhorn S. S. Cloning in Streptococcus pneumoniae of the gene for DpnII DNA methylase. J Bacteriol. 1984 Mar;157(3):934–936. doi: 10.1128/jb.157.3.934-936.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S. A., Springhorn S. S. Transfer of recombinant plasmids containing the gene for DpnII DNA methylase into strains of Streptococcus pneumoniae that produce DpnI or DpnII restriction endonucleases. J Bacteriol. 1984 Jun;158(3):905–909. doi: 10.1128/jb.158.3.905-909.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S., Greenberg B. A deoxyribonuclease of Diplococcus pneumoniae specific for methylated DNA. J Biol Chem. 1975 Jun 10;250(11):4060–4066. [PubMed] [Google Scholar]
- Lacks S., Greenberg B. Complementary specificity of restriction endonucleases of Diplococcus pneumoniae with respect to DNA methylation. J Mol Biol. 1977 Jul;114(1):153–168. doi: 10.1016/0022-2836(77)90289-3. [DOI] [PubMed] [Google Scholar]
- Lacks S. Integration efficiency and genetic recombination in pneumococcal transformation. Genetics. 1966 Jan;53(1):207–235. doi: 10.1093/genetics/53.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez P., Espinosa M., Lacks S. A. Physical structure and genetic expression of the sulfonamide-resistance plasmid pLS80 and its derivatives in Streptococcus pneumoniae and Bacillus subtilis. Mol Gen Genet. 1984;195(3):403–410. doi: 10.1007/BF00341440. [DOI] [PubMed] [Google Scholar]
- Marinus M. G., Morris N. R. Biological function for 6-methyladenine residues in the DNA of Escherichia coli K12. J Mol Biol. 1974 May 15;85(2):309–322. doi: 10.1016/0022-2836(74)90366-0. [DOI] [PubMed] [Google Scholar]
- Marinus M. G., Morris N. R. Isolation of deoxyribonucleic acid methylase mutants of Escherichia coli K-12. J Bacteriol. 1973 Jun;114(3):1143–1150. doi: 10.1128/jb.114.3.1143-1150.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Muckerman C. C., Springhorn S. S., Greenberg B., Lacks S. A. Transformation of restriction endonuclease phenotype in Streptococcus pneumoniae. J Bacteriol. 1982 Oct;152(1):183–190. doi: 10.1128/jb.152.1.183-190.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukkila P. J., Peterson J., Herman G., Modrich P., Meselson M. Effects of high levels of DNA adenine methylation on methyl-directed mismatch repair in Escherichia coli. Genetics. 1983 Aug;104(4):571–582. doi: 10.1093/genetics/104.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Schwartz R. M., Dayhoff M. O. Origins of prokaryotes, eukaryotes, mitochondria, and chloroplasts. Science. 1978 Jan 27;199(4327):395–403. doi: 10.1126/science.202030. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Stassi D. L., Lopez P., Espinosa M., Lacks S. A. Cloning of chromosomal genes in Streptococcus pneumoniae. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7028–7032. doi: 10.1073/pnas.78.11.7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vovis G. F., Lacks S. Complementary action of restriction enzymes endo R-DpnI and Endo R-DpnII on bacteriophage f1 DNA. J Mol Biol. 1977 Sep 25;115(3):525–538. doi: 10.1016/0022-2836(77)90169-3. [DOI] [PubMed] [Google Scholar]



