Abstract
In vitro blocking the spontaneous activity of primary cultures of chicken embryo myotubes with tetrodotoxin increases approximately equal to 2-fold their content in surface acetylcholine receptor. To investigate this effect at the level of gene expression, chicken genomic DNA sequences coding for the acetylcholine receptor alpha subunit were isolated and characterized. They were shown to belong to a single-copy, polymorphic gene with at least two alleles in the chicken strain utilized. Probes derived from these genomic clones were used to quantitate levels of alpha-subunit mRNA. In culture, a 2-day exposure to tetrodotoxin increased these mRNA levels up to 13-fold, a value similar to that observed after denervation of chick leg muscle (approximately equal to 17-fold). Actin mRNA levels varied little in any of these experiments. These results support the notion that membrane electrical activity affects acetylcholine receptor expression by regulating the accumulation of the corresponding mRNAs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., THESLEFF S. A study of supersensitivity in denervated mammalian skeletal muscle. J Physiol. 1959 Jun 23;147(1):178–193. doi: 10.1113/jphysiol.1959.sp006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Barnard E. A., Beeson D., Bilbe G., Brown D. A., Constanti A., Conti-Tronconi B. M., Dolly J. O., Dunn S. M., Mehraban F., Richards B. M. Acetylcholine and GABA receptors: subunits of central and peripheral receptors and their encoding nucleic acids. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):109–124. doi: 10.1101/sqb.1983.048.01.014. [DOI] [PubMed] [Google Scholar]
- Betz H., Changeux J. P. Regulation of muscle acetylcholine receptor synthesis in vitro by cyclic nucleotide derivatives. Nature. 1979 Apr 19;278(5706):749–752. doi: 10.1038/278749a0. [DOI] [PubMed] [Google Scholar]
- Betz H. Regulation of alpha-bungarotoxin receptor accumulation in chick retina cultures: effects of membrane depolarization, cyclic nucleotide derivatives, and Ca2+. J Neurosci. 1983 Jul;3(7):1333–1341. doi: 10.1523/JNEUROSCI.03-07-01333.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M., Farrell P. J., Barrell B. G. Transcription and DNA sequence of the BamHI L fragment of B95-8 Epstein-Barr virus. EMBO J. 1984 May;3(5):1083–1090. doi: 10.1002/j.1460-2075.1984.tb01933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum M., Reis M. A., Shainberg A. Role of calcium in the regulation of acetylcholine receptor synthese in cultured muscle cells*. Pflugers Arch. 1980 May;385(1):37–43. doi: 10.1007/BF00583913. [DOI] [PubMed] [Google Scholar]
- Black I. B., Adler J. E., Dreyfus C. F., Jonakait G. M., Katz D. M., LaGamma E. F., Markey K. M. Neurotransmitter plasticity at the molecular level. Science. 1984 Sep 21;225(4668):1266–1270. doi: 10.1126/science.6147894. [DOI] [PubMed] [Google Scholar]
- Botstein D., White R. L., Skolnick M., Davis R. W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980 May;32(3):314–331. [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Burden S. Acetylcholine receptors at the neuromuscular junction: developmental change in receptor turnover. Dev Biol. 1977 Nov;61(1):79–85. doi: 10.1016/0012-1606(77)90343-8. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Devillers-Thiéry A., Chemouilli P. Acetylcholine receptor: an allosteric protein. Science. 1984 Sep 21;225(4668):1335–1345. doi: 10.1126/science.6382611. [DOI] [PubMed] [Google Scholar]
- Devillers-Thiery A., Giraudat J., Bentaboulet M., Changeux J. P. Complete mRNA coding sequence of the acetylcholine binding alpha-subunit of Torpedo marmorata acetylcholine receptor: a model for the transmembrane organization of the polypeptide chain. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2067–2071. doi: 10.1073/pnas.80.7.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devreotes P. N., Fambrough D. M. Acetylcholine receptor turnover in membranes of developing muscle fibers. J Cell Biol. 1975 May;65(2):335–358. doi: 10.1083/jcb.65.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodgson J. B., Strommer J., Engel J. D. Isolation of the chicken beta-globin gene and a linked embryonic beta-like globin gene from a chicken DNA recombinant library. Cell. 1979 Aug;17(4):879–887. doi: 10.1016/0092-8674(79)90328-3. [DOI] [PubMed] [Google Scholar]
- Drachman D. B., Witzke F. Trophic regulation of acetylcholine sensitivity of muscle: effect of electrical stimulation. Science. 1972 May 5;176(4034):514–516. doi: 10.1126/science.176.4034.514. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M. Control of acetylcholine receptors in skeletal muscle. Physiol Rev. 1979 Jan;59(1):165–227. doi: 10.1152/physrev.1979.59.1.165. [DOI] [PubMed] [Google Scholar]
- Fertuck H. C., Salpeter M. M. Quantitation of junctional and extrajunctional acetylcholine receptors by electron microscope autoradiography after 125I-alpha-bungarotoxin binding at mouse neuromuscular junctions. J Cell Biol. 1976 Apr;69(1):144–158. doi: 10.1083/jcb.69.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudat J., Devillers-Thiery A., Auffray C., Rougeon F., Changeux J. P. Identification of a cDNA clone coding for the acetylcholine binding subunit of Torpedo marmorata acetylcholine receptor. EMBO J. 1982;1(6):713–717. doi: 10.1002/j.1460-2075.1982.tb01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall Z. W., Roisin M. P., Gu Y., Gorin P. D. A developmental change in the immunological properties of acetylcholine receptors at the rat neuromuscular junction. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):101–108. doi: 10.1101/sqb.1983.048.01.013. [DOI] [PubMed] [Google Scholar]
- Henderson C. E., Huchet M., Changeux J. P. Denervation increases a neurite-promoting activity in extracts of skeletal muscle. Nature. 1983 Apr 14;302(5909):609–611. doi: 10.1038/302609a0. [DOI] [PubMed] [Google Scholar]
- Kao F. T., Hartz J. A., Law M. L., Davidson J. N. Isolation and chromosomal localization of unique DNA sequences from a human genomic library. Proc Natl Acad Sci U S A. 1982 Feb;79(3):865–869. doi: 10.1073/pnas.79.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
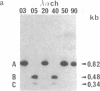
- Klarsfeld A., Devillers-Thiéry A., Giraudat J., Changeux J. P. A single gene codes for the nicotinic acetylcholine receptor alpha-subunit in Torpedo marmorata: structural and developmental implications. EMBO J. 1984 Jan;3(1):35–41. doi: 10.1002/j.1460-2075.1984.tb01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomo T., Westgaard R. H. Control of ACh sensitivity in rat muscle fibers. Cold Spring Harb Symp Quant Biol. 1976;40:263–274. doi: 10.1101/sqb.1976.040.01.027. [DOI] [PubMed] [Google Scholar]
- Merlie J. P. Biogenesis of the acetylcholine receptor, a multisubunit integral membrane protein. Cell. 1984 Mar;36(3):573–575. doi: 10.1016/0092-8674(84)90335-0. [DOI] [PubMed] [Google Scholar]
- Merlie J. P., Isenberg K. E., Russell S. D., Sanes J. R. Denervation supersensitivity in skeletal muscle: analysis with a cloned cDNA probe. J Cell Biol. 1984 Jul;99(1 Pt 1):332–335. doi: 10.1083/jcb.99.1.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlie J. P., Sebbane R., Gardner S., Lindstrom J. cDNA clone for the alpha subunit of the acetylcholine receptor from the mouse muscle cell line BC3H-1. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3845–3849. doi: 10.1073/pnas.80.12.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Minty A. J., Alonso S., Guénet J. L., Buckingham M. E. Number and organization of actin-related sequences in the mouse genome. J Mol Biol. 1983 Jun 15;167(1):77–101. doi: 10.1016/s0022-2836(83)80035-7. [DOI] [PubMed] [Google Scholar]
- Noda M., Furutani Y., Takahashi H., Toyosato M., Tanabe T., Shimizu S., Kikyotani S., Kayano T., Hirose T., Inayama S. Cloning and sequence analysis of calf cDNA and human genomic DNA encoding alpha-subunit precursor of muscle acetylcholine receptor. 1983 Oct 27-Nov 2Nature. 305(5937):818–823. doi: 10.1038/305818a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Furutani Y., Hirose T., Asai M., Inayama S., Miyata T., Numa S. Primary structure of alpha-subunit precursor of Torpedo californica acetylcholine receptor deduced from cDNA sequence. Nature. 1982 Oct 28;299(5886):793–797. doi: 10.1038/299793a0. [DOI] [PubMed] [Google Scholar]
- Norman R. I., Mehraban F., Barnard E. A., Dolly J. O. Nicotinic acetylcholine receptor from chick optic lobe. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1321–1325. doi: 10.1073/pnas.79.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. N., Glaser L., Merlie J. P., Lindstrom J. Expression of acetylcholine receptor alpha-subunit mRNA during differentiation of the BC3H1 muscle cell line. J Biol Chem. 1984 Mar 10;259(5):3330–3336. [PubMed] [Google Scholar]
- Oswald R. E., Freeman J. A. Alpha-bungarotoxin binding and central nervous system nicotinic acetylcholine receptors. Neuroscience. 1981;6(1):1–14. doi: 10.1016/0306-4522(81)90239-6. [DOI] [PubMed] [Google Scholar]
- Pezzementi L., Schmidt J. Rapid modulation of acetylcholine receptor synthesis. FEBS Lett. 1981 Nov 30;135(1):103–106. doi: 10.1016/0014-5793(81)80953-2. [DOI] [PubMed] [Google Scholar]
- Rackwitz H. R., Zehetner G., Frischauf A. M., Lehrach H. Rapid restriction mapping of DNA cloned in lambda phage vectors. Gene. 1984 Oct;30(1-3):195–200. doi: 10.1016/0378-1119(84)90120-3. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robert B., Barton P., Minty A., Daubas P., Weydert A., Bonhomme F., Catalan J., Chazottes D., Guénet J. L., Buckingham M. Investigation of genetic linkage between myosin and actin genes using an interspecific mouse back-cross. Nature. 1985 Mar 14;314(6007):181–183. doi: 10.1038/314181a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. J., Rothblum K. N. Gene switching in myogenesis: differential expression of the chicken actin multigene family. Biochemistry. 1981 Jul 7;20(14):4122–4129. doi: 10.1021/bi00517a027. [DOI] [PubMed] [Google Scholar]
- Shainberg A., Burstein M. Decrease of acetylcholine receptor synthesis in muscle cultures by electrical stimulation. Nature. 1976 Nov 25;264(5584):368–369. doi: 10.1038/264368a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]