Abstract
A nontoxigenic strain isolated from a fatal human case of bacterial sepsis was identified as a Clostridium strain from Clostridium botulinum group III, based on the phenotypic characters and 16S rRNA gene sequence, and was found to be related to the mosaic C. botulinum D/C strain according to a multilocus sequence analysis of 5 housekeeping genes.
CASE REPORT
An 83-year-old woman was hospitalized in a hospital geriatric ward in Paris for a cardiac decompensation. She had a mental disorder and a transient motor weakness on the day planned for her relapse, and she received a benzodiazepine treatment. The same day evening, she fell off her bed and had a fracture of the neck of the femur. Two days later, she developed sepsis (fever, hypotension of 11/5, 33,000 leukocytes/mm3, and C reactive protein level of 30 mg/liter) and received an antibiotic treatment (cefotaxime, 2 g/day, metronidazole, 0.5 g/8 h intravenously [i.v.]). No characteristic symptom of flaccid paralysis was evidenced. Surgery was delayed, and death occurred the following day. A blood culture performed during the septic phase yielded an anaerobic bacterium called strain AIP981.10.
Bacteria were grown in Trypticase yeast extract-glucose-hemin (TYGH) broth in an anaerobic atmosphere at 37°C. Phenotypic identification was performed with reference methods (1), and metabolic end products (volatile and nonvolatile fatty acids) were assayed by quantitative gas chromatography, as described previously (2). Toxicity was tested using a mouse bioassay (3), and cytotoxicity on Vero cells was performed as previously described (4). The 16S rRNA gene sequence was determined as described previously (5) and was compared to all eubacterial 16S rRNA gene sequences available in the GenBank database by using the multisequence Advanced BLAST comparison software from the National Center for Biotechnology Information (6).
Multilocus sequence typing (MLST) analysis was based on five housekeeping genes (the CTP synthetase [CTPs] gene, glpK, rpoB, gyrA, and dnaK) (see Table S1 in the supplemental material). Genome sequences of Clostridium botulinum D strain 1873 (NZ_ACSJ01000007) were used as the templates for sequence alignment of the clostridial genes, which have been analyzed, and primers were designed for the conserved sequences with Primer3 software (http://biotools.umassmed.edu/bioapps/primer3_www.cgi). C. botulinum type A ATCC 3502 and Clostridium perfringens strain 13 were used as outgroups in gene analysis (see Table S2 in the supplemental material). In addition, the botulinolysin, C. botulinum C2 toxin, and Clostridium novyi hemolysin (termed novyilysin) genes, as well as flagellin genes from C. novyi A and B, Clostridium haemolyticum, and Clostridium chauvoei according to reference 7, were investigated (see Table S1). Gene fragments were PCR amplified and sequenced. Sequence alignments and phylogenetic analysis were conducted using Molecular Evolutionary Genetics Analysis (MEGA) software (version 5) (http://www.megasoftware.net) (8). The phylogenetic inference was based on the neighbor-joining distance method (9). Gene trees were constructed by the neighbor-joining method, using the Kimura two-parameter model (10) and bootstrapping algorithms contained in MEGA software (11).
Strain AIP981.10 was a strictly anaerobic, Gram-positive, spore-forming bacillus that produced lipase and protease but not lecithinase. Gas was produced. Tests for catalase, urease, indole from tryptophan, and reduction of nitrates and nitrites were found negative. Hemolysis on sheep blood agar was observed. A commercial gallery (Rapid ID 32A; bioMérieux, Marcy l'Etoile, France) was inoculated and gave the resulting code 4006400000, which does not correspond to a known species. However, these results indicated that AIP981.10 might belong to the C. botulinum-C. novyi-Clostridium sporogenes group of bacteria. Major volatile and nonvolatile fatty acids were propionic (35.7 mM), lactic (12.9 mM), and butyric (6.0 mM) acids, with small amounts of 2-hydroxybutyric and 2-hydroxyvaleric acids. Production of propionic acid as a major metabolism end product is characteristic of C. botulinum group III, including C. botulinum C and D as well as related species, such as C. novyi and C. haemolyticum (1, 12). Thus, AIP981.10 was tentatively assigned to a C. botulinum from group III or a related species.
Strain AIP981.10 was not toxic, as monitored by injection of 1 ml of culture supernatant intraperitoneally into mice, and there was no cytotoxicity on Vero cells. Botulinum neurotoxin (BoNT) A-to-G genes, as well as flagellin genes of C. novyi A and B, C. haemolyticum, and C. chauvoei were not detected by PCR. Among the toxin genes tested, AIP981.10 gave a PCR amplification only with botulinolysin primers (Table 1). Botulinolysin and novyilysin, produced by C. botulinum and Clostridium novyi, respectively, are related hemolysins from the cholesterol-dependent cytolysin family, which also encompasses Clostridium tetani tetanolysin (13). Botulinolysin primers (see Table S1 in the supplemental material) yielded a PCR detection with all of the C. botulinum, C. novyi, and C. haemolyticum strains tested, whereas novyilysin primers were specific to C. novyi (Table 1) suggesting that AIP981.10 is more related to C. botulinum C and D, C. novyi B, or C. haemolyticum than to C. novyi A. However, AIP981.10 did not contain C2 toxin genes. The 16S rRNA gene sequence from strain AIP981.10 (1,332 bp) clustered within Clostridium cluster I, as defined by Collins et al. (14), in the branch containing C. botulinum C, C. botulinum D, C. botulinum C/D and D/C mosaic strains, C. novyi and C. haemolyticum (Fig. 1) (7, 14, 15). Sequence from AIP981.10 was more related to those of C. botulinum C/D and D/C mosaic isolates (99.9% identity) compared to the other Clostridium sequences: C. botulinum C strain 468 (99.3%), C. botulinum D strain 1873 (99.6%), C. haemolyticum ATCC 9650 (99.5%), C. novyi A (98.8), and C. botulinum C strain Eklund (98.1%).
TABLE 1.
PCR detection of novyilysin, botulinolysin, C2 toxin, and clostridiolysin S genes in strain AIP981.10, C. botulinum C and D, C. novyi A and B, and C. haemolyticum
| Strain | Result for gene coding for: |
||||||
|---|---|---|---|---|---|---|---|
| Novyilysin | Botulinolysin | Phospholipase C | C2 toxin component I | C2 toxin component II | C3 exoenzyme | Clostridiolysin S | |
| AIP981.10 | − | + | + | − | − | + | − |
| C. botulinum | |||||||
| C strain 468 | − | + | + | + | + | + | − |
| D strain 1873 | − | + | + | + | + | + | − |
| C/D mosaic | − | + | + | + | + | + | − |
| D/C mosaic | − | + | NAa | − | − | NA | NA |
| C. haemolyticum AIP10052T | − | + | + | − | − | NA | NA |
| C. novyi | |||||||
| A strain AIP10062T | + | + | + | − | − | − | − |
| B strain AIP212.86 | − | + | NA | − | − | NA | NA |
NA, not available.
FIG 1.
16S rRNA gene analysis. The dendrogram was reconstructed from the nucleotide sequence of the gene by the unweighted-pair group method using average linkages (UPGMA) method. The genetic distances were computed by using the Kimura two-parameter model. The scale bar indicates the genetic distance. The number shown next to each node indicates the percent bootstrap value of 1,000 replicates. Evolutionary analyses were conducted in MEGA5 (8). Strain AIP981.10 is highlighted on the tree by a black square.
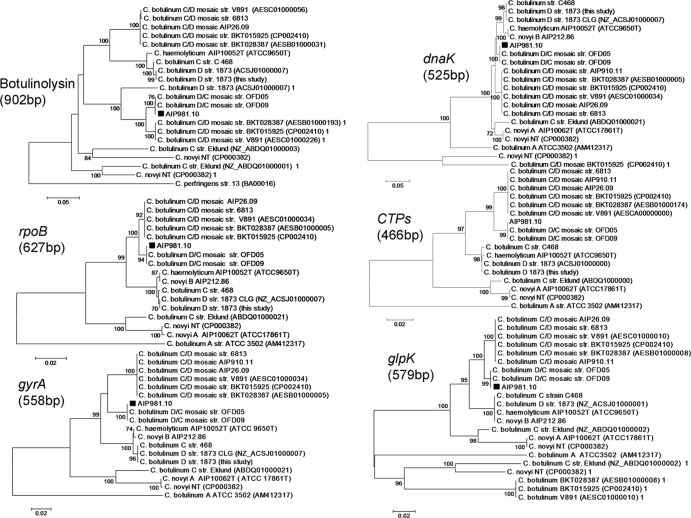
Analysis of the five housekeeping genes and botulinolysin gene gave the same patterns for AIP981.10 as the 16S rRNA sequence phylogeny tree (Fig. 2). AIP981.10 is located on a distinct branch containing C. botulinum D/C mosaic strains between two closely related branches—one containing C. botulinum C/D strains and another one encompassing C. novyi B, C. botulinum C strain 468, C. botulinum D, and C. haemolyticum. Interestingly, the housekeeping gene sequences of C. botulinum C strain Eklund clustered within the branch containing C. novyi A, which is distantly related to the one containing the C. botulinum C strain, 468. These results are in agreement with those from the phylogenomic analysis of Skarin et al. (16), which show that C. botulinum C strain Eklund is closely related to C. novyi A. The two C. botulinum C strains share 97.2% 16S rRNA gene sequence identity, suggesting that strain Eklund might be assigned to a different species. Nucleotide differences in housekeeping gene and botulinolysin gene sequences between AIP981.10 and the Clostridium reference strains (Table 2) indicate that AIP981.10 is more related to the mosaic C. botulinum D/C strains than to the other C. botulinum and C. novyi strains.
FIG 2.
Housekeeping gene and botulinolysin gene analyses. The dendrogram was reconstructed from the nucleotide sequence of the gene by the neighbor-joining method. The genetic distances were computed by using the Kimura two-parameter model. The scale bar indicates the genetic distance. The number shown next to each node indicates the percent bootstrap value of 1,000 replicates. Evolutionary analyses were conducted in MEGA5 (8). Strain AIP981.10 is highlighted on the trees by black squares.
TABLE 2.
Nucleotide differences between AIP981.10 gene sequences and corresponding sequences from C. botulinum C or D and C/D mosaic, C. haemolyticum, and C. novyi NT
| AIP981.10 gene/product (source) | Size (bp) | No. of nucleotide differences between AIP981.10 and corresponding Clostridium sequences |
||||
|---|---|---|---|---|---|---|
| C. botulinum D/C Japanese bovine strain group | C. botulinum C/D mosaic avian strains | C. botulinum C-C. botulinum D-C. novyi B-C. haemolyticum | C. botulinum C strain Eklund | C. novyi NT | ||
| CTPs (PCR amplicon) | 466 | 0 | 10 | 19 | 49 | 50 |
| glpK (PCR amplicon) | 579 | 0 | 9/121a | 23 | 35/121a | 56 |
| rpoB (PCR amplicon) | 627 | 0 | 6 | 23 | 49 | 56 |
| gyrA (PCR amplicon) | 558 | 0 | 26 | 29 | 72 | 82 |
| dnaK (PCR amplicon) | 525 | 0 | 11/217a | 15 | 33 | 24/213a |
| Botulinolysin (PCR amplicon) | 902 | 2 | 26/154a | 176 | 168/195a | 210 |
| Phospholipase C (gene sequence from WGS) | 1,200b | NA | 55/234a | 74/77/NA/105c | 388 | 394 |
| C3 exoenzyme (gene sequence from WGS) | 735b | NA | 16 | 16/1/NA/NAd | 11 | NA |
Some genomes show two gene copies of dnaK, glpK, and the botulinolysin and phospholipase C genes that are distantly related. (Differences in both gene copies are separated by a slash.) The corresponding GenBank accession numbers of these genomes are listed in Table S2 in the supplemental material.
Data obtained from the whole-genome sequencing (WGS) of strain AIP981.10.
In addition, whole-genome sequencing of AIP981.10 has been performed with Illumina single-read sequencing technology. Illumina library preparation and sequencing followed standard protocols developed by the supplier (TrueSeq DNA sample preparation). Briefly, genomic DNA was sheared by nebulization, and sheared fragments were end repaired and phosphorylated. Blunt end fragments were A-tailed, and sequencing adapters were ligated to the fragments. Fragments with an insert size of around 400 bp were gel extracted and enriched with 10 cycles of PCR. Hybridization of the library to the flow cell and bridge amplification was performed to generate clusters, and single reads of 50 cycles were collected on a HiSeq 2000 (Illumina, San Diego, CA). After sequencing was complete, image analysis, base calling, and error estimation were performed using Illumina Analysis Pipeline version 1.7. High-quality filtered reads were assembled using CLC Assembly Cell (CLC bio). A total of 266 contigs ranging from 310 to 109,956 bp were obtained. DNA sequences derived from standard PCR for 16S rRNA genes, botulinolysin genes, and the five housekeeping genes were identical to those from the whole-genome sequencing. Again, the putative botulinum toxin gene and C2 toxin genes have not been identified by sequence similarity searching. However, C3 exoenzyme and phospholipase C genes related to those found in C. botulinum C and D (17, 18) have been evidenced in AIP981.10 (Table 1 and 2). An additional toxin gene, coding for clostridiolysin S, has been recently characterized in C. botulinum. Clostridiolysin S is related to streptolysin S from Streptococcus and is a posttranslationally modified toxin resulting from a cluster of nine genes (closA, closB, closC, closD, closE, closF, closG, closH, and closI) (19). Among the C. botulinum/C. sporogenes whole-genome sequences available, clostridiolysin S genes have been found only in C. botulinum strains from group I and C. sporogenes, whereas no related genes have been evidenced in C. botulinum group III strains or AIP981.10.
Anaerobic bacteria are responsible for various types of infections, including bacteremia. Bloodstream infections with anaerobes (1 to 17% of positive blood cultures) are mainly due to Gram-negative bacilli. Clostridium account for 8 to 18% (up to 46% in some studies) of anaerobic bacteremia in adults (reviewed in reference 20). The most frequently identified Clostridium species are Clostridium perfringens and Clostridium septicum, which are often associated with a dramatic outcome (20, 21). Other toxigenic clostridia might also be involved, such as Clostridium sordellii (22), to a lower extent Clostridium difficile (23), and one case of Clostridium tetani (24). However, nontoxigenic clostridia from the environment, like Clostridium tertium, Clostridium aldenense, or Clostridium cadaveris, are occasionally isolated in blood infections (25–27).
Here we report an atypical Clostridium strain related to Clostridium botulinum group III, which has been identified in a blood culture. Phenotypic and genotypic analyses indicate that strain AIP981.10 belongs to the physiological group III of C. botulinum (reviewed in references 28 and 29), which encompasses C. botulinum type C, C. botulinum type D, and C. botulinum mosaic strains C/D and D/C and which is closely related to C. novyi and C. haemolyticum. Indeed, the chromosome of the group III C. botulinum isolates is highly conserved and is related to that of C. novyi (16). However, based on the 16S rRNA sequence, AIP981.10 is on a phylogenetic subbranch distinct from those of the C. botulinum and C. novyi strains, and multisequence analysis of housekeeping genes shows a close relatedness to the C. botulinum D/C mosaic strain and to a lower extent to C. botulinum C/D mosaic strain. AIP981.10, which was isolated from a blood culture from a fatal case of infection, was found to not produce lethal toxin. This strain contains botulinolysin, phospholipase C, and C3 exoenzyme genes but not clostridiolysin S genes, supporting that AIP981.10 is phylogenetically related to C. botulinum group III strains. In addition, the sequences of the botulinolysin and phospholipase C genes are highly related to those of C. botulinum C and D and less similar to those of C. novyi and C. haemolyticum (Table 2). Both BoNT genes of types C and D and the C. novyi alpha-toxin gene (tcnA) are localized on phage DNAs that are not integrated into the chromosome. These phages can be easily lost upon subcultures and can be interchanged between C. botulinum C or D and C. novyi (30–34). Similarly, C2 toxin genes are localized on large plasmids in C. botulinum C and D that can be lost or acquired (35). It could be hypothesized that AIP981.10 has lost a phage harboring a BoNT gene during the isolation and subcultures of the strain, and is therefore no longer toxigenic. Indeed a C. botulinum type B-like nontoxigenic strain has been isolated from an infant botulism case (36). However, C. botulinum C or D strains have been involved only in a few cases of human botulism (37), and no characteristic symptoms of flaccid paralysis were observed in this patient. Alternatively, AIP981.10 could have lost either a phage harboring tcnA, a plasmid or another mobile genetic element carrying C2 toxin genes or other unknown toxin gene(s), since the group III C. botulinum strains possess various plasmids and mobile elements (35, 38). An environmental nontoxigenic Clostridium strain might also be the causative agent in compromised patients, as already evidenced by other nontoxic Clostridium species (25–27).
Nucleotide sequence accession numbers.
16S rRNA, phospholipase C, and C3 exoenzyme gene sequences from strain AIP981.10 have been deposited in the GenBank database under accession no. KC589740, KF662728, and KF662729, respectively.
Supplementary Material
ACKNOWLEDGMENTS
We thank S. Kozaki for kindly providing C. botulinum D/C mosaic isolates.
This work was supported by Institut Pasteur funding.
Footnotes
Published ahead of print 2 October 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00390-13.
REFERENCES
- 1.Jousimies-Somer HR, Summanen P, Citron DM, Baron EJ, Wexler HM, Finegold SM. 2002. Anaerobic bacteriology manual, 6th ed. Star Publishing Company, Belmont, CA [Google Scholar]
- 2.Carlier JP. 2002. The metabolic end-products—a rapid identification tool for common anaerobic bacteria isolated in clinical microbiology. Recent Res. Dev. Microbiol. 6:157–175 [Google Scholar]
- 3.Lindström M, Korkeala H. 2006. Laboratory diagnosis of botulism. Clin. Microbiol. Rev. 19:298–314. 10.1128/CMR.19.2.298-314.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knapp O, Maier E, Mkaddem SB, Benz R, Bens M, Chenal A, Geny B, Vandewalle A, Popoff MR. 2010. Clostridium septicum alpha-toxin forms pores and induces rapid cell necrosis. Toxicon 55:61–72. 10.1016/j.toxicon.2009.06.037 [DOI] [PubMed] [Google Scholar]
- 5.Carlier JP, K'Ouas G, Bonne I, Lozniewski A, Mory F. 2004. Oribacterium sinus gen. nov., sp. nov., within the family 'Lachnospiraceae' (phylum Firmicutes). Int. J. Syst. Evol. Microbiol. 54:1611–1615. 10.1099/ijs.0.63060-0 [DOI] [PubMed] [Google Scholar]
- 6.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402. 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasaki Y, Kojima A, Aoki H, Ogikubo Y, Takikawa N, Tamura Y. 2002. Phylogenetic analysis and PCR detection of Clostridium chauvoei, Clostridium haemolyticum, Clostridium novyi types A and B, and Clostridium septicum based on the flagellin gene. Vet. Microbiol. 86:257–267. 10.1016/S0378-1135(02)00002-0 [DOI] [PubMed] [Google Scholar]
- 8.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406–425 [DOI] [PubMed] [Google Scholar]
- 10.Kimura M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111–120. 10.1007/BF01731581 [DOI] [PubMed] [Google Scholar]
- 11.Kumar S, Tamura K, Jakobsen IB, Nei M. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244–1245. 10.1093/bioinformatics/17.12.1244 [DOI] [PubMed] [Google Scholar]
- 12.Collins MD, East AK. 1998. Phylogeny and taxonomy of the food-borne pathogen Clostridium botulinum and its neurotoxins. J. Appl. Microbiol. 84:5–17. 10.1046/j.1365-2672.1997.00313.x [DOI] [PubMed] [Google Scholar]
- 13.Alouf JE. 2000. Cholesterol-binding cytolytic protein toxins. Int. J. Med. Microbiol. 290:351–356. 10.1016/S1438-4221(00)80039-9 [DOI] [PubMed] [Google Scholar]
- 14.Collins MD, Lawson PA, Willems A, Cordoba JJ, Fernandez-Garayzabal J, Garcia P, Cai J, Hippe H, Farrow JA. 1994. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int. J. Syst. Bacteriol. 44:812–826. 10.1099/00207713-44-4-812 [DOI] [PubMed] [Google Scholar]
- 15.Nakamura K, Kohda T, Umeda K, Yamamoto H, Mukamoto M, Kozaki S. 2010. Characterization of the D/C mosaic neurotoxin produced by Clostridium botulinum associated with bovine botulism in Japan. Vet. Microbiol. 140:147–154. 10.1016/j.vetmic.2009.07.023 [DOI] [PubMed] [Google Scholar]
- 16.Skarin H, Hafstrom T, Westerberg J, Segerman B. 2011. Clostridium botulinum group III: a group with dual identity shaped by plasmids, phages and mobile elements. BMC Genomics 12:185. 10.1186/1471-2164-12-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fatmawati NN, Sakaguchi Y, Suzuki T, Oda M, Shimizu K, Yamamoto Y, Sakurai J, Matsushita O, Oguma K. 2013. Phospholipase C produced by Clostridium botulinum types C and D: comparison of gene, enzymatic, and biological activities with those of Clostridium perfringens alpha-toxin. Acta Med. Okayama 67:9–18 http://escholarship.lib.okayama-u.ac.jp/amo/ [DOI] [PubMed] [Google Scholar]
- 18.Popoff MR, Hauser D, Boquet P, Eklund MW, Gill DM. 1991. Characterization of the C3 gene of Clostridium botulinum types C and D and its expression in Escherichia coli. Infect. Immun. 59:3673–3679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez DJ, Lee SW, Hensler ME, Markley AL, Dahesh S, Mitchell DA, Bandeira N, Nizet V, Dixon JE, Dorrestein PC. 2010. Clostridiolysin S, a post-translationally modified biotoxin from Clostridium botulinum. J. Biol. Chem. 285:28220–28228. 10.1074/jbc.M110.118554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brook I. 2010. The role of anaerobic bacteria in bacteremia. Anaerobe 16:183–189. 10.1016/j.anaerobe.2009.12.001 [DOI] [PubMed] [Google Scholar]
- 21.Rechner PM, Agger WA, Mruz K, Cogbill TH. 2001. Clinical features of clostridial bacteremia: a review from a rural area. Clin. Infect. Dis. 33:349–353. 10.1086/321883 [DOI] [PubMed] [Google Scholar]
- 22.Abdulla A, Yee L. 2000. The clinical spectrum of Clostridium sordellii bacteraemia: two case reports and a review of the literature. J. Clin. Pathol. 53:709–712. 10.1136/jcp.53.9.709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Libby DB, Bearman G. 2009. Bacteremia due to Clostridium difficile—review of the literature. Int. J. Infect. Dis. 13:e305–e309. 10.1016/j.ijid.2009.01.014 [DOI] [PubMed] [Google Scholar]
- 24.Hallit RR, Afridi M, Sison R, Salem E, Boghossian J, Slim J. 2013. Clostridium tetani bacteraemia. J. Med. Microbiol. 62:155–156. 10.1099/jmm.0.044941-0 [DOI] [PubMed] [Google Scholar]
- 25.Schade RP, Van Rijn M, Timmers HJ, Dofferhoff AS, Klaassen CH, Meis JF. 2006. Clostridium cadaveris bacteraemia: two cases and review. Scand. J. Infect. Dis. 38:59–62. 10.1080/00365540500388792 [DOI] [PubMed] [Google Scholar]
- 26.Vanderhofstadt M, Andre M, Lonchay C, Levecque P, Holemans X, Canon JL, D'Hondt L. 2010. Clostridium tertium bacteremia: contamination or true pathogen? A report of two cases and a review of the literature. Int. J. Infect. Dis. 14(Suppl 3):e335–e337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams OM, Brazier J, Peraino V, Goldstein EJ. 2010. A review of three cases of Clostridium aldenense bacteremia. Anaerobe 16:475–477. 10.1016/j.anaerobe.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 28.Poulain B, Popoff MR, Molgo J. 2008. How do the botulinum neurotoxins block neurotransmitter release: from botulism to the molecular mechanism of action. Botulinum J. 1:14–87. 10.1504/TBJ.2008.018951 [DOI] [Google Scholar]
- 29.Montecucco C, Rossetto O, Popoff MR. 2006. Neurotoxigenic clostridia, p 679–697 In Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E. (ed), The prokaryotes, vol 4 Springer, New York, NY [Google Scholar]
- 30.Eklund MW, Poysky FT. 1974. Interconversion of type C and D strains of Clostridium botulinum by specific bacteriophages. Appl. Microbiol. 27:251–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eklund MW, Poysky FT, Peterson ME, Meyers JA. 1976. Relationship of bacteriophage to alpha toxin production in Clostridium novyi types A and B. Infect. Immun. 14:798–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eklund MW, Poysky FT, Reed SM, Smith CA. 1971. Bacteriophage and the toxigenicity of Clostridium botulinum type C. Science 172:480–482. 10.1126/science.172.3982.480 [DOI] [PubMed] [Google Scholar]
- 33.Sakaguchi Y, Hayashi T, Kurokawa K, Nakayama K, Oshima K, Fujinaga Y, Ohnishi M, Ohtsubo E, Hattori M, Oguma K. 2005. The genome sequence of Clostridium botulinum type C neurotoxin-converting phage and the molecular mechanisms of unstable lysogeny. Proc. Natl. Acad. Sci. U. S. A. 102:17472–17477. 10.1073/pnas.0505503102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schallehn G, Eklund MW, Brandis H. 1980. Phage conversion of Clostridium novyi type A. Zentralbl. Bakteriol. 247:95–100 [PubMed] [Google Scholar]
- 35.Sakaguchi Y, Hayashi T, Yamamoto Y, Nakayama K, Zhang K, Ma S, Arimitsu H, Oguma K. 2009. Molecular analysis of an extrachromosomal element containing the C2 toxin gene discovered in Clostridium botulinum type C. J. Bacteriol. 191:3282–3291. 10.1128/JB.01797-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamakawa K, Karasawa T, Kakinuma H, Maruyama H, Takahashi H, Nakamura S. 1997. Emergence of Clostridium botulinum type B-like nontoxigenic organisms in a patient with type B infant botulism. J. Clin. Microbiol. 35:2163–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oguma K, Yokota K, Hayashi S, Takeshi K, Kumagai M, Itoh N, Tachi N, Chiba S. 1990. Infant botulism due to Clostridium botulinum type C toxin. Lancet 336:1449–1450 [DOI] [PubMed] [Google Scholar]
- 38.Skarin H, Segerman B. 2011. Horizontal gene transfer of toxin genes in Clostridium botulinum: involvement of mobile elements and plasmids. Mob. Genet. Elements 1:213–215. 10.4161/mge.1.3.17617 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.