Abstract
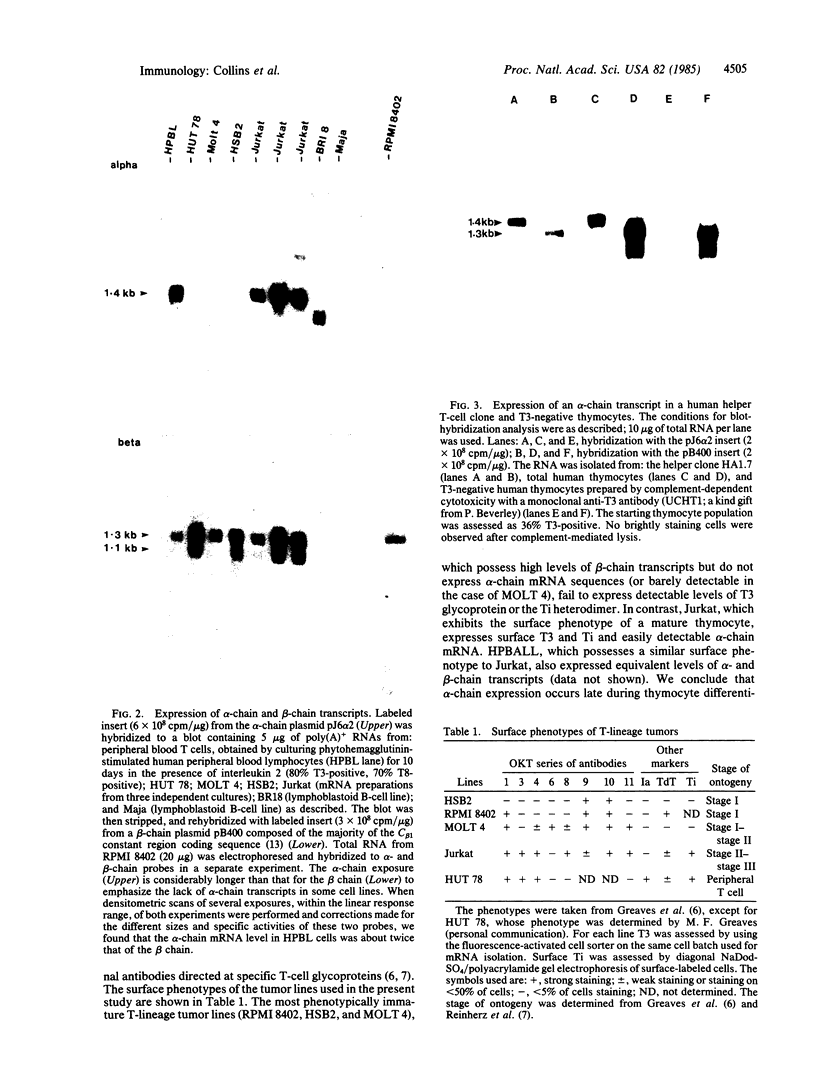
A cDNA clone encoding the alpha chain of the human T-cell antigen receptor was isolated by screening a library from the human T-cell line Jurkat with a mouse alpha-chain cDNA clone. This human alpha-chain clone, together with a human antigen receptor beta-chain cDNA clone, was used to determine the stage of T-cell development at which antigen receptor mRNAs first appear. Blot-hybridization analysis of mRNA isolated from a panel of human thymic tumor lines clearly demonstrated that beta-chain transcripts could be detected in all T-lineage cells. However, alpha-chain transcripts were only found in the most phenotypically mature lines, which express the antigen receptor-associated molecule T3. Furthermore, beta-chain transcripts were abundant in RNA prepared from purified T3-negative thymocytes, whereas alpha-chain transcripts were virtually absent. From these results we conclude that alpha-chain expression occurs later in thymic ontogeny than that of the beta chain and propose that it controls surface expression of the antigen receptor-T3 complex.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acuto O., Hussey R. E., Fitzgerald K. A., Protentis J. P., Meuer S. C., Schlossman S. F., Reinherz E. L. The human T cell receptor: appearance in ontogeny and biochemical relationship of alpha and beta subunits on IL-2 dependent clones and T cell tumors. Cell. 1983 Oct;34(3):717–726. doi: 10.1016/0092-8674(83)90528-7. [DOI] [PubMed] [Google Scholar]
- Broder S., Edelson R. L., Lutzner M. A., Nelson D. L., MacDermott R. P., Durm M. E., Goldman C. K., Meade B. D., Waldmann T. A. The Sézary syndrome: a malignant proliferation of helper T cells. J Clin Invest. 1976 Dec;58(6):1297–1306. doi: 10.1172/JCI108585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien Y., Becker D. M., Lindsten T., Okamura M., Cohen D. I., Davis M. M. A third type of murine T-cell receptor gene. Nature. 1984 Nov 1;312(5989):31–35. doi: 10.1038/312031a0. [DOI] [PubMed] [Google Scholar]
- Chilson O. P., Boylston A. W., Crumpton M. J. Phaseolus vulgaris phytohaemagglutinin (PHA) binds to the human T lymphocyte antigen receptor. EMBO J. 1984 Dec 20;3(13):3239–3245. doi: 10.1002/j.1460-2075.1984.tb02285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gascoigne N. R., Chien Y., Becker D. M., Kavaler J., Davis M. M. Genomic organization and sequence of T-cell receptor beta-chain constant- and joining-region genes. Nature. 1984 Aug 2;310(5976):387–391. doi: 10.1038/310387a0. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Rao J., Hariri G., Verbi W., Catovsky D., Kung P., Goldstein G. Phenotypic heterogeneity and cellular origins of T cell malignancies. Leuk Res. 1981;5(4-5):281–299. doi: 10.1016/0145-2126(81)90001-1. [DOI] [PubMed] [Google Scholar]
- Kappler J., Kubo R., Haskins K., White J., Marrack P. The mouse T cell receptor: comparison of MHC-restricted receptors on two T cell hybridomas. Cell. 1983 Oct;34(3):727–737. doi: 10.1016/0092-8674(83)90529-9. [DOI] [PubMed] [Google Scholar]
- Kavaler J., Davis M. M., Chien Y. Localization of a T-cell receptor diversity-region element. Nature. 1984 Aug 2;310(5976):421–423. doi: 10.1038/310421a0. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Eckels D. D., Lake P., Johnson A. H., Hartzman R. J., Woody J. N. Antigen-specific human T lymphocyte clones: induction, antigen specificity, and MHC restriction of influenza virus-immune clones. J Immunol. 1982 Jan;128(1):233–238. [PubMed] [Google Scholar]
- Malissen M., Minard K., Mjolsness S., Kronenberg M., Goverman J., Hunkapiller T., Prystowsky M. B., Yoshikai Y., Fitch F., Mak T. W. Mouse T cell antigen receptor: structure and organization of constant and joining gene segments encoding the beta polypeptide. Cell. 1984 Jul;37(3):1101–1110. doi: 10.1016/0092-8674(84)90444-6. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McIntyre B. W., Allison J. P. The mouse T cell receptor: structural heterogeneity of molecules of normal T cells defined by xenoantiserum. Cell. 1983 Oct;34(3):739–746. doi: 10.1016/0092-8674(83)90530-5. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Acuto O., Hussey R. E., Hodgdon J. C., Fitzgerald K. A., Schlossman S. F., Reinherz E. L. Evidence for the T3-associated 90K heterodimer as the T-cell antigen receptor. Nature. 1983 Jun 30;303(5920):808–810. doi: 10.1038/303808a0. [DOI] [PubMed] [Google Scholar]
- Raulet D. H., Garman R. D., Saito H., Tonegawa S. Developmental regulation of T-cell receptor gene expression. Nature. 1985 Mar 7;314(6006):103–107. doi: 10.1038/314103a0. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer H. D., Acuto O., Fabbi M., Tizard R., Ramachandran K., Smart J. E., Reinherz E. L. Genes encoding the Ti beta subunit of the antigen/MHC receptor undergo rearrangement during intrathymic ontogeny prior to surface T3-Ti expression. Cell. 1984 Dec;39(2 Pt 1):261–266. doi: 10.1016/0092-8674(84)90003-5. [DOI] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. A third rearranged and expressed gene in a clone of cytotoxic T lymphocytes. Nature. 1984 Nov 1;312(5989):36–40. doi: 10.1038/312036a0. [DOI] [PubMed] [Google Scholar]
- Sim G. K., Yagüe J., Nelson J., Marrack P., Palmer E., Augustin A., Kappler J. Primary structure of human T-cell receptor alpha-chain. Nature. 1984 Dec 20;312(5996):771–775. doi: 10.1038/312771a0. [DOI] [PubMed] [Google Scholar]
- Weiss A., Stobo J. D. Requirement for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. J Exp Med. 1984 Nov 1;160(5):1284–1299. doi: 10.1084/jem.160.5.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikai Y., Anatoniou D., Clark S. P., Yanagi Y., Sangster R., Van den Elsen P., Terhorst C., Mak T. W. Sequence and expression of transcripts of the human T-cell receptor beta-chain genes. Nature. 1984 Dec 6;312(5994):521–524. doi: 10.1038/312521a0. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Yanagi Y., Suciu-Foca N., Mak T. W. Presence of T-cell receptor mRNA in functionally distinct T cells and elevation during intrathymic differentiation. Nature. 1984 Aug 9;310(5977):506–508. doi: 10.1038/310506a0. [DOI] [PubMed] [Google Scholar]