Abstract
Hematopoietic stem cells (HSCs) give rise to progenitors with potential to produce multiple cell types, including dendritic cells (DCs). DCs are the principal antigen-presenting cells and represent the crucial link between innate and adaptive immune responses. Bluetongue virus (BTV), an economically important Orbivirus of the Reoviridae family, causes a hemorrhagic disease mainly in sheep and occasionally in other species of ruminants. BTV is transmitted between its mammalian hosts by certain species of biting midges (Culicoides spp.) and is a potent alpha interferon (IFN-α) inducer. In the present report, we show that BTV infects cells of hematopoietic origin but not HSCs in immunocompetent sheep. However, BTV infects HSCs in the absence of type I IFN (IFN-I) signaling in vitro and in vivo. Infection of HSCs in vitro results in cellular death by apoptosis. Furthermore, BTV infects bone marrow-derived DCs (BM-DCs), interfering with their development to mature DCs in the absence of type I IFN signaling. Costimulatory molecules CD80 and CD86 and costimulatory molecules CD40 and major histocompatibility complex class II (MHC-II) are affected by BTV infection, suggesting that BTV interferes with DC antigen-presenting capacity. In vivo, different DC populations are also affected during the course of infection, probably as a result of a direct effect of BTV replication in DCs and the production of infectious virus. These new findings suggest that BTV infection of HSCs and DCs can impair the immune response, leading to persistence or animal death, and that this relies on IFN-I.
INTRODUCTION
Bluetongue virus (BTV) is the prototype member of the genus Orbivirus, family Reoviridae, that causes a major infectious disease in ruminants transmitted by biting midges (1, 2). The genome consists of 10 segments of double-stranded RNA, encoding 7 structural (VP1 to -7) and 4 nonstructural (NS1 to NS4) proteins (3, 4). Traditionally, bluetongue virus infections have been endemic almost exclusively in temperate and tropical areas. However, since 1998 BTV has been introduced into the Mediterranean area and Northern Europe, having a negative economic impact (5). Symptoms of BT disease result from the damage to small blood vessels and include oral ulceration, facial and pulmonary edema, vascular thrombosis, and necrosis of infected tissues (6). After a BTV-infected insect bites a susceptible host, the virus replicates initially in the draining lymph node and disseminates to secondary organs (7). Conventional dendritic cells (cDCs) have been implicated in virus dissemination from the skin to the lymph nodes (8), contributing to the establishment of viremia, which can persist during long periods in some infected animals, in spite of concurrent specific humoral and cellular immunity (9).
Mammalian innate immunity to viral infection depends critically on a successful type I interferon (IFN-I) response (10). IFN-I consists of 15 subtypes of alpha IFN (IFN-α), 1 subtype of IFN-β, and 1 subtype of IFN-ω, all sharing a common type I IFN receptor (11). IFN-I is produced by almost all virus-infected cells in response to stimulation by intracellular double-stranded RNA (dsRNA), which is detected by the RNA helicases retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (MDA-5) (12–14). In some cell types (mainly macrophages and DCs), type I IFN is produced in response to the triggering of the transmembrane Toll-like receptors (TLR) by exogenous viral RNA or DNA (15). The production of type I IFNs depends on an autocrine feedback mechanism that involves signaling by IFN-I receptor (16). Mice deficient in the functional IFN-I receptor (IFNAR−/−), generated by genetic disruption of IFN receptor 1 (17), are highly susceptible to viral infections because of the absence of the direct viral control effects of IFN-I. The importance of the IFN-I response for restriction of BTV infection is implied by the observations indicating that IFNAR−/− mice exhibit increased viral replication and spread to almost all the organs, causing rapid death (18). Recently, it has been reported that RIG-I and MDA5 contribute to triggering the IFN-I response to BTV in A549 cells (19). Immune cells play a key role in the production of type I IFN in vivo during the infection by other members of the Reoviridae family such as reovirus (20). Similarly, BTV induces IFN-I production in plasmacytoid DCs (pDCs) through signaling of the MyD88 adaptor (21). Thus, hematopoietic cells might play a relevant role in response to BTV infection and IFN-I. In addition, several reports have pointed out that BTV can induce lymphopenia at the peak of infection, leading to immunosuppression, and can increase susceptibility to secondary microbial infection (22–24). One of the mechanisms proposed for BTV-induced immunosuppression and lymphoid depletion in lymph nodes includes lymphocyte apoptosis (22, 25).
The bone marrow (BM) is the primary site of hematopoiesis, a process that relies on hematopoietic stem cells (HSCs) and ultimately gives rise to all the blood cells from myeloid and lymphoid lineages (reviewed in reference 26). Viruses such as parvovirus (27), coxsackievirus B3 (28), and dengue virus (29) have developed different strategies to disrupt BM function. Infection of HSCs is one of the strategies used by viruses to disrupt the hematopoiesis as a means to cause immunosuppression (30). Furthermore, during systemic viral infections, IFN-I from HSCs has been proved to be essential for the control of viral replication and dissemination to different organs (31). Here we have investigated the role of IFN-I in the infection of HSCs by BTV serotype 8 (BTV-8). Our results indicate that BTV-8 infects BM-derived DCs and HSCs from IFNAR−/− mice in vitro and in vivo, reducing the restorative capacity of specific DC subsets in peripheral lymphatic organs.
MATERIALS AND METHODS
Viruses, cells, and infections.
All virus stocks were grown in baby hamster kidney (BHK) cells, and viral titers were determined by plaque assay on Vero cells in semisolid agar medium as previously described (18). BTV-8 (Belgium/06), originally isolated from an infected calf in Belgium in 2006 (32), was used in the experiments. Inactivated BTV was prepared by incubation with freshly prepared 3 mM binary ethyleneimine (BEI) for 24 h at 37°C, and the process was stopped by adding 0.02 M sodium thiosulfate (33). Murine bone marrow cells (BMCs) were incubated for 1 h at 37°C with virus at a multiplicity of infection (MOI) of 0.1 PFU/cell at day 0 of culture, after 6 days (day 6) or after 10 days (day 10) in culture. Thereafter, cells were washed once with phosphate-buffered saline (PBS) and resuspended in complete RPMI 1640 medium supplemented with 200 U/ml of recombinant mouse granulocyte-macrophage colony-stimulating factor (rmGM-CSF) and cultivated as described below. Similarly, ovine BMCs were prepared as described below and infected with BTV-8 at MOI 0.1 PFU/cell for 1 h at 37°C. Cells were washed once with PBS and put in culture in complete medium supplemented with ovine GM-CSF for 7 days. Ovine splenocytes and thymocytes were isolated by mechanical disruption of the spleen and thymus, respectively, and maintained in complete RPMI medium supplemented with 10% T-STIM medium (BD). Cells were infected with BTV-8 at MOI of 0. 1 PFU/cell.
Mice and inoculations.
C57BL/6 mice were purchased from Harlan Interfauna Ibérica S.L. IFN-α/β Ro/o IFNAR−/− mice, on a C57BL/6 genetic background, were generously provided by R. Zinkernagel (Institute of Experimental Medicine, Zurich, Switzerland). Animals were maintained under pathogen-free conditions and allowed to acclimatize to the biosafety level 3 (BSL3) animal facilities at the Centro de Investigación en Sanidad Animal (INIA, Madrid, Spain) for 1 week before being used in the experiments. Mice were infected at 8 to 10 weeks of age by intravenous inoculation of 10 PFU/ml of BTV-8 and were examined for clinical symptoms daily. Whole blood was collected in EDTA from all animals at regular intervals after inoculation. At 2, 4, and 6 days postinfection (dpi), mice were sacrificed. All experiments with live animals were performed under the guidelines of the European Community (86/609) and were approved by the site ethical review committee at the Centro de Investigación en Sanidad Animal (CISA-INIA).
Generation and culture of ovine BM-DCs.
Twelve-month-old sheep of the Spanish Alacarreña breed were used in this study. Animals were maintained in the biosafety level 3 (BSL3) animal facilities at the Centro de Investigación en Sanidad Animal (INIA, Madrid, Spain) and were euthanized in accordance with the guidelines of the European Community (86/609) as approved by the site ethical review committee at the Centro de Investigación en Sanidad Animal (CISA-INIA). Bone marrow was prepared from the sternum after longitudinal sectioning in two halves and scraping of the red marrow. BM-DCs were prepared according to the method described in reference 34. Briefly, BMCs were released by peeling the marrow and, after lysis of red blood cells with ammonium chloride, resuspended in complete RPMI 1640 (Invitrogen GmbH, Eggenstein, Germany) supplemented with 10% fetal calf serum (FCS), l-glutamine (Sigma-Aldrich) (2 mM), HEPES (Sigma-Aldrich) (5 mM), and 2-mercaptoethanol (Sigma-Aldrich) (50 μM) and cultured in a 100-mm-diameter petri dish at 37°C in 5% CO2 for 7 days. RPMI medium was supplemented with 20 ng/ml of ovine recombinant GM-CSF (BioSource). Fresh medium containing the same amount of cytokine was added every 3 days. BMCs were incubated for final activation with 1 μg/ml lipopolysaccharide (LPS; Sigma-Aldrich) during 16 h.
Generation and culture of murine BM-DCs.
BMCs were isolated as previously described (35). Briefly, BMCs were obtained by flushing tibias and femurs with PBS. The BMCs were resuspended in ammonium chloride to lyse red blood cells and then mixed with RPMI 1640 (Invitrogen GmbH, Eggenstein, Germany) supplemented with 10% FCS, l-glutamine (Sigma-Aldrich) (2 mM), HEPES (Sigma-Aldrich) (5 mM), and 2-mercaptoethanol (Sigma-Aldrich) (50 μM). Cells were seeded at a dose of 2 × 106 cells per 100-mm-diameter dish in 10 ml of culture medium containing 200 U/ml of rmGM-CSF (Peprotech). After every 3 days of culture, half of the medium was carefully removed and replaced with medium supplemented with rmGM-CSF. To drive BMCs to complete maturation, nonadherent cells were collected at day 10 of culture and resuspended in 10 ml of culture medium containing 100 U/ml of rmGM-CSF and LPS (Sigma-Aldrich) at 1 μg/ml.
Flow cytometric analysis.
BM-DCs were pelleted by centrifugation and resuspended in staining buffer (PBS containing 2% [vol/vol] FCS and 0.02% [wt/vol] NaN3) for flow cytometry acquisition. Expression of cell surface molecules on murine DCs was quantified using the following monoclonal antibodies (MAbs): CD11c-phycoerythrin (PE), CD11b-allophycocyanin (APC), I-A–PE, CD80-PE, CD86-APC, B220-APC, CD8-peridinin chlorophyll protein (PerCP), CD4-fluorescein isothiocyanate (FITC), and CD40-FITC (BD Pharmingen). For ovine DCs, the following antibodies were used: anti-CD11c from VMRD, anti-mouse IgG1–R-phycoerythrin (PE) (BD Biosciences), FITC-conjugated anti-major histocompatibility complex class II (MHC-II) DQ/DR (Serotec), and A647-conjugated CD14 (Serotec). Cells were then washed twice in staining buffer and fixed in PBS–1% FCS–4% paraformaldehyde (PFA) (wt/vol). Cells were acquired using a FACSCalibur flow cytometer (Becton, Dickinson, Franklin Lakes, NJ). Dead cells were excluded on the basis of forward and side light scatter. Data were analyzed with FlowJo (Tree Star Inc., San Francisco, CA).
Determination of cell viability and apoptosis.
Viability and apoptosis in BM-DCs were determined using an annexin V-PE apoptosis detection kit from Pharmingen (BD Pharmingen) according to the manufacturer's instructions. Briefly, cells were centrifuged and resuspended in 100 μl of binding buffer in the presence of 5 μl of annexin V-PE and 5 μl of 7-aminoactinomycin D (7-AAD). After 15 min incubation at room temperature, 400 μl of binding buffer was added, and cells were analyzed by flow cytometry using a FACSCalibur flow cytometer (Becton, Dickinson, Franklin Lakes, NJ) and CellQuest Pro software.
Statistical analyses.
Data handling, analyses, and graphic representations were done using Prism 2.01 (GraphPad Software Inc.). Statistical differences were determined using a Student t test or one-way analysis of variance (ANOVA) (P < 0.05).
RESULTS
BTV infects ovine thymus and spleen cells but not HSCs.
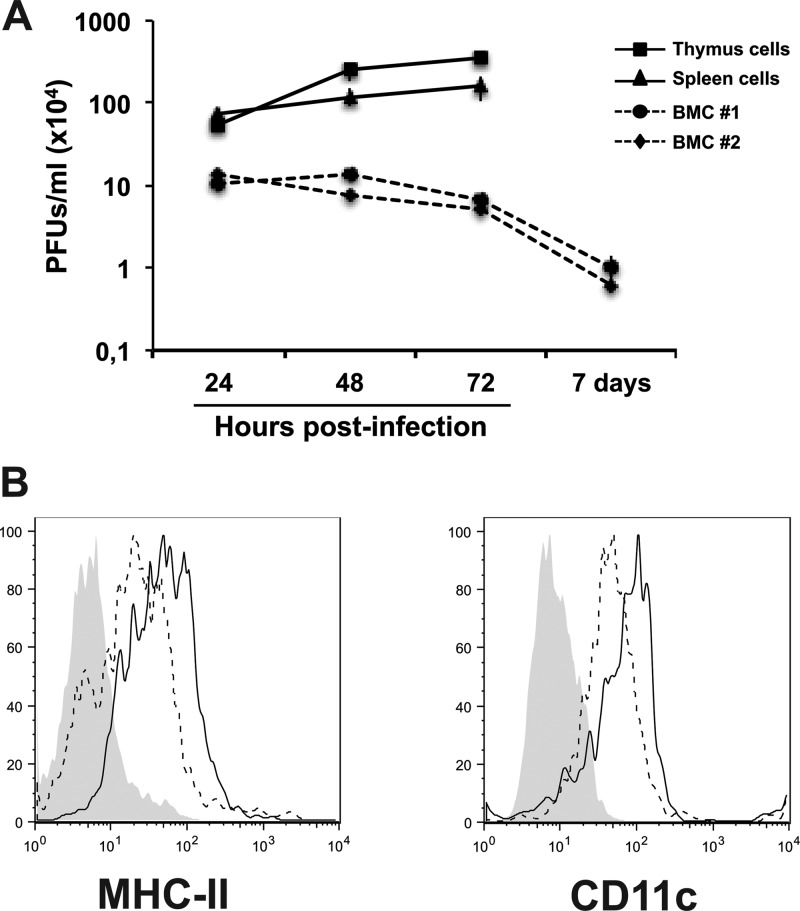
To investigate the susceptibility of hematopoietic cells to BTV infection, ovine BMCs (OvBMCs) were obtained from the sternum and were cultured in OvGM-CSF as indicated in Materials and Methods. OvBMCs were infected with BTV-8 at MOI of 0.1 PFU/cell and kept in culture with OvGM-CSF for 8 additional days. In parallel, ovine cells of hematopoietic origin such as cells from spleen and thymus (SSC and STC, respectively) were isolated and infected with BTV-8 at MOI of 0.1 PFU/cell. Viral production was determined by plaque assay in Vero cells at different times postinfection. BTV replicated productively in SSC and STC cells but failed to replicate in OvBMCs (Fig. 1A). Thus, we can conclude that BTV-8 infects cells of hematopoietic origin but not HSCs.
FIG 1.
BTV infects ovine splenocytes and thimocytes but not ovine HSCs. (A) Ovine BMCs were infected with BTV-8 at MOI of 0.1 PFU/cell. In parallel, cells isolated from ovine spleen and thymus were infected. The viral load in the supernatants of BMCs and spleen/thymus cells from 24 h to 7 days postinfection is shown as determined by plaque assay in Vero cells. (B) Histograms show the results of one representative experiment analyzing the expression of cell surface MHC-II and CD11c molecules on OvBM-DCs after 8 days in culture with GM-CSF. Shaded histograms indicate background staining with isotype control antibodies, black-lined histograms represent BTV-infected cultures, and dashed histograms represent mock-infected cultures.
To determine whether DC development was affected by BTV attachment/internalization without viral replication, we looked for OvBM-DC morphology by microscopy and found that BTV-infected OvBM-DCs showed morphology similar or identical to mock-infected OvBM-DC morphology (data not shown), indicating that the addition of BTV to OvBMCs did not affect their development into mature OvBM-DCs. Furthermore, we measured the expression of MHC-II and CD11c molecules by flow cytometry on mature OvBM-DCs after 7 days in culture with OvGM-CSF. Interestingly, the pattern of expression of MHC-II was upregulated on BTV-infected OvBM-DCs compared with mock-infected OvBM-DCs (mean fluorescence intensity [MFI] of mock-infected BM-DCs, 47.8 ± 2.3; MFI of BTV-infected BM-DCs, 85.3 ± 5.5) (statistically significant by Student t test, P < 0.0001) (Fig. 1B), suggesting that BTV induce maturation of OvBM-DCs in the absence of viral replication due to BTV binding to a cellular receptor or other secreted factors that may induce OvBM-DC maturation.
HSCs and immature BM-derived DCs are highly susceptible to BTV infection in the absence of IFN-I receptor.
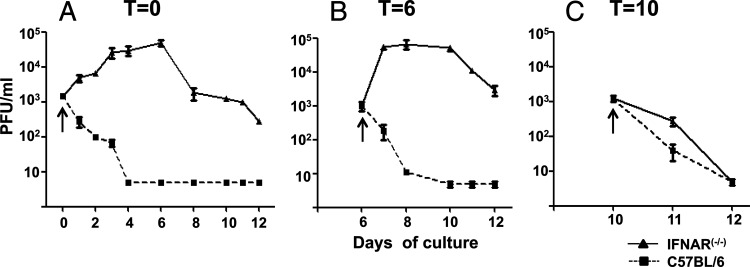
We have previously shown that mice with the IFN-I receptor knocked out (IFNAR−/−) are highly susceptible to BTV infection (18). In sheep, we observed that BTV did not replicate in BMCs but that the virus was able to bypass the IFN-I block and infect cells of hematopoietic origin in spite of their IFN-I competence. Since IFN-I plays an important role in systemic viral infections (31, 36), we investigated whether IFN-I played a role in protecting HSCs from BTV infection. Thus, BMCs were isolated from femurs and tibias of C57BL/6 or IFNAR−/− mice and were cultured in RPMI medium in the presence of 200 U/ml of GM-CSF. First, HSCs (t = 0) were infected with BTV-8 at an MOI of 0.1 PFU/cell and grown during 11 days in the presence of GM-CSF, and the amount of virus produced was measured in supernatants by titration on Vero cells. Supernatants from IFNAR−/− HSCs showed increased viral production starting at day 1 postculture to reach a maximum of a 2-log10-fold increase by day 6 (Fig. 2A). At this time point, the viral production started to decline, mainly due to the loss of viable cells in the culture. In contrast, the viral titers decreased in C57BL/6 mice by day 1 postculture and were at undetectable levels by day 4 postculture (Fig. 2A) (5 PFU/ml, the detection limit of the assay), indicating that BTV are able to replicate in HSCs from IFNAR−/− mice but not in those from wild-type mice.
FIG 2.
BTV replicates in HSCs and immature BM-DCs in the absence of IFN-I receptor. Supernatants of HSC and BM-DC cultures from IFNAR−/− or C57BL/6 mice were taken at different times post-BTV infection, and viral titer was determined by plaque assay in Vero cells as indicated in Materials and Methods. (A) HSCs were infected with BTV-8 at MOI of 0.01 PFU/cell (t = 0). Supernatants were taken at days 1, 2, 3, 4, 6, 8, 10, 11, and 12 postculture. (B) BM-DC cultures were infected at day 6 postculture (immature phenotype) with BTV-8 at MOI of 0.01 PFU/cell (t = 6). Supernatants were taken at postculture days 7 (24 hpi), 8 (48 hpi), 10 (4 dpi), 11 (5 dpi), and 12 (6 dpi). (C) Mature BM-DC cultures, after 10 days in culture, were infected with BTV at MOI of 0.01 PFU/cell (t = 10). Supernatants were taken at day 11 postculture (24 hpi) and day 12 postculture (48 hpi). Each arrow indicates the viral input in each infection. The data are representative of the results of 4 independent experiments.
To establish whether BTV-8 could infect at multiple stages along the developmental pathway of BM-DCs and whether this infection relied on IFN-I, the same experimental design was used but cells were previously cultured with GM-CSF during 6 days and then infected (t = 6). At day 6, BM-DCs showed an immature phenotype according to CD11c/MHC-II staining results (data not shown). Thus, immature C57BL/6 or IFNAR−/− BM-DCs were infected with BTV-8 (MOI of 0.01 PFU/cell) and viral production was quantified by plaque assay on Vero cells. Supernatants from C57BL/6 BM-DCs did not show any viral production whereas IFNAR−/− BM-DCs showed a 2-log increase at 2 dpi (Fig. 2B), suggesting that BTV-8 is capable of replicating on immature IFNAR−/− BM-DCs. An experiment of the same design was performed using BM-DCs from C57BL/6 or IFNAR−/− mice at day 10 postculture and infected on that day (t = 10) in which BM-DCs showed a mature phenotype according to CD11c/MHC-II staining results (data not shown). Neither C57BL/6 nor IFNAR−/− mature BM-DCs were infected by BTV-8 as indicated by the reduction in virus titer detected on the supernatants of these cells after 24 hpi (Fig. 2C). Although cells start to die after 24 h of addition of LPS, cells were kept for additional 24 h (48 hpi) in order to determine whether viral replication was detected at later times postinfection. Viral replication was not detected either at 24 hpi or at 48 hpi in mature BM-DCs from IFNAR−/− or C57BL/6 mice infected with BTV. Thus, mature BM-DCs were not susceptible to BTV-8 infection independently of the expression of IFN-I receptor.
BTV replication interferes with BM-DC development.
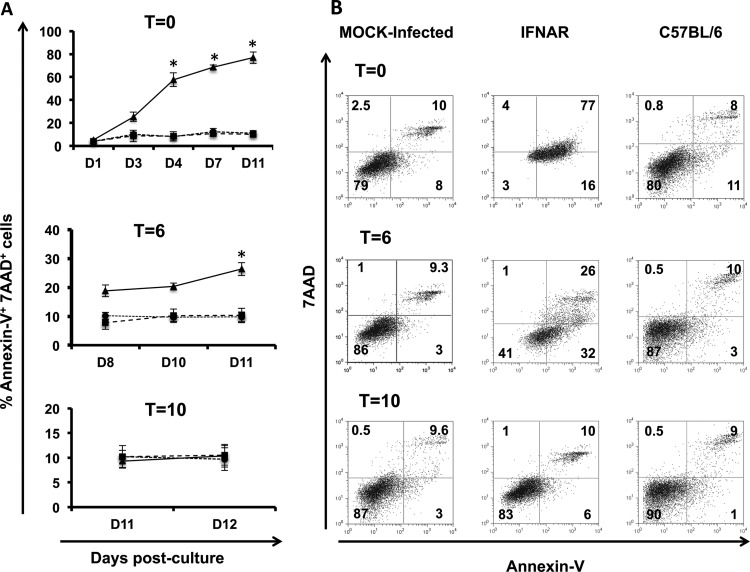
In order to determine whether viral infection had any effect on cell differentiation or surface expression of proteins required for T-cell stimulation, we next analyzed the effect(s) of BTV-8 replication on cell maturation and expression of costimulatory molecules CD80 and CD86 (CD80/86), CD40, and MHC class II. C57BL/6 or IFNAR−/− BM cells infected in vitro with BTV-8 at days 0, 6, and 10 postdevelopment (t = 0, t = 6, and t = 10) were cultured in the presence of GM-CSF until day 10 and brought to maturation by addition of LPS for an additional 24 h. Initially, we studied the expression of CD11c and CD11b to determine if BTV-infected BM-DCs acquired a mature phenotype. Mock-infected cultures (IFNAR−/− and C57BL/6) showed an increase in size and granularity after LPS treatment (as determined by forward and side scatter) and were CD11c+ CD11b+ (data not shown). These data indicated that the lack of signaling mediated by IFN-I was not affecting the development of BM-DCs from HSCs in IFNAR−/− mice. BTV-infected BM-DCs from IFNAR−/− mice at day 0 did not show an increase in size after the addition of LPS; furthermore, their forward-to-side-scatter light profile suggested dying cells (data not shown). Morphologically, these cells showed rapid cell shrinkage and increased cell granularity, which are changes often associated with apoptosis. Therefore, we monitored cell apoptosis by 7-AAD and annexin V staining during the development of BM-DCs after infection (Fig. 3A). There were statistically significant (Student t test, P < 0.05 [infected IFNAR−/− BM-DCs versus wild-type BM-DCS]) increases in the numbers of late apoptotic cells (annexin V+ and 7-AAD+) in the BTV-infected IFNAR−/− BM-DCs at t = 0 starting at day 4 postmaturation and t = 6 at day 11 postmaturation. Those infected at a later stage of maturation (t = 10) did not display a significant increase in apoptosis. All these results confirmed that BTV infection caused the apoptosis of infected immature BM-DCs and HSCs that lack IFN-I receptor signaling.
FIG 3.
BTV induces apoptosis in BM-DCs from IFNAR−/− mice. (A) Percentages of annexin V+ and 7-AAD+ BM-DCs during development from HSC to mature BM-DCs of cells infected with BTV at t = 0, t = 6, and t = 10. BM-DCs infected with BTV are indicated by black lines and triangles, mock-infected cells are indicated by dashed lines and squares, and BM-DCs from C57BL/6 mice infected with BTV are indicated by dotted lines and circles. Asterisks indicate that the difference between BTV-infected and mock-infected cells is statistically significant (P < 0.01 unpaired Student t test). (B) Dot plots show staining for annexin V and 7-AAD on mature BM-DCs (10 days postculture). Numbers indicate the percentage of cells per quadrant: 7-AAD+ annexin V− (dead cells); 7-AAD− annexin V− (live cells); 7-AAD− annexin V+ (early apoptotic cells); and 7-AAD+ annexin V+ (late apoptotic cells).
On the basis of these results, we analyzed the expression of costimulatory molecules and of MHC class II only on mock- and BTV-infected BM-DCs at day 6 (t = 6) and 10 (t = 10) from IFNAR−/− and C57BL/6 mice after incubation with LPS because most BTV-infected BM-DCs at day 0 were dead at the time of maturation. As we have shown before, IFNAR−/− BM-DCs were not productively infected with BTV at day 10 postmaturation. However, the binding of BTV to the cellular receptor might have an effect on DC function. Thus, we have included BM-DCs infected at t = 10 in these experiments. The results showed an increase in the expression of costimulatory molecules in IFNAR−/− BM-DCs infected at day 10 (t = 10) similar to that seen with mock-infected IFNAR−/− BM-DCs (Fig. 4). Meanwhile, IFNAR−/− BM-DCs infected at day 6 postmaturation did not upregulate the expression of CD80, CD86, and MHC-II, and CD40 expression remained as high as on immature DCs, suggesting that BTV infection inhibits the maturation of BM-DCs. In contrast, BTV-infected BM-DCs from C57BL/6 mice showed upregulation of CD80, CD86, CD40, and MHC-II to levels similar to those seen with mock-infected BM-DCs (data not shown). Thus, infection of immature but not mature BM-DCs from IFNAR−/− mice interfered with the expression of molecules required for T-cell stimulation.
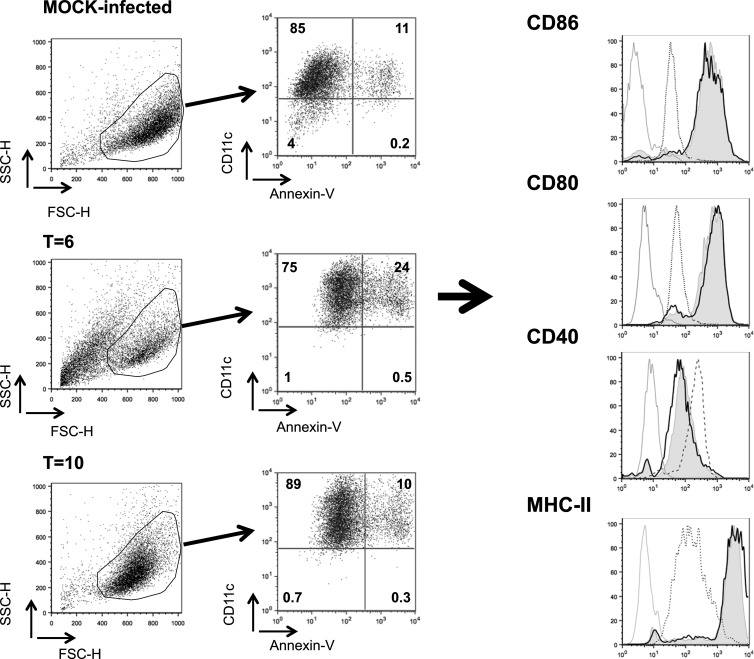
FIG 4.
BTV infection of BM-DCs from IFNAR−/− mice interferes with their expression of stimulatory surface molecules. Dot plots show profiles of mature IFNAR−/− BM-DCs at day 10 postculture in mock-infected cultures, BTV-infected BM-DCs at day 6 postculture (t = 6), and BTV-infected BM-DCs at day 10 postculture (t = 10). Live cells have been gated and stained for CD11c and annexin V. Determination of the expression of costimulatory molecules and MHC-II has been done on live cells (CD11c+, annexin V−) as indicated by the arrows. Histograms show the expression of cell surface molecules (CD86, CD80, CD40, and MHC-II) on BM-DCs from C57BL/6 or IFNAR−/− mice for mock-infected cells (black lines), BTV-infected cells at day 6 (t = 6, dashed lines), and BTV-infected cells at day 10 (t = 10, shaded histograms). The gray lines indicate background staining with isotype control antibodies. Note that BTV-infected BM-DCs at t = 0 from IFNAR−/− mice are not shown since all cells were dead after 10 days in culture.
To determine if viral replication was necessary for this observed interference in BM-DC development, BEI-inactivated BTV was used to infect BM-DCs from IFNAR−/− mice at days 0, 6, and 10 postdifferentiation. These cultures underwent an increase in size and granularity as well as upregulation of CD80/86 and MHC-II similar to mock-infected controls (data not shown), indicating that virus replication is needed to trigger the impairment of BM-DC development.
HSCs from IFNAR−/− mice are susceptible to BTV infection in vivo.
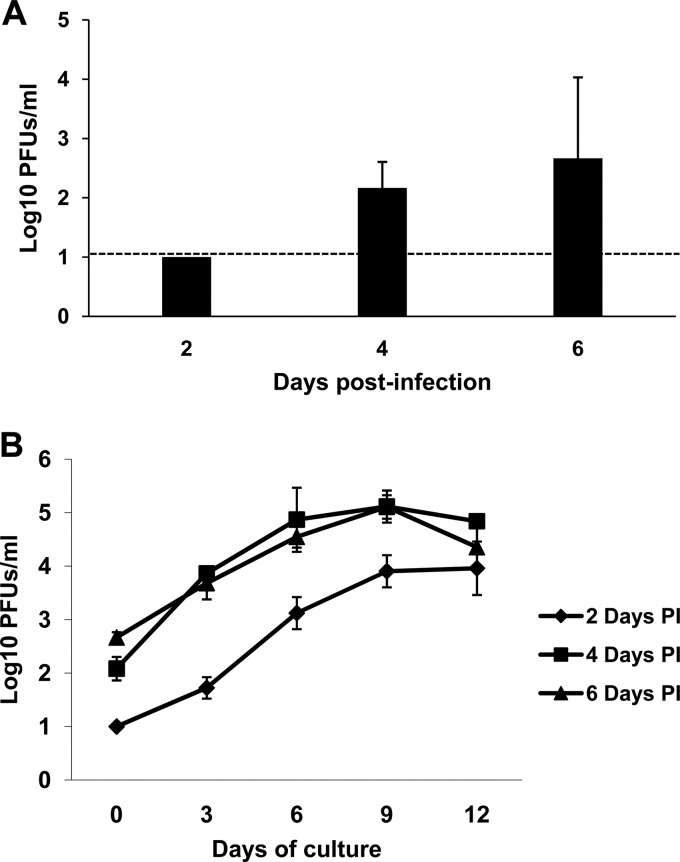
The in vitro data indicated that BTV is able to replicate in HSCs and immature DCs in the absence of type I IFN receptor signaling. To determine whether HSCs were infected in vivo, 24 IFNAR−/− mice and 24 C57BL/6 mice were inoculated with 10 PFU of BTV-8 and 8 mice per day were sacrificed at 2, 4, and 6 dpi. Six animals were used as mock-infected controls. All IFNAR−/− mice developed clinical signs characterized by ocular discharges and apathy starting at 48 hpi. Meanwhile, C57BL/6 did not show any symptoms, as previously described (18). HSCs were obtained from femurs and tibias of individual mice, and 2 × 106 cells were placed in a 100-mm-diameter dish with medium containing GM-CSF (as described in Materials and Methods). After 2 h of culture, supernatants were taken to quantify viral production as determined by plaque assay in susceptible Vero cells. Infectious virus was recovered from supernatants of HSCs from IFNAR−/− mice sacrificed at 4 and 6 dpi but not from those sacrificed at 2 dpi (Fig. 5A). No infectious virus from C57BL/6 mice was detected at any time postinfection (data not shown). Additionally, supernatants were taken at different times postdifferentiation to determine if HSCs from infected mice maintained in culture during 10 days in the presence of GM-CSF and kept for an additional 24 h with LPS were producing virus. All HSCs from IFNAR−/− mice sacrificed at 4 or 6 dpi showed an increase in viral titers at up to day 9 of culture (Fig. 5B). Interestingly, although HSCs from mice sacrificed at 2 dpi had no detectable virus after 2 h, at day 3 of culture the amount of virus increased 3-log10-fold (Fig. 5B). No viral production was detected at any time point in C57BL/6 mice infected with BTV (data not shown). These data suggest that BTV replicates in HSC in vivo in the absence of type I IFN receptor signaling.
FIG 5.
BTV infects HSCs in vivo in IFNAR−/− mice. (A) HSCs isolated from BTV-infected IFNAR−/− mice at 2, 4, and 6 dpi were kept in culture during 2 h, and viral production in the supernatants was tested in plaque assay on Vero cells according to the method described in Materials and Methods. The dashed line indicates the detection limit of the plaque assay. (B) HSCs isolated from BTV-infected IFNAR−/− mice at 2, 4, and 6 dpi were maintained in culture during 10 days in GM-CSF and stimulated the last 24 h with LPS (see Materials and Methods). Viral production in the supernatants during these 10 days of culture was evaluated by plaque assay on Vero cells.
HSCs from BTV-infected IFNAR−/− mice do not develop to mature DCs.
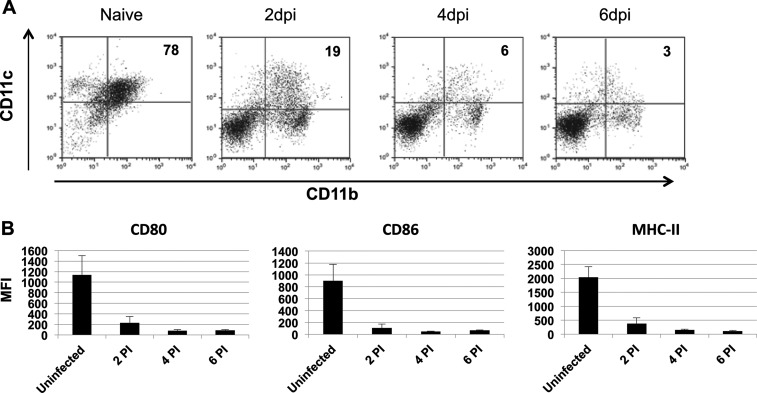
Based on the previous data showing that BTV infected HSCs from IFNAR−/− mice in vivo, we next analyzed whether these HSCs developed to mature BM-DCs. Thus, HSCs were obtained from BTV-infected IFNAR−/− or C57BL/6 mice at 2, 4, and 6 dpi and were cultured in GM-CSF for 10 days. The expression of CD11c and CD11b was assessed by flow cytometry after treatment with LPS to induce complete maturation at day 11 postculture (Fig. 6A). Cultures from naive IFNAR−/− HSCs started with 7% CD11c+ cells and showed a dramatic increase in the percentage of CD11c+ cells (up to 80%) after LPS treatment. In stark contrast, HSC cultures from BTV-infected IFNAR−/− mice did not increase the amount of CD11c+ cells (Fig. 6A). This lack of increase in the percentage of CD11c+ cells was not observed in cultures from BTV-infected C57BL/6 mice (data not shown). To expand upon this observation and define the impact that BTV has on the development of BM-DCs, we evaluated the expression of MHC-II and costimulatory molecules CD80 and CD86 by flow cytometry on live CD11c+ cells after 11 days in culture as explained above. Compared with BM-DCs from naive IFNAR−/− mice, BM-DCs derived from BTV-infected IFNAR−/− mice at day 2, 4, and 6 postinfection showed a significant reduction in MHC-II, CD80, and CD86 expression (Fig. 6B). Thus, these results indicated that HSC from BTV-infected IFNAR−/− mice were not able to differentiate into mature BM-DCs in vitro.
FIG 6.
BTV infection of HSC in vivo impairs BM-DC development in vitro. HSCs isolated from BTV-infected IFNAR−/− mice at 2, 4, and 6 dpi were cultured in GM-CSF during 10 days and kept an additional 24 h in culture with LPS. (A) Dot plots show double staining for CD11c and CD11b molecules on mature BM-DCs from naive or BTV-infected IFNAR−/− mice. The numbers in the upper right quadrant indicate the percentage of doubly positive CD11c and CD11b cells. (B) The MFI of surface molecules expressed on CD11c+ CD11b+ BM-DCs after LPS treatment is displayed. Each bar represents the MFI of a given surface molecule on CD11c+ CD11b+ BM-DCs from uninfected or BTV-infected mice HSCs isolated at 2, 4, and 6 dpi. Data are presented as means ± standard deviations (SD).
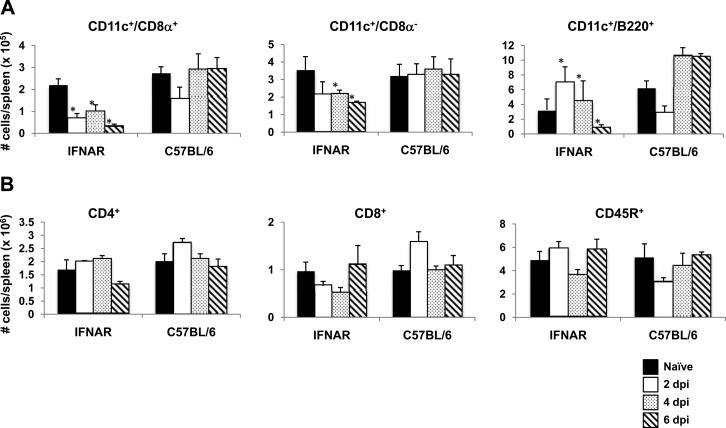
Given that HSCs from BTV-infected IFNAR−/− mice did not develop into mature DCs in vitro, the generation of distinct DC subsets in vivo from HSCs may also be compromised during BTV infection. To address this issue, spleens from BTV-infected IFNAR−/− mice were obtained at 2, 4, and 6 dpi and DC subsets were analyzed by flow cytometry (Fig. 7). CD11c+ CD8α+ and CD11c+ CD8α− DC population numbers decreased in the spleen of BTV-infected IFNAR−/− mice compared to noninfected mice. In contrast, as the infection progressed in C57BL/6 mice (4 and 6 dpi), the number of CD11c+ CD8α+ DCs in the spleen increased. CD11c+ CD8α− DC numbers did not appear altered in C57BL/6 mice. A different pattern for plasmacytoid DCs (CD11c+ B220+) was observed in BTV-infected IFNAR−/− mice, in which they increased their number at early times postinfection but declined in number sharply before animal death occurred (6 dpi) (Fig. 7A). To determine whether the differentiation pathway from HSC of lymphoid lineage was also compromised due to BTV infection, CD8+, CD4+, and CD45R+ populations were analyzed by flow cytometry through the sample period. None of these populations were shown to be depleted due to BTV infection (Fig. 7B); in fact, IFNAR−/− mice as well as C57BL/6 mice showed similar profiles in the numbers of cells. Thus, DC subsets are altered in vivo during BTV infection in the absence of type I IFN receptor.
FIG 7.
BTV-infected IFNAR−/− mice do not expand DCs in response to viral infection. (A) Splenic DCs were isolated from BTV-infected IFNAR−/− and C57BL/6 mice at 2, 4, and 6 dpi. (B) The numbers of the different lymphocyte populations in the spleen of BTV-infected IFNAR−/− and C57BL/6 mice were measured at 2, 4, and 6 dpi. Each bar indicates the number of cells in each population per spleen. Data are presented as means ± SD. Statistical differences denoted by asterisks were determined using a Student test (P < 0.05).
DISCUSSION
This report presents evidence that type I IFN is required to control BTV infection of BM hematopoietic cells. In the absence of type I IFN receptor, BM progenitors are infected not only in vitro but also in vivo. Interestingly, BTV infects BM progenitors in a restricted fashion, leading to a significant decrease in numbers of spleen DCs in IFNAR−/− mice, particularly in the myeloid DC subsets (CD11c+ CD8α+ and CD11c+ CD8α−). All these data indicate that a major effect of type I IFNs produced in BM cells is to induce antiviral proteins, resulting in limiting replication or absence of BTV in the BM compartment and, consequently, control of systemic viral dissemination. In addition, this control of viral replication exerted by type I IFN in BM progenitors might explain the different durations of viremia caused by BTV in individual animals. Thus, those animals that may have the IFN-I route compromised due to environmental factors or other asymptomatic infections might not control BTV replication in the HSC compartment, resulting in higher viremia and lack of BTV control.
We report that the expression of IFN-I receptor confers protection against BTV infection in BM-derived cells. First, we present data from in vitro experiments in which HSCs were infected by BTV-8 in the absence of IFN-I receptor, resulting in impairments in BM-DC development. Second, our in vivo data support the idea that HSC targeting by BTV in IFNAR−/− mice results in these cells failing to develop properly into DCs, suggesting that IFN-I is mediating protection of HSCs from BTV infection. The contribution of IFN-I produced in HSCs to viral protection has also been studied in other mouse models. Infection of mice with reovirus T1L, another member of the Reoviridae family, led to fatal disease when BM cells were not able to respond to the IFN-I they produced (20). Similarly, IFN-I secreted by HSCs seems to be essential to control systemic viral infections such as those by mouse hepatitis virus (MHV) or vesicular stomatitis virus (VSV) (31).
One possible hypothesis regarding the mechanism for suppression of HSC development is that disruption of hematopoiesis occurs only when HSCs are infected at a particular differentiation stage. Since we are generating in vitro BM-derived DCs in the presence of GM-CSF (see Materials and Methods), restricting our study to myeloid precursors, we have approached this issue by infecting BM cells (HSCs) and BM-derived DCs at different developmental stages. First, we have shown that the infection of BM cells from IFNAR−/− mice by BTV-8 in vitro interferes significantly with CD11c+ DC development, showing a reduced cell surface expression of proteins involved in antigen presentation (MHC class II) and costimulation (CD80 and CD86 molecules). The expression of these molecules is associated with maturation of DCs and is required for the generation of an effective immune response. Thus, the lack of costimulation via CD80 and/or CD86 may impair the development of immune responses by inducing T cell anergy or apoptosis instead of activation (37). The reduction in the expression of MHC class II molecules is also likely to have a profound effect on the priming and development of cellular immune responses. The disruption in DC development is observed only when the infection is established at early time points during culture. When BTV is added at day 0 (HSCs) or at an intermediate DC maturation stage (t = 6), DC development is impaired, but when the virus is added at 10 days postmaturation (t = 10), the development remains intact. Although we have not detected viral infection when the virus is added at day 10 either in DCs from IFNAR−/− mice or in those from C57BL/6 mice, we cannot discard the possibility that these cells may be infected but require more than 48 h to complete a replication cycle. In fact, this may be the case, as such a finding has been described in ovine DCs, where BTV infects mature DCs and promotes cell survival, suggesting that the virus dissemination is coupled to DC (8). Therefore, BTV replication in the absence of type I IFN response occurs mainly in early hematopoietic precursors, leading to apoptosis and/or lack of maturation of myeloid progenitors. Interestingly, BM cells from C57BL/6 mice, with an intact IFN-I receptor, are not infected by BTV and DC development is not affected. Moreover, addition of inactivated BTV to BM-DC cultures of IFNAR−/− mice did not have any effect on DC differentiation, suggesting that viral replication is required to interfere with DC development.
Interestingly, HSCs are also infected by BTV in vivo in the absence of IFN-I receptor. Thus, HSCs isolated from BTV-infected IFNAR−/− mice, and grown with GM-CSF, do not develop into mature DCs in vitro. At day 2 postinfection, when the viral infection of HSCs is undetectable, viral particles appear in culture only at later times postmaturation (day 4) (D4; Fig. 4). This suggests that HSCs may be among the primary targets of BTV in vivo and that this compartment is partially protected from viral infection by the IFN-I it produces in response to viral infection. Nonetheless, once the virus has infected HSCs, the detrimental effect mainly affects the myeloid progenitor subset, as observed in vitro. Accordingly, we have observed that myeloid DC populations are depleted in spleens of BTV-infected IFNAR−/− mice whereas CD8+, CD4+, and CD45R+ cell populations expand similarly to those of BTV-infected C57BL/6 mice. Other viruses with immunosuppressive capacity have been reported to disrupt hematopoiesis (27, 30, 38). Various studies have shown that BTV induces a transient immunosuppression with a predisposition to secondary microbial infections (7, 22, 39). Therefore, our new findings in which BTV targets HSCs suggest that this phenomenon is likely to contribute to the transient immunosuppression observed in the pathogenesis of BTV at early time points of infection. In turn, the absence or failure of response to type I IFN may lead to animal death.
Finally, the protective role of IFN-I against BTV infection of HSCs has also been demonstrated in ovine BM cells. Ovine BM cells with an intact IFN-I receptor are not infected by BTV; neither is their fate compromised by BTV replication. Actually, we have found that BTV is not able to replicate in ovine BM cells, probably due to the antiviral action of IFN-I. Therefore, the presented results suggest that as long as the IFN-I response remains intact, BTV is unlikely to infect HSCs productively, resulting in no apparent deleterious effect on hematopoiesis. However, our results demonstrate that a small failure (congenital or due to other concomitant infections) in the IFN-I response has detrimental effects in the animals, resulting in defective antigen presentation, severe immunosuppression, and, ultimately, animal death.
ACKNOWLEDGMENTS
This work was supported by grants AGL2009-07353 from Ministerio de Ciencia e Innovación (MCINN) and 228394-NADIR-Integrating Activities 7th EU Program. T.R.-C. was supported by a contract from Comunidad Autónoma de Madrid. V.M. was supported by a Ramon y Cajal contract (RyC2010-06516).
We thank Félix Valcárcel for providing sheep bone marrows and Alí Alejo for critical reading of the manuscript.
Footnotes
Published ahead of print 30 October 2013
REFERENCES
- 1.Schwartz-Cornil I, Mertens PP, Contreras V, Hemati B, Pascale F, Breard E, Mellor PS, MacLachlan NJ, Zientara S. 2008. Bluetongue virus: virology, pathogenesis and immunity. Vet. Res. 39:46. 10.1051/vetres:2008023 [DOI] [PubMed] [Google Scholar]
- 2.Mellor PS, Baylis M, Mertens PP. 2009. Bluetongue. Academic Press, London, United Kingdom [Google Scholar]
- 3.Roy P. 2005. Bluetongue virus proteins and particles and their role in virus entry, assembly, and release. Adv. Virus Res. 64:69–123. 10.1016/S0065-3527(05)64004-3 [DOI] [PubMed] [Google Scholar]
- 4.Ratinier M, Caporale M, Golder M, Franzoni G, Allan K, Nunes SF, Armezzani A, Bayoumy A, Rixon F, Shaw A, Palmarini M. 2011. Identification and characterization of a novel non-structural protein of bluetongue virus. PLoS Pathog. 7:e1002477. 10.1371/journal.ppat.1002477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maclachlan NJ, Guthrie AJ. 2010. Re-emergence of bluetongue, African horse sickness, and other orbivirus diseases. Vet. Res. 41:35. 10.1051/vetres/2010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacLachlan NJ. 1994. The pathogenesis and immunology of bluetongue virus infection of ruminants. Comp. Immunol. Microbiol. Infect. Dis. 17:197–206. 10.1016/0147-9571(94)90043-4 [DOI] [PubMed] [Google Scholar]
- 7.Barratt-Boyes SM, MacLachlan NJ. 1994. Dynamics of viral spread in bluetongue virus infected calves. Vet. Microbiol. 40:361–371. 10.1016/0378-1135(94)90123-6 [DOI] [PubMed] [Google Scholar]
- 8.Hemati B, Contreras V, Urien C, Bonneau M, Takamatsu HH, Mertens PP, Breard E, Sailleau C, Zientara S, Schwartz-Cornil I. 2009. Bluetongue virus targets conventional dendritic cells in skin lymph. J. Virol. 83:8789–8799. 10.1128/JVI.00626-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeggo MH, Wardley RC. 1982. Generation of cross-reactive cytotoxic T lymphocytes following immunization of mice with various bluetongue virus types. Immunology 45:629–635 [PMC free article] [PubMed] [Google Scholar]
- 10.Randall RE, Goodbourn S. 2008. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 89:1–47. 10.1099/vir.0.83391-0 [DOI] [PubMed] [Google Scholar]
- 11.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. 2005. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu. Rev. Immunol. 23:307–336. 10.1146/annurev.immunol.23.021704.115843 [DOI] [PubMed] [Google Scholar]
- 12.Kato H, Sato S, Yoneyama M, Yamamoto M, Uematsu S, Matsui K, Tsujimura T, Takeda K, Fujita T, Takeuchi O, Akira S. 2005. Cell type-specific involvement of RIG-I in antiviral response. Immunity 23:19–28. 10.1016/j.immuni.2005.04.010 [DOI] [PubMed] [Google Scholar]
- 13.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101–105. 10.1038/nature04734 [DOI] [PubMed] [Google Scholar]
- 14.Loo YM, Fornek J, Crochet N, Bajwa G, Perwitasari O, Martinez-Sobrido L, Akira S, Gill MA, Garcia-Sastre A, Katze MG, Gale M., Jr 2008. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J. Virol. 82:335–345. 10.1128/JVI.01080-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawai T, Akira S. 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11:373–384. 10.1038/ni.1863 [DOI] [PubMed] [Google Scholar]
- 16.Clarke CJ, Trapani JA, Johnstone RW. 2001. Mechanisms of interferon mediated anti-viral resistance. Curr. Drug Targets Immune Endocr. Metabol. Disord. 1:117–130. 10.2174/1568008013341361 [DOI] [PubMed] [Google Scholar]
- 17.Müller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918–1921. 10.1126/science.8009221 [DOI] [PubMed] [Google Scholar]
- 18.Calvo-Pinilla E, Rodriguez-Calvo T, Anguita J, Sevilla N, Ortego J. 2009. Establishment of a bluetongue virus infection model in mice that are deficient in the alpha/beta interferon receptor. PLoS One 4:e5171. 10.1371/journal.pone.0005171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chauveau E, Doceul V, Lara E, Adam M, Breard E, Sailleau C, Viarouge C, Desprat A, Meyer G, Schwartz-Cornil I, Ruscanu S, Charley B, Zientara S, Vitour D. 2012. Sensing and control of bluetongue virus infection in epithelial cells via RIG-I and MDA5 helicases. J. Virol. 86:11789–11799. 10.1128/JVI.00430-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson C, Wetzel JD, He J, Mikacenic C, Dermody TS, Kelsall BL. 2007. Type I interferons produced by hematopoietic cells protect mice against lethal infection by mammalian reovirus. J. Exp. Med. 204:1349–1358. 10.1084/jem.20061587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruscanu S, Pascale F, Bourge M, Hemati B, Elhmouzi-Younes J, Urien C, Bonneau M, Takamatsu H, Hope J, Mertens P, Meyer G, Stewart M, Roy P, Meurs EF, Dabo S, Zientara S, Breard E, Sailleau C, Chauveau E, Vitour D, Charley B, Schwartz-Cornil I. 2012. The double-stranded RNA bluetongue virus induces type I interferon in plasmacytoid dendritic cells via a MYD88-dependent TLR7/8-independent signaling pathway. J. Virol. 86:5817–5828. 10.1128/JVI.06716-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umeshappa CS, Singh KP, Nanjundappa RH, Pandey AB. 2010. Apoptosis and immuno-suppression in sheep infected with bluetongue virus serotype-23. Vet. Microbiol. 144:310–318. 10.1016/j.vetmic.2010.02.033 [DOI] [PubMed] [Google Scholar]
- 23.Odeón AC, Schore CE, Osburn BI. 1997. The role of cell-mediated immunity in the pathogenesis of bluetongue virus serotype 11 in the experimental infection of vaccine/sensitized calves. Comp. Immunol. Microbiol. Infect. Dis. 20:219–231. 10.1016/S0147-9571(97)00004-0 [DOI] [PubMed] [Google Scholar]
- 24.Quist CF, Howerth EW, Bounous DI, Stallknecht DE. 1997. Cell-mediated immune response and IL-2 production in white-tailed deer experimentally infected with hemorrhagic disease viruses. Vet. Immunol. Immunopathol. 56:283–297. 10.1016/S0165-2427(96)05747-9 [DOI] [PubMed] [Google Scholar]
- 25.Owens RJ, Limn C, Roy P. 2004. Role of an arbovirus nonstructural protein in cellular pathogenesis and virus release. J. Virol. 78:6649–6656. 10.1128/JVI.78.12.6649-6656.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mercier FE, Ragu C, Scadden DT. 2012. The bone marrow at the crossroads of blood and immunity. Nat. Rev. Immunol. 12:49–60. 10.1038/nri3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segovia JC, Guenechea G, Gallego JM, Almendral JM, Bueren JA. 2003. Parvovirus infection suppresses long-term repopulating hematopoietic stem cells. J. Virol. 77:8495–8503. 10.1128/JVI.77.15.8495-8503.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Althof N, Whitton JL. 2013. Coxsackievirus b3 infects the bone marrow and diminishes the restorative capacity of erythroid and lymphoid progenitors. J. Virol. 87:2823–2834. 10.1128/JVI.03004-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.La Russa VF, Innis BL. 1995. Mechanisms of dengue virus-induced bone marrow suppression. Baillieres Clin. Haematol. 8:249–270. 10.1016/S0950-3536(05)80240-9 [DOI] [PubMed] [Google Scholar]
- 30.Manchester M, Smith KA, Eto DS, Perkin HB, Torbett BE. 2002. Targeting and hematopoietic suppression of human CD34+ cells by measles virus. J. Virol. 76:6636–6642. 10.1128/JVI.76.13.6636-6642.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lang PA, Cervantes-Barragan L, Verschoor A, Navarini AA, Recher M, Pellegrini M, Flatz L, Bergthaler A, Honda K, Ludewig B, Ohashi PS, Lang KS. 2009. Hematopoietic cell-derived interferon controls viral replication and virus-induced disease. Blood 113:1045–1052. 10.1182/blood-2007-10-117861 [DOI] [PubMed] [Google Scholar]
- 32.Elbers AR, Backx A, Meroc E, Gerbier G, Staubach C, Hendrickx G, van der Spek A, Mintiens K. 2008. Field observations during the bluetongue serotype 8 epidemic in 2006. I. Detection of first outbreaks and clinical signs in sheep and cattle in Belgium, France and the Netherlands. Prev. Vet. Med. 87:21–30. 10.1016/j.prevetmed.2008.06.004 [DOI] [PubMed] [Google Scholar]
- 33.Rojas JM, Rodriguez-Calvo T, Pena L, Sevilla N. 2011. T cell responses to bluetongue virus are directed against multiple and identical CD4+ and CD8+ T cell epitopes from the VP7 core protein in mouse and sheep. Vaccine 29:6848–6857. 10.1016/j.vaccine.2011.07.061 [DOI] [PubMed] [Google Scholar]
- 34.Foulon E, Foucras G. 2008. Two populations of ovine bone marrow-derived dendritic cells can be generated with recombinant GM-CSF and separated on CD11b expression. J. Immunol. Methods 339:1–10. 10.1016/j.jim.2008.07.012 [DOI] [PubMed] [Google Scholar]
- 35.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223:77–92. 10.1016/S0022-1759(98)00204-X [DOI] [PubMed] [Google Scholar]
- 36.Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M, Schreiber RD, de la Torre JC, Oldstone MB. 2013. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science 340:207–211. 10.1126/science.1235214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Gool SW, Vermeiren J, Rafiq K, Lorr K, de Boer M, Ceuppens JL. 1999. Blocking CD40 - CD154 and CD80/CD86 - CD28 interactions during primary allogeneic stimulation results in T cell anergy and high IL-10 production. Eur. J. Immunol. 29:2367–2375. [DOI] [PubMed] [Google Scholar]
- 38.Moses AV, Williams S, Heneveld ML, Strussenberg J, Rarick M, Loveless M, Bagby G, Nelson JA. 1996. Human immunodeficiency virus infection of bone marrow endothelium reduces induction of stromal hematopoietic growth factors. Blood 87:919–925 [PubMed] [Google Scholar]
- 39.Barratt-Boyes SM, Rossitto PV, Stott JL, MacLachlan NJ. 1992. Flow cytometric analysis of in vitro bluetongue virus infection of bovine blood mononuclear cells. J. Gen. Virol. 73(Pt 8):1953–1960. 10.1099/0022-1317-73-8-1953 [DOI] [PubMed] [Google Scholar]