Abstract
CD8+ T cell responses are thought to play an important role during HIV infection, particularly in HIV controllers (HIC) in whom viral replication is spontaneously controlled without any treatment. We have demonstrated that CD8+ T cells from these subjects are able to suppress viral replication in vitro. In parallel, HIV-specific CD8+ responses were shown to be strong and of high quality, with proliferative abilities and cytotoxic capacities, in HIC. The HLA-B*57 allele, which is associated with a better clinical outcome in HIV infection, is overrepresented in HIC. However, we showed that these patients constitute a heterogeneous group that includes subjects who present weak suppression of viral replication in vitro and HIV-specific responses. We performed an extensive study of 101 HIC (49 HLA-B*57+ and 52 HLA-B*57−) to determine the impact of HLA-B*57 on the HIV-specific CD8+ response. The HLA-B*57-restricted response displayed better qualitative features, such as higher functional avidity, higher proliferation capacity, and a higher level of cytokine production, than responses not restricted by HLA-B*57. However, the highest frequencies of HIV-specific CD8+ T cells were observed only in a subset of HLA-B*57+ subjects. They were tightly associated with the ability to suppress viral replication in vitro. In contrast, the subset of HLA-B*57+ subjects with a weak ability to suppress viral replication had significantly lower ultrasensitive viral loads than all the other groups of controllers. In conclusion, both HLA-B*57 and the amount of ultrasensitive viral load seem to play a role in HIV-specific CD8+ T cell responses in HIC.
INTRODUCTION
Despite the lack of eradication of HIV, it is well documented that CD8+ T cells play a major role in controlling viral replication, and this has been shown in HIV-infected individuals (1–3) as well as in simian models of infection (4, 5) and at different periods during the course of infection (6, 7). This is particularly the case in the subset of patients who spontaneously control infection in the absence of treatment for very long periods and who have been referred to as HIV controllers (HIC) or elite controllers (8, 9). The role of CD8+ T cells in these patients was first suggested by an overrepresentation of patients bearing specific major histocompatibility complex class I (MHC-class I) alleles, such as HLA-B*57, HLA-B*27, or a few other alleles (10–14), all described as protective. We and others have further shown a powerful antiviral activity of HIV-specific CD8+ T cells from HIC which are able ex vivo to kill virally infected autologous CD4+ T cells and therefore suppress viral replication (15, 16). However, this CD8+ T cell viral suppression activity is not present in all HIC (17). Most studies have been performed with HLA-B*57+ or HLA-B*27+ patients, since they represent a large part of HIC and the HIV-specific CD8+ T cell responses restricted by these alleles are well documented. However, only a minority of HLA-B*57+ or HLA-B*27+ patients become HIC when infected, even though plasma HIV RNA levels are lower in these patients than in those who do not bear protective HLA alleles (18–20). In addition, 15% to 70% of HIC in the different cohorts are not HLA-B*57+ (14, 21–23), and in the Agence Nationale de Recherche sur le SIDA (ANRS), French National Agency for Research on AIDS, cohort of controllers, 43% of HIC patients are HLA-B*57− and HLA-B*27− subjects.
To gain greater insight into the role of HLA-B*57 in the HIV-specific CD8+ response in HIC and the heterogeneity of this response, we conducted an extensive study of the HIV-specific CD8+ T cell response in a large cohort of HIC (the French ANRS CODEX cohort). We compared the responses observed in HLA-B*57+ and HLA-B*57− individuals as well as the responses restricted or not restricted by HLA-B*57 for activation and differentiation markers, proliferation potential, and cytokine production as well as for HIV-suppressive capacity. In addition, as a group control, we studied HLA-B*57-restricted responses in a group of HLA-B*57+ chronic progressors.
MATERIALS AND METHODS
Study population.
With their informed consent, we collected samples from 121 HIC enrolled in the multicenter CODEX cohort of the ANRS. Inclusion criteria were (i) known infection for more than 5 years, (ii) viral load of <400 copies/ml for at least the last five consecutive measurements, and (iii) never having had any antiretroviral treatment.
In this study, we focused on CD8+ T cell responses in HLA-B*57+ and HLA-B*57− HIC. Of the 121 subjects studied, 55 (45%) were HLA-B*57+ and 20 (17%) HLA-B*27+. Six subjects were both HLA-B*57+ and HLA-B*27+. Fifty-nine subjects were women, 59 were men, and 2 were transsexuals; we had no documentation on the gender of 1 subject. At inclusion, the median age was 47 years (interquartile range [IQR], 41 to 52 years), and the subjects had been infected for a median of at least 13 years (IQR, 9 to 16 years). Seventy-two subjects presented undetectable viral loads (the threshold was from 10 to 50 copies/ml) at inclusion; the median number of copies of HIV RNA per milliliter of plasma was 74 (IQR, 32 to 164 copies/ml) for the others, and the median CD4+ T cell count was 723 cells/μl (IQR, 529 to 946 cells/μl). All data presented are from samples collected at inclusion in the CODEX cohort.
We also studied nine HLA-B*57+ untreated chronic progressors from the ANRS Co-6 PRIMO cohort (eight men and one woman with a median age of 32 years; IQR, 31 to 38 years). The median number of copies per milliliter of plasma HIV RNA was 3.85 log10 (IQR, 3.44 to 4.39 log10 copies/ml), and the median CD4+ T cell count was 670 cells/μl (IQR, 517 to 828 cells/μl). The subjects had been untreated since primary HIV infection, and the median duration of infection was 42 months (IQR, 24 to 48 months).
Cells.
Peripheral blood mononuclear cells (PBMC) were isolated from EDTA-anticoagulated blood by Ficoll density gradient centrifugation and stored in liquid nitrogen. Human leukocyte antigen typing was done with the complement-dependent microlymphocytotoxic technique (InGen [International Genetic Technologies]).
In vitro HIV suppression assay.
The method used to assess the capacity of CD8+ T cells to suppress HIV-1 infection of autologous CD4+ T cells ex vivo is described in detail in reference 24. Briefly, CD4+ and CD8+ T cells were isolated from freshly purified PBMC by, respectively, positive and negative magnetic-bead sorting (Stemcell Technologies). CD4+ T cells were activated with phytohemagglutinin (PHA; 1 mg/ml) and interleukin-2 (IL-2; 100 IU/ml) for 3 days. They were then infected in vitro with HIV-1 BaL using a spinoculation protocol (24) and cultured alone or cocultured with autologous CD8+ T cells in a 1:1 ratio. Nonsuperinfected CD4+ T cells were also cultured in parallel to assess replication of autologous virus. Viral replication was measured by p24 production in culture supernatants as determined by enzyme-linked immunosorbent assay (ELISA; ZeptoMetrix). The index of in vitro superinfection for each experiment was determined by comparing the peak level of p24 in culture supernatants of PHA-activated CD4+ T cells infected in vitro with HIV-1 BaL with that in culture supernatants of PHA-activated CD4+ T cells not exposed to HIV-1 BaL. The capacity of CD8+ T cells to suppress HIV infection was calculated as the log drop in p24 production when superinfected CD4+ T cells were cultured in the presence of CD8+ T cells. Experiments were conducted in triplicate with cells from each patient.
IFN-γ ELISpot assay and functional avidity.
Gamma interferon (IFN-γ) enzyme-linked immunosorbent spot (ELISpot) assays were used to measure specific responses to peptides corresponding to optimal HIV cytotoxic T lymphocyte (CTL) epitopes (National Institutes of Health HIV Molecular Immunology Database; http://www.hiv.lanl.gov/content/immunology/index) according to the subjects' HLA type. Optimal peptides were used at 2 μg/ml in pools according to the HLA allele and the HIV protein and were tested in duplicate. Data were expressed as numbers of spot-forming cells (SFC) per 106 PBMC. Results were considered positive if there were >50 SFC/106 PBMC after subtraction of the mean background obtained with cells alone. Data are presented as the cumulative data observed for all specificities.
Functional avidity was measured in an IFN-γ ELISpot assay using single epitopic peptides with limiting dilutions with concentrations ranging from 10−5 to 10−11 M for each peptide. Functional avidity was defined as the peptide concentration required to achieve 50% of the maximal response (EC50%) and expressed as log EC50%.
Peptide-HLA class I multimers.
HIV-specific CD8+ T cells were identified by using soluble allophycocyanin (APC)-labeled peptide-HLA class I multimers (Proimmune; Beckman Coulter) or dextramer (Immudex) derived from the HIV Gag, Nef, Pol, and Env proteins. The following epitopes were used: HLA-A*0201-restricted peptide ligands SLYNTVATL (Gag 77 to 85) and ILKEPVHGV (Pol 476 to 484), A*0301-restricted peptide ligands RLRPGGKKK (Gag 20 to 28) and QVPLRPMTYK (Nef 73 to 82), A*1101-restricted ligand AVDLSHFLK (Nef 84 to 92), A*2402-restricted peptide ligand RYPLTFGWCY (Nef 134 to 143), B*0702-restricted peptide ligand IPRRIRQGL (Env 848 to 856), B*0801-restricted peptide ligands GEIYKRWII (Gag 259 to 267) and FLKEKGGL (Nef 90 to 97), B*2705-restricted peptide ligand KRWIILGLNK (Gag 263 to 272), and B*5701-restricted peptide ligands ISPRTLNAW (Gag 147 to 155), KAFSPEVIPMF (Gag 162 to 172), TSTLQEQIGW (Gag 240 to 249), QASQDVKNW (Gag 308 to 316), and HTQGYFPDW (Nef 116 to 124).
Flow cytometry.
PBMC were analyzed by 6-color flow cytometry. Samples were first stained with APC-conjugated HLA class I peptide multimers for 20 min at room temperature and then incubated with labeled specific antibodies for 15 min at 4°C. The following antibodies were used to characterize HIV-specific CD8+ T cells: antibodies coupled to fluorescein isothiocyanate (FITC), CCR7, CD27, and CD38 (BD Pharmingen); to phycoerythrin (PE), CD127 (Beckman Coulter) and CD57 (BD Pharmingen); to peridin chlorophyll protein-cyanine 5.5 (PerCP-Cy5.5), CD8 (BD Pharmingen); to PE-cyanine 7 (PE-Cy7), CD45RO and HLA-DR (BD Pharmingen); and to allophycocyanin-H7 (APC-H7), CD3 (BD Pharmingen). Samples were acquired on a BD FACSCanto flow cytometer (Becton, Dickinson) and analyzed with DIVA software (Becton, Dickinson).
CFSE proliferation assay.
PBMC were stained with 0.35 μM carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes) for 10 min at 37°C. After washes, they were cultured for 6 days in RPMI medium containing 10% fetal calf serum and antibiotics, as well as HIV-specific peptides (2 μg/ml). After incubation, the cells were washed and stained as described above (see “Flow cytometry”). A negative control (medium) and a positive-control staphylococcal enterotoxin B (SEB) were included in each experiment. Samples were acquired on a BD FACSCanto flow cytometer (Becton, Dickinson) and analyzed with DIVA software (Becton, Dickinson).
Intracellular cytokine production.
PBMC were stimulated for 15 h in medium containing the relevant optimal HIV peptide (2 μg/ml). After 1 h of stimulation, cytokine secretion was blocked by adding brefeldin A (10 μg/ml; Sigma-Aldrich Chemie). After further incubation, samples were stained using the following antibodies to characterize HIV-specific CD8+ T cells: antibody coupled to FITC, CCR7 (R&D Systems Europe, Lille, France); to PerCP-Cy5.5, CD8 (BD Pharmingen); to PE-Cy7, CD45RO (BD Pharmingen); and to APC-H7, CD3 (BD Pharmingen). To detect intracellular cytokines, we incubated samples with anti-IFN-γ-APC and anti-IL-2-PE antibodies (BD Biosciences) for 30 min at 4°C after incubation with fluorescence-activated cell sorter (FACS) permeabilizing solution (BD Biosciences). A negative control (medium) and a positive-control SEB were included in each experiment. Samples were acquired on a BD FACSCanto flow cytometer (Becton, Dickinson) and analyzed with DIVA software (Becton, Dickinson).
Statistical analysis.
Data were analyzed with GraphPad Prism 5 software (GraphPad Software, Inc.). Qualitative variables (HLA-B*57 frequencies, strong or weak suppressor status) were compared as categorical data using Fisher's exact test or the chi-square test when appropriate. All quantitative variables were analyzed as continuous data with the nonparametric Mann-Whitney U test. Correlations were identified with Spearman's correlation test. P values of ≤0.05 were considered to denote significant differences in multiple comparisons (Fig. 2A and 3 to 6; *, P < 0.05; **, P < 0.01; ***, P < 0.001).
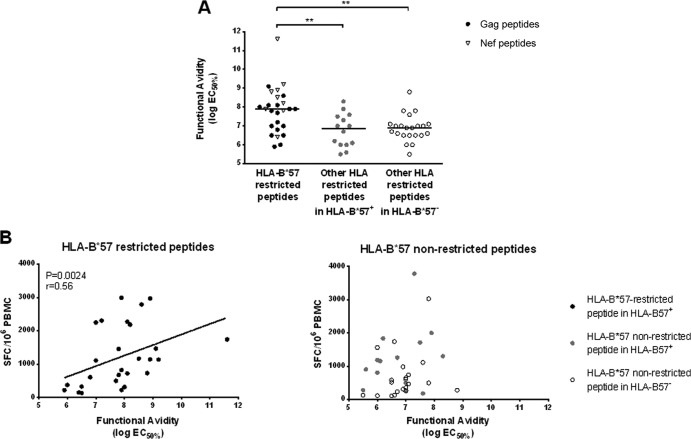
FIG 2.
Functional avidity was determined in an IFN-γ ELISpot assay using peptide dilutions and defined as the peptide concentration required to induce 50% of the maximal response (EC50%). (A) Functional avidity was measured for HLA-B*57-restricted peptides (black circles for peptides derived from Gag protein and gray triangles for peptides derived from Nef protein), HLA-B57-nonrestricted peptides in HLA-B*57+ subjects (gray circles), and HLA-B*57-nonrestricted peptides in HLA-B*57− subjects (clear circles). (B) Functional avidity is correlated with the frequency of HIV-specific CD8+ T cells measured by ELISpot IFN-γ assay for HLA-B*57-restricted peptides (left panel) but not for HLA-B*57-nonrestricted peptides (right panel) whether in HLA-B*57+ (gray circles) or HLA-B*57− subjects (clear circles).
FIG 3.
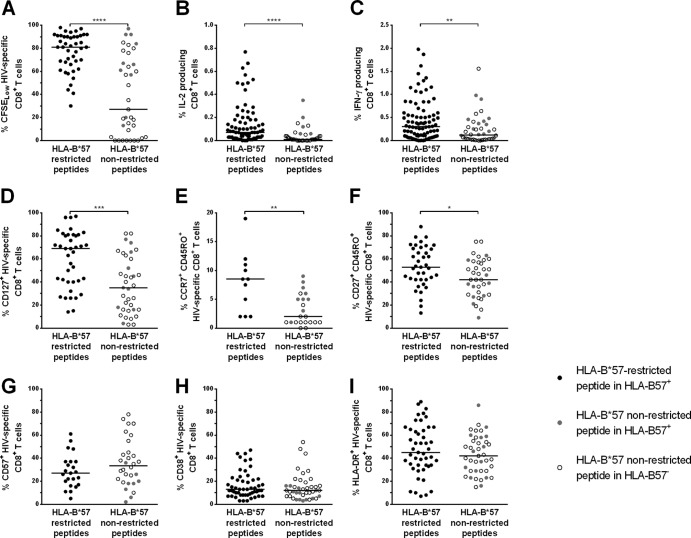
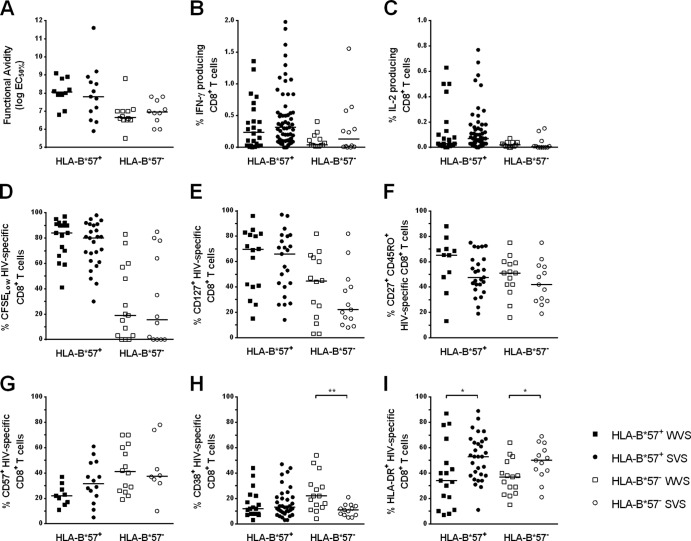
Qualitative features of HIV-specific CD8+ T cell responses restricted by HLA-B*57 (black circles) or nonrestricted by HLA-B*57 (gray circles in HLA-B*57+ subjects and clear circles in HLA-B*57− subjects). (A) Percentages of HIV-specific CD8+ T cells CFSELow after 6 days of culture with the appropriate peptide. (B and C) Percentages of cytokine-producing CD8+ T cells after 15 h of HIV-specific stimulation: IL-2 production (B) and IFN-γ production (C). (D to G) Differentiation phenotype of HIV-specific CD8+ T cells: CD127 expression (D), central memory phenotype (CCR7+ CD45RO+) (E), effector memory phenotype (CD27+ CD45RO+) (F), and expression of the senescence marker CD57 (G). (H and I) Expression of the activation markers CD38 (H) and HLA-DR (I) by HIV-specific CD8+ T cells.
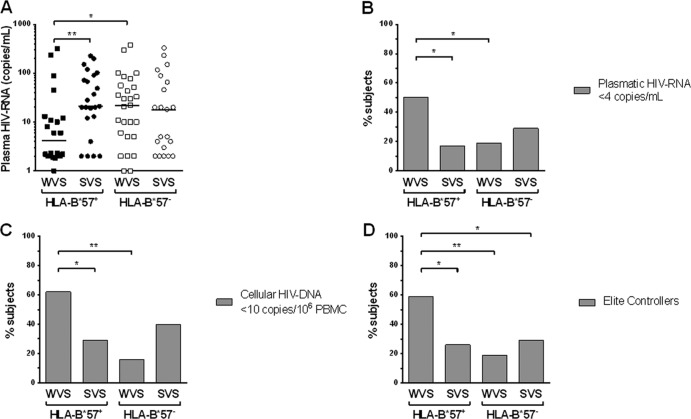
FIG 6.
Virological parameters in HLA-B*57+ WVS (black squares), HLA-B*57+ SVS (black circles), HLA-B*57− WVS (clear squares), and HLA-B*57− SVS (clear circles) were analyzed. (A) Ultrasensitive plasma HIV RNA was measured in the four groups at inclusion. (B to D) Percentages of subjects at inclusion with plasma HIV RNA of <4 copies/ml (B), cellular HIV DNA of <10 copies/106 PBMC (C), and with no viral blip during the clinical follow-up since HIV diagnosis (D) in the four groups.
RESULTS
Study population.
In this study, we focused on HIV-specific CD8+ responses and the role of the HLA-B*57 allele in this response. Accordingly, we excluded the 20 HLA-B*27+ subjects present in the cohort from the analyses, since HLA-B*27, whose frequency is increased in HIV controllers (17% in our series), is also associated with better control of HIV replication (10–13). The immunovirological characteristics of the 49 HLA-B*57+ subjects and 52 HLA-B*57− subjects are presented in Table 1. The 2 groups of patients did not differ in terms of gender, age, CD4 and CD8 counts, and plasma HIV RNA. The frequencies of subjects who never experienced a viral blip (referred to as elite controllers) or with cellular HIV DNA below 10 copies/106 PBMC were also similar for HLA-B*57+ and HLA-B*57− patients. We observed that HLA-B*57+ subjects had a longer follow-up, since the median time between the diagnosis of HIV infection and inclusion in the cohort was 13 years (10 to 17 years) compared to 11 years (7 to 14 years) in HLA-B*57− subjects (P = 0.02), and that they exhibit lower cellular HIV DNA viral load than HLA-B*57− subjects (11 copies/106 PBMC [10 to 27 copies/106 PBMC] versus 20 copies/106 PBMC [10 to 46 copies/106 PBMC]; P = 0.03).
TABLE 1.
Study population
| Characteristic | HLA-B*57+ subjects | HLA-B*57− subjects | P value |
|---|---|---|---|
| No. of subjects | 49 | 52 | |
| Gender (% men) | 52 | 37 | 0.16 |
| Age (yr) | 47 (42–51) | 46 (40–51) | 0.54 |
| Time since diagnosis (yr) | 13 (10–17) | 11 (7–14) | 0.02 |
| CD4/μl | 649 (465–951) | 752 (567–914) | 0.23 |
| % CD4 | 36 (28–47) | 37 (32–43) | 0.78 |
| CD8/μl | 677 (495–1,012) | 740 (586–918) | 0.54 |
| % CD8 | 37 (29–42) | 37 (33–44) | 0.58 |
| CD4/CD8 | 1.0 (0.7–1.4) | 1.0 (0.8–1.3) | 0.83 |
| Plasma RNA (copies/ml) | 12 (2–67) | 20 (4–62) | 0.52 |
| % elite controllersa | 40 | 24 | 0.13 |
| Amt of cellular HIV DNA (copies/106 PBMC) | 11 (10–27) | 20 (10–46) | 0.03 |
| % with <10 copies/106 PBMC of cellular HIV DNA | 43 | 30 | 0.20 |
Elite controllers have always had a viral load of <50 copies/ml at clinical follow-up since HIV diagnosis.
High frequencies of HIV-specific CD8+ T cells in HIV controllers.
HIV-specific CD8+ T cell responses were quantified by means of an IFN-γ ELISpot assay in 98 subjects. Cumulative frequencies of these responses were higher in HLA-B*57+ individuals than in HLA-B*57− ones (3,250 [969 to 7,625] versus 1,308 [115 to 2,331] SFC/106 PBMC, respectively; P = 0.0003) (Fig. 1A). This difference was mainly driven by Gag-specific responses that were higher in the HLA-B*57+ group (1,637 [518 to 4,180] versus 118 [0 to 1,216] SFC/106 PBMC, respectively; P < 0.0001) (Fig. 1B). In contrast, the frequencies of responses directed at Env, Pol, and Nef antigens were comparable in the 2 groups. The median number of peptides tested in HLA-B*57+ and HLA-B*57− subjects was 40 and 38 peptides, respectively, which makes it unlikely that a bias in peptide selection could explain the difference. However, the breadth of the repertoire (evaluated based on the percentages of positive responses among the peptides tested) was higher in the HLA-B*57+ group than in the HLA-B*57− group (frequencies of recognition of 33% [17% to 47%] and 23% [8% to 36%], respectively; P = 0.02). The contribution of HLA-B*57-restricted responses accounted for 80% [50% to 100%] of the HIV-specific CD8+ T cell response in HLA-B*57+ subjects. Most of this response was directed to Gag antigens (60% [44% to 94%]), but substantial responses were also directed to Nef (16% [0% to 40%]); the peptides Gag 147 to 155 and Gag 162 to 172 were the most frequently recognized (76% and 79% of cases, respectively), while Gag 240 to 249, Gag 308 to 316, and Nef 116 to 124 were recognized in nearly half of samples and Env 424 to 432 and Nef 120 to 128 were less frequently recognized (see the upper panel of Fig. S1 in the supplemental material). Responses not restricted by HLA-B*57 were also broad, with comparable frequencies between HLA-B*57+ and HLA-B*57− subjects (Fig. S1, lower panel).
FIG 1.
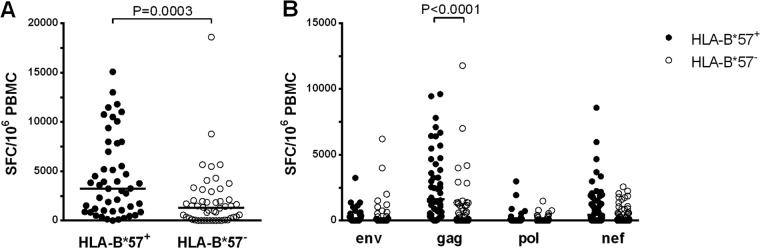
CD8+ T cell responses were measured by IFN-γ ELISpot assay in HLA-B*57+ (black circles) and HLA-B*57− (clear circles) subjects and are expressed as SFC/106 PBMC. (A) Total HIV-specific response. (B) Response directed against Env, Gag, Pol, and Nef proteins. Horizontal bars represent median values.
High functional avidity of HLA-B*57-restricted HIV-specific CD8+ T cells.
Since plasma viral loads were comparable between the two groups of HIC (Table 1), we checked whether the higher frequencies of HIV-specific CD8+ T cell responses could be linked to a higher functional avidity of the T cell receptor (TCR)-peptide interactions previously described in the context of HLA-B*27 and HLA-B*57 molecules (25–27). Indeed, a higher functional avidity would allow strong HIV-specific responses even in the context of small amounts of antigen. To address this hypothesis, we measured the functional avidity of HIV-specific CD8+ T cells in ELISpot assays with limiting dilution of antigenic peptides. Functional avidity was then expressed as the molar concentration of peptide giving 50% of the maximal response and referred to as EC50%. HLA-B*57-restricted responses were 1 log more sensitive than responses not restricted by HLA-B*57, whether the latter were evaluated in HLA-B*57+ or HLA-B*57− subjects: 10−7.9 [7.0–8.5] M for HLA-B*57-restricted responses versus 10−6.9 [6.0–7.5] M for responses not restricted by HLA-B*57 in HLA-B*57+ subjects (P = 0.005) and 10−6.9 [6.5–7.1] M in HLA-B*57− subjects (P = 0.002) (Fig. 2A). We also observed that peptide functional avidity (expressed as EC50%) and HIV-specific CD8+ T cell frequencies were highly correlated (Spearman's correlation, P = 0.0004, r = 0.43), further supporting our hypothesis. Interestingly, this correlation was found when considering HLA-B*57-restricted responses (Spearman's correlation, P = 0.002, r = 0.56), whereas it was not maintained for the responses not restricted by HLA-B*57 (Spearman's correlation, P = 0.23) (Fig. 2B and C). Since HLA-B*57 responses were characterized by a significantly higher functional avidity than responses not restricted by HLA-B*57, we then compared the functional profiles of HIV-specific CD8+ T cells whether restricted or not restricted by HLA-B*57.
Improved functionality of HLA-B*57-restricted HIV-specific CD8+ T cells.
We first evaluated whether the higher avidity of HIV-specific CD8+ T cells could lead to a greater ability of these cells to proliferate. We observed a strong correlation between the functional avidity to a viral peptide (expressed as EC50%) and the percentage of cells proliferating in response to this peptide (assessed by CFSE labeling) (Spearman's correlation, P < 0.0001, r = 0.61). Accordingly, the percentage of proliferating cells was higher for HLA-B*57-restricted responses than for those restricted by other alleles (81% [67% to 91%] versus 27% [3% to 72%], respectively; P < 0.0001) (Fig. 3A). This increased ability to proliferate in vitro could also account for the higher frequencies of HIV-specific CD8+ T cells detected ex vivo, since the proliferation rate and the number of SFC were correlated (Spearman's correlation, P = 0.004, r = 0.32).
To gain further insight into the characteristics of HIV-specific CD8+ T cells, we evaluated several functional and phenotypic features associated with high proliferative rates in the two groups of responses. Proliferation is known to be associated with IL-2 production. Accordingly, IL-2 production by HLA-B*57-restricted T cells was higher than that observed for responses not restricted by HLA-B*57 (0.07% [0.02% to 0.18%] versus 0.01% [0.00% to 0.04%], respectively; P < 0.0001) (Fig. 3B). In addition, proliferative potential and the magnitude of IL-2 production were highly correlated (Spearman's correlation, P < 0.0001, r = 0.58). In the same way, at the peptide level, the frequency of IFN-γ-producing CD8+ T cells was higher for HLA-B*57-restricted peptides than for peptides not restricted by HLA-B*57 (0.30% [0.10% to 0.60%] versus 0.12% [0.03% to 0.36%], respectively; P = 0.002) (Fig. 3C).
Several phenotypic markers associated with the degree of differentiation or senescence are also linked to functional abilities. Cells expressing CD127, the IL-7-Rα chain, are known as memory cells with high proliferating capacities (28, 29). Indeed, HLA-B*57-restricted CD8+ T cells had higher percentages of CD127-expressing cells than T cells not restricted by HLA-B*57 (69% [40% to 81%] and 35% [16% to 62%], respectively; P = 0.0002) (Fig. 3D). Expression of CCR7, CD27, and the RA/RO isoforms of CD45 are other independent markers associated with differentiation. Central memory cells, defined by CCR7 and CD45RO expression, are described as highly functional (30). A very low proportion of HIV-specific CD8+ T cells usually belongs to this subtype (31), and percentages of central memory HIV-specific CD8+ T cells were actually low in the patients studied. However, percentages were significantly higher for HLA-B*57-restricted responses than for responses not restricted by HLA-B*57 (9% [2% to 11%] and 2% [1% to 5%], respectively; P = 0.002) (Fig. 3E). Effector memory cells, which lack CCR7 but express CD27 and CD45RO, are more differentiated than the central memory ones but are considered to be endowed with greater proliferative abilities than the CD27− effector cells. Once again, phenotypic analysis of HIV-specific CD8+ T cells showed higher percentages of this subtype for HLA-B*57-restricted responses than for the other ones (53% [42% to 69%] versus 42% [29% to 58%], respectively; P = 0.016) (Fig. 3F). Finally, CD57 is a marker of senescence and its expression is associated with the lack of proliferative capacities (32). Figure 3G shows a slight difference, although not reaching significance, between HLA-B*57-restricted and nonrestricted responses (27% [17% to 37%] and 34% [20% to 48%], respectively; P = 0.22). Concerning the responses not restricted by HLA-B*57, no differences were ever observed in the phenotypes in HLA-B*57+ or HLA-B*57− patients.
As for the activation markers CD38 and HLA-DR, we confirmed the paradoxical profile of activation on HIV-specific CD8+ T cells that we reported previously (16), with a low level of expression of CD38 contrasting with a high level of expression of HLA-DR. Conversely, we did not observe any difference in the levels of expression of these markers between HLA-B*57-restricted and nonrestricted responses (13% [8% to 22%] and 45% [34% to 65%], 12% [7% to 18%] and 42% [29% to 57%] for CD38 and HLA-DR, in HLA-B*57-restricted and nonrestricted, respectively) (Fig. 3H and I).
Higher functional avidity and improved functionality of HLA-B*57-restricted HIV-specific CD8+ T cells are observed in HIC patients.
In order to evaluate whether the higher avidity and the improved functionality were associated with the HIC status or only with the presence of the HLA-B*57 allele, we analyzed the HLA-B*57-restricted CD8+ T cell responses in nine untreated viremic HLA-B*57+ chronic progressors and compared these responses to those observed in the HLA-B*57+ HIC group. As shown on Fig. S2 in the supplemental material, the functional avidity of HLA-B*57-restricted T cells for their cognate peptides was significantly higher in the HIC group than in the progressors (EC50% = 10−7.9 [7.0–8.5] M in the HIC group versus 10−7.2 [6.2–7.6] M in the progressor group; P = 0.02). However, the cumulative frequencies of HLA-B*57-specific CD8+ T cells were comparable in both groups and no correlation was found between functional avidity and these frequencies in the progressor group (Spearman's correlation, P = 0.84), in contrast to what we observed in the HIC group (Fig. 2B) (Spearman's correlation, P = 0.0004, r = 0.43). The proliferative capacity of HLA-B*57-restricted HIV-specific CD8+ T cells was decreased in the progressor group, the percentage of proliferating cells being 55% (41% to 78%) (compared to 81% [67 to 91%] in the HIC group; P = 0.001). In line with this decreased proliferative capacity, HIV-specific CD8+ T cells produced significantly less IL-2 and expressed lower levels of CD127 (see Fig. S2 in the supplemental material).
The capacity of CD8+ T cells to suppress viral replication ex vivo was not restricted to HLA-B*57+ HIC.
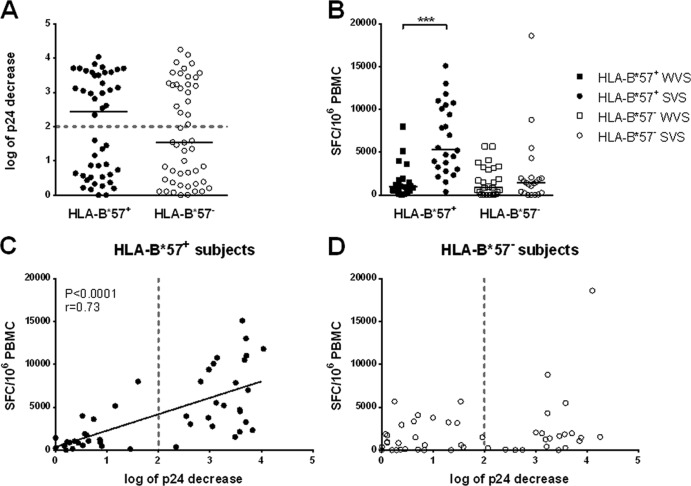
We then examined the capacity of CD8+ T cells to suppress HIV replication in autologous CD4+ T cells in the two HIC groups. As shown in Fig. 4A, no differences were observed between HLA-B*57+ and HLA-B*57− subjects, the decrease in p24 production in CD8+/CD4+ cocultures being 2.44 [0.56 to 3.52] log versus 1.55 [0.39 to 3.23] log, respectively (P = 0.41). We had previously defined a threshold of 2.0 log decrease in p24 production to distinguish subjects with a high and low capacity to suppress ex vivo HIV replication (17). According to this threshold, subjects were then defined as strong or weak viral suppressors (SVS or WVS). We observed a high level of suppression (i.e., >2.0 log decrease in p24 production) in 52% of samples from HLA-B*57+ subjects compared with 44% in HLA-B*57− subjects (Fisher's exact test; P = 0.54). SVS had higher frequencies of HIV-specific CD8+ T cells than WVS (3,031 [1,398 to 7,919] versus 920 [275 to 2,683] SFC/106 PBMC, respectively; P < 0.0001). This difference was, however, limited to HLA-B*57+ patients. As shown in Fig. 4B, HIV-specific CD8+ T cell frequencies were much higher in HLA-B*57+ SVS than in HLA-B*57+ WVS (5,360 [3,088 to 10,403] versus 950 [483 to 1,813] SFC/106 PBMC, respectively; P < 0.0001) and also significantly higher than in HLA-B*57− subjects whether they were WVS or SVS (853 [58 to 3151] and 1,440 [257 to 1,998] SFC/106 PBMC, respectively; P < 0.0001 for both comparisons). Moreover, in HLA-B*57+ subjects, the total frequency of HIV-specific CD8+ T cells producing IFN-γ was highly correlated with the capacity to suppress HIV replication in autologous CD4+ T cells (Spearman's correlation; P < 0.0001, r = 0.73) (Fig. 4C), whereas no such correlation was observed in HLA-B*57− subjects (Fig. 4D).
FIG 4.
Suppressive capacity was assessed in vitro in cocultures of infected or uninfected CD4+ and autologous CD8+ T cells and expressed as log of p24 decrease in culture supernatants. (A, C, and D) Suppressive capacity was measured in HLA-B*57+ (black circles) and HLA-B*57− subjects (clear circles) (A). The dotted line indicates the threshold of 2 log of p24 decrease that distinguishes weak viral suppressors (WVS) from strong viral suppressors (SVS). Suppressive capacity correlated with total HIV-specific response in HLA-B*57+ subjects (C), but there was no correlation in HLA-B*57− subjects (D). (B) The total HIV-specific response was measured by IFN-γ ELISpot assay in the four groups of subjects: HLA-B*57+ WVS (black squares), HLA-B*57+ SVS (black circles), HLA-B*57− WVS (clear squares), and HLA-B*57− SVS (clear circles).
To gain a better insight into the different profiles, we checked whether the characteristics of the HIV-specific CD8+ T cell response differed between SVS and WVS within each group of HLA-defined individuals. Results are shown in Fig. 5 and Table S1 in the supplemental material. Interestingly, the functional and differentiation profiles shown in Fig. 3 for the HLA-B*57-restricted responses were observed in all HLA-B*57+ subjects irrespective of their strong- or weak-suppressor status (see Table S1 in the supplemental material for details). HIV-specific CD8+ T cells in HLA-B*57+ subjects were more sensitive, produced more IFN-γ and IL-2, and proliferated more than cells from HLA-B*57− subjects. Similarly, the less favorable profiles observed in the HLA-B*57− patients were comparable in the strong and weak suppressor subgroups (see Table S1 for details).
FIG 5.
Qualitative features of HIV-specific CD8+ T cell responses in the 4 groups of subjects: HLA-B*57+ WVS (black squares), HLA-B*57+ SVS (black circles), HLA-B*57− WVS (clear squares), and HLA-B*57− SVS (clear circles). (A) Functional avidity determined by IFN-γ ELISPOT assay and expressed as log EC50% (50% of the maximal response). (B and C) Percentages of cytokine-producing CD8+ T cells after 15 h of HIV-specific stimulation: IFN-γ production (B) and IL-2 production (C). (D) Percentages of HIV-specific CD8+ T cells CFSELow after 6 days of culture with the appropriate peptide. (E to G) Differentiation phenotype of HIV-specific CD8+ T cells: CD127 expression (E), effector memory phenotype (CD27+ CD45RO+) (F), and expression of the senescence marker CD57 (G). (H and I) Expression of the activation markers CD38 (H) and HLA-DR (I) by HIV-specific CD8+ T cells.
Frequencies of HIV-specific CD8+ T cells are highly dependent on the viral burden in HLA-B*57+ patients.
HIV-specific CD8+ T cells from HLA-B*57+ WVS had the same characteristics as those from SVS in terms of functional avidity, IL-2 production, and ability to proliferate. Yet the frequencies of these cells were much lower in WVS. We checked whether these low frequencies could then be the consequence of lower stimulation. Viral parameters were unique in HLA-B*57+ WVS in several ways, as shown in Fig. 6. At the time of analysis, plasma viral load (4 [2 to 12] copies/ml) was significantly lower in these patients than the levels observed in HLA-B*57+ SVS (21 [12 to 91] copies/ml; P = 0.005), with 50% of the former having plasma viral RNA of <4 copies/ml compared to 17% in HLA-B*57+ SVS (Fisher's test, P = 0.003). Indeed, we observed in all HLA-B*57+ patients a positive correlation between plasma viral loads and the frequencies of HIV-specific CD8+ T cells (Spearman's correlation, P = 0.003, r = 0.43). Furthermore, 62% of patients of the HLA-B*57+ WVS group had undetectable cell-associated HIV DNA (<10 copies/106 PBMC) compared to only 29% in the HLA-B*57+ SVS group (Fisher's test, P = 0.03). In addition, thorough analysis of clinical follow-ups since HIV diagnosis demonstrated that the HLA-B57+ WVS group had a higher frequency of subjects who never experienced plasma viral RNA higher than 50 copies/ml (elite controllers) compared with the HLA-B*57+ SVS group (59% versus 26% of patients, respectively; Fisher's test, P = 0.04). Conversely, we did not observe any differences in the two subgroups of HLA-B*57− patients, neither in plasma viral RNA levels, nor in cell-associated HIV-DNA levels, nor in the frequency of elite controllers, and no correlation was observed between viral loads and frequencies of HIV-specific CD8+ T cells. Finally, viral quantitation gave comparable data for the HLA-B*57+ SVS group and the two HLA-B*57− groups (chi-square test; P > 0.1 by all comparisons).
DISCUSSION
As already reported, HLA-B*57+ individuals infected with HIV have a more favorable outcome, and this allele is often overrepresented in the cohorts of HIC (10, 13, 33). In this article we report on a large cohort of HIC with regard to their HIV-specific CD8+ T cell properties, focusing on the comparison between HLA-B*57+ and HLA-B*57− individuals. Many hypotheses, not necessarily exclusive, have been proposed to explain the role of these protective alleles during HIV infection. While some of these refer to non-CD8+ T cell-mediated mechanisms, including viral characteristics and restriction at the cellular level (34–37), most hypotheses point out the major influence of the HIV-specific CD8+ T cell response (38–41). Particular features of the MHC-peptide and TCR-peptide interactions have been underlined (42, 43), and functional avidity has been shown to be a major feature of the HIV-specific CD8+ T cell response. This was elegantly first reported in the context of HLA-B*27 (25, 26, 44), another protective allele, and more recently extended in the context of HLA-B*57 (45, 46). We report here that both enhanced functional avidity and the level of antigen contribute to better quantitative and qualitative CD8+ T cell responses in HLA-B*57+ individuals.
The HIV-specific CD8+ T cell response in HIC is markedly impacted by the presence of the HLA-B*57 allele. The magnitude of the response is greater in HLA-B*57+ patients than in HLA-B*57− patients. In the former, the responses restricted by HLA-B*57 represent about three-fourths of the total response. The HLA-B*57 repertoire is broad, mainly directed at Gag peptides, but responses to Nef peptides were often observed in the same range of magnitude. All these HLA-B*57-restricted responses, directed either at Gag or Nef, were found to be highly sensitive, contrasting with responses restricted by other alleles whether observed in HLA-B*57+ or HLA-B*57− individuals.
This higher functional avidity may play a key role in the magnitude of the response in HLA-B*57+ patients, since we observed, only in HLA-B*57+ subjects, positive correlations between functional avidity and the magnitude of the CD8+ T cell response on the one hand and between functional avidity and the ability to proliferate in vitro on the other hand. This may explain the high frequencies of HIV-specific CD8+ T cell responses in HLA-B*57+ patients despite the low level of antigen.
The higher functional avidity may also impact the quality of the response. Almeida et al. have reported a tight link between functional avidity and the functional abilities of HIV-specific CD8+ T cells on HIV-specific CD8+ T cell clones in HLA-B*27+ patients, and polyfunctionality has been described as the hallmark of a favorable HIV-specific CD8+ T cell response (25, 47). We showed that the highly sensitive HLA-B*57-restricted responses have better functional profiles than other responses. They produce more IFN-γ on an individual level. They express a differentiation profile, in terms of CCR7, CD27, CD45RO, CD127 expression, which is compatible with high proliferation capacities. High proliferation rates were observed, confirming original data from Migueles et al. (14), and were correlated with increased production of IL-2. Therefore, the high functional avidity observed in the context of HLA-B*57 responses is associated with favorable quantitative and qualitative features.
Functional avidity was lower in our group of HLA-B*57+ progressors than in HIC. Such a difference was not observed by Berger et al. (45) but was reported for other groups of HIC and progressors (46). Differences in functional avidity levels between HIC and progressors could be due to selection of different clonotypes (46). Alternatively, the low functional avidity in progressors may be the consequence of the preferential deletion of the most sensitive cells in early infection (27). The lower functional avidity observed in progressors was not associated with quantitative but with qualitative differences. The persistent viremia in the progressor group and the activation usually associated with it likely explain most of these differences, as they stimulate the response while impairing its quality.
Another function of CD8+ T cells, and especially of HIV-specific CD8+ T cells, is the capacity to suppress viral replication. Our group was the first to describe this feature of HIC, which is the ability of their CD8+ T cells to suppress viral replication directly without the need for in vitro stimulation. This is due to HIV-specific CD8+ T cells through cell-to-cell cytotoxic mechanisms leading to the elimination of infected CD4+ T cells. The high cytotoxic potential of HIV-specific CD8+ T cells has also been reported by others after in vitro culture with HIV antigens (15, 48, 49). However, our first report described only 11 subjects, the majority of whom expressed HLA-B*57 or HLA-B*27 or both. We then reported heterogeneity of the CD8+ T cell response (17). Some patients presented a high capacity to suppress viral replication and were identified as SVS, while others were described as WVS, and the present study extends these data. Overall, the presence or absence of the HLA-B*57 allele does not impact the capacity to suppress viral replication. Nearly half of the patients in each group could be identified as either SVS or WVS, leading to the definition of four groups either bearing HLA-B*57 or not and being either SVS or WVS.
Thorough study of the four groups revealed a more subtle effect of HLA-B*57. The high frequencies of HIV-specific CD8+ T cells observed in HLA-B*57+ patients were observed only in the subgroup of SVS, while frequencies were comparable and moderate in the group of HLA-B*57+ WVS and in the two HLA-B*57− subgroups whether they were classified as SVS or WVS. The correlation between the frequencies of HIV-specific CD8+ T cells and the capacity to suppress viral replication was detected only in the HLA-B*57+ group and not in the other one. Therefore, the positive effect of HLA-B*57 on the quantitative HIV-specific CD8+ T cell response is not unequivocal. By contrast, the favorable qualitative features observed in the whole group of HLA-B*57+ patients (high functional avidity, IFN-γ and IL-2 production, ability to proliferate, levels of CD127 and CD57 expression) were found in the two subgroups, whether they were SVS or WVS. The lack of suppression in the HLA-B*57+ WVS group despite a substantial level of functional avidity may appear to contrast with the results of Almeida et al. (26), who observed, in the HLA-B*27 context, a correlation between functional avidity and viral suppression. However, the study of Almeida et al. was performed with T cell clones which allowed normalization of the number of potential effector CD8+ T cells in cocultures. In addition, our suppression assay was performed with bulk cocultures of CD4:CD8 and thus we could not correlate the functional avidity of specific CD8+ T cells to the suppressive activity of a bulk culture often including several specificities. All these characteristics observed in HLA-B*57+ subjects differed from what was observed in the two subgroups of HLA-B*57− patients in whom these properties were less favorable, in WVS as well as in SVW.
The higher and comparable functional avidity observed in both HLA-B*57+ SVS and WVS was associated with similar qualitative features but not with comparable frequencies of HIV-specific CD8+ T cells. The low frequencies observed in the HLA-B*57+ WVS group were likely in line with the very low levels of viral stimulation. Indeed low levels of HIV-RNA plasma viral loads and higher frequencies of patients with undetectable plasma viral RNA or cellular-associated viral DNA in ultrasensitive measurements were observed in the HLA-B*57+ WVS group. In addition, the high frequencies of patients whose plasma viral loads had always been undetectable in the WVS group argue for a long history of tight viral control. Conversely, the higher frequencies of HIV-specific CD8+ T cells in the HLA-B*57+ SVS group are likely to be explained by the presence of low doses of antigens and viral blips which, together with the high functional avidity, allow these cells to expand. This is not the case for the low-sensitivity HIV-specific CD8+ T cells not restricted by HLA-B*57. This implies that the high avidity of HLA-B*57-restricted CD8+ T cells could not induce by itself a strong response in HIC if the control of virus is too tight and prevents any antigen stimulation.
All these points also raise the question of the mechanisms involved in viral control, and our data may question the relationship between the HIV-specific CD8+ T cell response and viral control. All patients described here are HIC in vivo and had been so for many years and most have remained controllers during the follow-up of the cohort. However, the HLA-B*57− patients, who account for about 50% of them, do not have a high specific CD8+ T cell response. In addition, half of the HLA-B*57+ patients do not have a marked CD8+ T cell response, particularly those who perfectly and durably control the virus. Therefore, alternative hypotheses on the origin of control may be discussed, and other mechanisms may be linked to the virus (35, 50, 51) or to cellular characteristics or to alternative immune parameters that have been reported as possible clues to the control of viral replication (52, 53; reviewed in reference 54). These alternative mechanisms do not exclude, however, a possible role of the HIV-specific CD8+ T cell response at an earlier time point, possibly during HIV primary infection (55), in controlling viral replication. A tight control would lead, by lack of antigen stimulation, to a decrease in the frequencies of HIV-specific CD8+ effector T cells, as observed in highly active antiretroviral therapy (HAART)-treated patients (56). However, even at low frequencies, HIV-specific CD8+ T cells may participate in the persistence of control as memory cells able to rapidly expand (49) and differentiate into effector cells in the case of viral reactivation.
In summary, a high functional avidity such as that observed in HLA-B*57+ patients and also in the HLA-B*27+ context (25, 26) provides efficient qualitative features to HIV-specific CD8+ T cells which may represent a key factor in controlling viral replication. This high avidity would allow viral reactivations to enhance the CD8+ T cell response, which may maintain the control for long periods. Further studies must be performed to better understand the implication of the CD8+ T cell response in the control of HIV replication, particularly in the context of nonprotective HLA alleles.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sandie Gérard and Aurélia Lamine for their technical support and Azeb Tadesse for data monitoring. The authors also thank the clinicians and patients from all of the participating centers of the French CODEX Cohort (Agence Nationale de Recherche sur le SIDA) for their efficient collaboration.
This work was supported by institutional grants from the Agence Nationale de Recherche sur le SIDA.
Footnotes
Published ahead of print 16 October 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.02098-13.
REFERENCES
- 1.Ogg GS, Jin X, Bonhoeffer S, Dunbar PR, Nowak MA, Monard S, Segal JP, Cao Y, Rowland-Jones SL, Cerundolo V, Hurley A, Markowitz M, Ho DD, Nixon DF, McMichael AJ. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103–2106. 10.1126/science.279.5359.2103 [DOI] [PubMed] [Google Scholar]
- 2.Phillips RE, Rowland-Jones S, Nixon DF, Gotch FM, Edwards JP, Ogunlesi AO, Elvin JG, Rothbard JA, Bangham CR, Rizza CR, McMichael AJ. 1991. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature 354:453–459. 10.1038/354453a0 [DOI] [PubMed] [Google Scholar]
- 3.Walker BD, Chakrabarti S, Moss B, Paradis TJ, Flynn T, Durno AG, Blumberg RS, Kaplan JC, Hirsch MS, Schooley RT. 1987. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature 328:345–348. 10.1038/328345a0 [DOI] [PubMed] [Google Scholar]
- 4.Matano T, Shibata R, Siemon C, Connors M, Lane HC, Martin MA. 1998. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 72:164–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, Ghrayeb J, Forman MA, Montefiori DC, Rieber EP, Letvin NL, Reimann KA. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857–860. 10.1126/science.283.5403.857 [DOI] [PubMed] [Google Scholar]
- 6.Evans DT, O'Connor DH, Jing P, Dzuris JL, Sidney J, da Silva J, Allen TM, Horton H, Venham JE, Rudersdorf RA, Vogel T, Pauza CD, Bontrop RE, DeMars R, Sette A, Hughes AL, Watkins DI. 1999. Virus-specific cytotoxic T-lymphocyte responses select for amino-acid variation in simian immunodeficiency virus Env and Nef. Nat. Med. 5:1270–1276. 10.1038/15224 [DOI] [PubMed] [Google Scholar]
- 7.Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, Blanchard J, Irwin CE, Safrit JT, Mittler J, Weinberger L, Kostrikis LG, Zhang L, Perelson AS, Ho DD. 1999. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991–998. 10.1084/jem.189.6.991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deeks SG, Walker BD. 2007. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity 27:406–416. 10.1016/j.immuni.2007.08.010 [DOI] [PubMed] [Google Scholar]
- 9.Lambotte O, Boufassa F, Madec Y, Nguyen A, Goujard C, Meyer L, Rouzioux C, Venet A, Delfraissy JF. 2005. HIV controllers: a homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin. Infect. Dis. 41:1053–1056. 10.1086/433188 [DOI] [PubMed] [Google Scholar]
- 10.Carrington M, O'Brien SJ. 2003. The influence of HLA genotype on AIDS. Annu. Rev. Med. 54:535–551. 10.1146/annurev.med.54.101601.152346 [DOI] [PubMed] [Google Scholar]
- 11.Carrington M, Walker BD. 2012. Immunogenetics of spontaneous control of HIV. Annu. Rev. Med. 63:131–145. 10.1146/annurev-med-062909-130018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goulder PJ, Walker BD. 2012. HIV and HLA class I: an evolving relationship. Immunity 37:426–440. 10.1016/j.immuni.2012.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goulder PJ, Watkins DI. 2008. Impact of MHC class I diversity on immune control of immunodeficiency virus replication. Nat. Rev. Immunol. 8:619–630. 10.1038/nri2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Migueles SA, Sabbaghian MS, Shupert WL, Bettinotti MP, Marincola FM, Martino L, Hallahan CW, Selig SM, Schwartz D, Sullivan J, Connors M. 2000. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. U. S. A. 97:2709–2714. 10.1073/pnas.050567397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Migueles SA, Osborne CM, Royce C, Compton AA, Joshi RP, Weeks KA, Rood JE, Berkley AM, Sacha JB, Cogliano-Shutta NA, Lloyd M, Roby G, Kwan R, McLaughlin M, Stallings S, Rehm C, O'Shea MA, Mican J, Packard BZ, Komoriya A, Palmer S, Wiegand AP, Maldarelli F, Coffin JM, Mellors JW, Hallahan CW, Follman DA, Connors M. 2008. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity 29:1009–1021. 10.1016/j.immuni.2008.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sáez-Cirión A, Lacabaratz C, Lambotte O, Versmisse P, Urrutia A, Boufassa F, Barre-Sinoussi F, Delfraissy JF, Sinet M, Pancino G, Venet A. 2007. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc. Natl. Acad. Sci. U. S. A. 104:6776–6781. 10.1073/pnas.0611244104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sáez-Cirión A, Sinet M, Shin SY, Urrutia A, Versmisse P, Lacabaratz C, Boufassa F, Avettand-Fenoel V, Rouzioux C, Delfraissy JF, Barre-Sinoussi F, Lambotte O, Venet A, Pancino G. 2009. Heterogeneity in HIV suppression by CD8 T cells from HIV controllers: association with Gag-specific CD8 T cell responses. J. Immunol. 182:7828–7837. 10.4049/jimmunol.0803928 [DOI] [PubMed] [Google Scholar]
- 18.Altfeld M, Addo MM, Rosenberg ES, Hecht FM, Lee PK, Vogel M, Yu XG, Draenert R, Johnston MN, Strick D, Allen TM, Feeney ME, Kahn JO, Sekaly RP, Levy JA, Rockstroh JK, Goulder PJ, Walker BD. 2003. Influence of HLA-B57 on clinical presentation and viral control during acute HIV-1 infection. AIDS 17:2581–2591. 10.1097/00002030-200312050-00005 [DOI] [PubMed] [Google Scholar]
- 19.Dalmasso C, Carpentier W, Meyer L, Rouzioux C, Goujard C, Chaix ML, Lambotte O, Avettand-Fenoel V, Le Clerc S, de Senneville LD, Deveau C, Boufassa F, Debre P, Delfraissy JF, Broet P, Theodorou I. 2008. Distinct genetic loci control plasma HIV-RNA and cellular HIV-DNA levels in HIV-1 infection: the ANRS Genome Wide Association 01 study. PLoS One 3:e3907. 10.1371/journal.pone.0003907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fellay J, Shianna KV, Ge D, Colombo S, Ledergerber B, Weale M, Zhang K, Gumbs C, Castagna A, Cossarizza A, Cozzi-Lepri A, De Luca A, Easterbrook P, Francioli P, Mallal S, Martinez-Picado J, Miro JM, Obel N, Smith JP, Wyniger J, Descombes P, Antonarakis SE, Letvin NL, McMichael AJ, Haynes BF, Telenti A, Goldstein DB. 2007. A whole-genome association study of major determinants for host control of HIV-1. Science 317:944–947. 10.1126/science.1143767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flores-Villanueva PO, Yunis EJ, Delgado JC, Vittinghoff E, Buchbinder S, Leung JY, Uglialoro AM, Clavijo OP, Rosenberg ES, Kalams SA, Braun JD, Boswell SL, Walker BD, Goldfeld AE. 2001. Control of HIV-1 viremia and protection from AIDS are associated with HLA-Bw4 homozygosity. Proc. Natl. Acad. Sci. U. S. A. 98:5140–5145. 10.1073/pnas.071548198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pereyra F, Addo MM, Kaufmann DE, Liu Y, Miura T, Rathod A, Baker B, Trocha A, Rosenberg R, Mackey E, Ueda P, Lu Z, Cohen D, Wrin T, Petropoulos CJ, Rosenberg ES, Walker BD. 2008. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J. Infect. Dis. 197:563–571. 10.1086/526786 [DOI] [PubMed] [Google Scholar]
- 23.Tang Y, Huang S, Dunkley-Thompson J, Steel-Duncan JC, Ryland EG, St John MA, Hazra R, Christie CD, Feeney ME. 2010. Correlates of spontaneous viral control among long-term survivors of perinatal HIV-1 infection expressing human leukocyte antigen-B57. AIDS 24:1425–1435. 10.1097/QAD.0b013e32833a2b5b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sáez-Cirión A, Shin SY, Versmisse P, Barre-Sinoussi F, Pancino G. 2010. Ex vivo T cell-based HIV suppression assay to evaluate HIV-specific CD8+ T-cell responses. Nat. Protoc. 5:1033–1041. 10.1038/nprot.2010.73 [DOI] [PubMed] [Google Scholar]
- 25.Almeida JR, Price DA, Papagno L, Arkoub ZA, Sauce D, Bornstein E, Asher TE, Samri A, Schnuriger A, Theodorou I, Costagliola D, Rouzioux C, Agut H, Marcelin AG, Douek D, Autran B, Appay V. 2007. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J. Exp. Med. 204:2473–2485. 10.1084/jem.20070784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almeida JR, Sauce D, Price DA, Papagno L, Shin SY, Moris A, Larsen M, Pancino G, Douek DC, Autran B, Saez-Cirion A, Appay V. 2009. Antigen sensitivity is a major determinant of CD8+ T-cell polyfunctionality and HIV-suppressive activity. Blood 113:6351–6360. 10.1182/blood-2009-02-206557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lichterfeld M, Yu XG, Mui SK, Williams KL, Trocha A, Brockman MA, Allgaier RL, Waring MT, Koibuchi T, Johnston MN, Cohen D, Allen TM, Rosenberg ES, Walker BD, Altfeld M. 2007. Selective depletion of high-avidity human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T cells after early HIV-1 infection. J. Virol. 81:4199–4214. 10.1128/JVI.01388-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, Busch DH. 2004. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc. Natl. Acad. Sci. U. S. A. 101:5610–5615. 10.1073/pnas.0308054101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4:1191–1198. 10.1038/ni1009 [DOI] [PubMed] [Google Scholar]
- 30.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708–712. 10.1038/44385 [DOI] [PubMed] [Google Scholar]
- 31.Rufer N, Zippelius A, Batard P, Pittet MJ, Kurth I, Corthesy P, Cerottini JC, Leyvraz S, Roosnek E, Nabholz M, Romero P. 2003. Ex vivo characterization of human CD8+ T subsets with distinct replicative history and partial effector functions. Blood 102:1779–1787. 10.1182/blood-2003-02-0420 [DOI] [PubMed] [Google Scholar]
- 32.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA. 2003. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 101:2711–2720. 10.1182/blood-2002-07-2103 [DOI] [PubMed] [Google Scholar]
- 33.Kaslow RA, Carrington M, Apple R, Park L, Munoz A, Saah AJ, Goedert JJ, Winkler C, O'Brien SJ, Rinaldo C, Detels R, Blattner W, Phair J, Erlich H, Mann DL. 1996. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat. Med. 2:405–411. 10.1038/nm0496-405 [DOI] [PubMed] [Google Scholar]
- 34.Elahi S, Dinges WL, Lejarcegui N, Laing KJ, Collier AC, Koelle DM, McElrath MJ, Horton H. 2011. Protective HIV-specific CD8+ T cells evade Treg cell suppression. Nat. Med. 17:989–995. 10.1038/nm.2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Granier C, Battivelli E, Lecuroux C, Venet A, Lambotte O, Schmitt-Boulanger M, Delaugerre C, Molina JM, Chakrabarti LA, Clavel F, Hance AJ. 2013. Pressure from TRIM5α contributes to control of HIV-1 replication by individuals expressing protective HLA-B alleles. J. Virol. 87:10368–10380. 10.1128/JVI.01313-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kosmrlj A, Read EL, Qi Y, Allen TM, Altfeld M, Deeks SG, Pereyra F, Carrington M, Walker BD, Chakraborty AK. 2010. Effects of thymic selection of the T-cell repertoire on HLA class I-associated control of HIV infection. Nature 465:350–354. 10.1038/nature08997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomescu C, Duh FM, Hoh R, Viviani A, Harvill K, Martin MP, Carrington M, Deeks SG, Montaner LJ. 2012. Impact of protective killer inhibitory receptor/human leukocyte antigen genotypes on natural killer cell and T-cell function in HIV-1-infected controllers. AIDS 26:1869–1878. 10.1097/QAD.0b013e32835861b0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bailey JR, Brennan TP, O'Connell KA, Siliciano RF, Blankson JN. 2009. Evidence of CD8+ T-cell-mediated selective pressure on human immunodeficiency virus type 1 nef in HLA-B*57+ elite suppressors. J. Virol. 83:88–97. 10.1128/JVI.01958-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brennan CA, Ibarrondo FJ, Sugar CA, Hausner MA, Shih R, Ng HL, Detels R, Margolick JB, Rinaldo CR, Phair J, Jacobson LP, Yang OO, Jamieson BD. 2012. Early HLA-B*57-restricted CD8+ T lymphocyte responses predict HIV-1 disease progression. J. Virol. 86:10505–10516. 10.1128/JVI.00102-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen H, Ndhlovu ZM, Liu D, Porter LC, Fang JW, Darko S, Brockman MA, Miura T, Brumme ZL, Schneidewind A, Piechocka-Trocha A, Cesa KT, Sela J, Cung TD, Toth I, Pereyra F, Yu XG, Douek DC, Kaufmann DE, Allen TM, Walker BD. 2012. TCR clonotypes modulate the protective effect of HLA class I molecules in HIV-1 infection. Nat. Immunol. 13:691–700. 10.1038/ni.2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miura T, Brockman MA, Schneidewind A, Lobritz M, Pereyra F, Rathod A, Block BL, Brumme ZL, Brumme CJ, Baker B, Rothchild AC, Li B, Trocha A, Cutrell E, Frahm N, Brander C, Toth I, Arts EJ, Allen TM, Walker BD. 2009. HLA-B57/B*5801 human immunodeficiency virus type 1 elite controllers select for rare Gag variants associated with reduced viral replication capacity and strong cytotoxic T-lymphocyte recognition. J. Virol. 83:2743–2755. 10.1128/JVI.02265-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gillespie GM, Kaul R, Dong T, Yang HB, Rostron T, Bwayo JJ, Kiama P, Peto T, Plummer FA, McMichael AJ, Rowland-Jones SL. 2002. Cross-reactive cytotoxic T lymphocytes against a HIV-1 p24 epitope in slow progressors with B*57. AIDS 16:961–972. 10.1097/00002030-200205030-00002 [DOI] [PubMed] [Google Scholar]
- 43.Goulder PJ, Tang Y, Pelton SI, Walker BD. 2000. HLA-B57-restricted cytotoxic T-lymphocyte activity in a single infected subject toward two optimal epitopes, one of which is entirely contained within the other. J. Virol. 74:5291–5299. 10.1128/JVI.74.11.5291-5299.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ladell K, Hashimoto M, Iglesias MC, Wilmann PG, McLaren JE, Gras S, Chikata T, Kuse N, Fastenackels S, Gostick E, Bridgeman JS, Venturi V, Arkoub ZA, Agut H, van Bockel DJ, Almeida JR, Douek DC, Meyer L, Venet A, Takiguchi M, Rossjohn J, Price DA, Appay V. 2013. A molecular basis for the control of preimmune escape variants by HIV-specific CD8+ T cells. Immunity 38:425–436. 10.1016/j.immuni.2012.11.021 [DOI] [PubMed] [Google Scholar]
- 45.Berger CT, Frahm N, Price DA, Mothe B, Ghebremichael M, Hartman KL, Henry LM, Brenchley JM, Ruff LE, Venturi V, Pereyra F, Sidney J, Sette A, Douek DC, Walker BD, Kaufmann DE, Brander C. 2011. High-functional-avidity cytotoxic T lymphocyte responses to HLA-B-restricted Gag-derived epitopes associated with relative HIV control. J. Virol. 85:9334–9345. 10.1128/JVI.00460-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mothe B, Llano A, Ibarrondo J, Zamarreno J, Schiaulini M, Miranda C, Ruiz-Riol M, Berger CT, Herrero MJ, Palou E, Plana M, Rolland M, Khatri A, Heckerman D, Pereyra F, Walker BD, Weiner D, Paredes R, Clotet B, Felber BK, Pavlakis GN, Mullins JI, Brander C. 2012. CTL responses of high functional avidity and broad variant cross-reactivity are associated with HIV control. PLoS One 7:e29717. 10.1371/journal.pone.0029717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781–4789. 10.1182/blood-2005-12-4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buckheit RW, III, Salgado M, Silciano RF, Blankson JN. 2012. Inhibitory potential of subpopulations of CD8+ T cells in HIV-1-infected elite suppressors. J. Virol. 86:13679–13688. 10.1128/JVI.02439-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ndhlovu ZM, Proudfoot J, Cesa K, Alvino DM, McMullen A, Vine S, Stampouloglou E, Piechocka-Trocha A, Walker BD, Pereyra F. 2012. Elite controllers with low to absent effector CD8+ T cell responses maintain highly functional, broadly directed central memory responses. J. Virol. 86:6959–6969. 10.1128/JVI.00531-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen H, Li C, Huang J, Cung T, Seiss K, Beamon J, Carrington MF, Porter LC, Burke PS, Yang Y, Ryan BJ, Liu R, Weiss RH, Pereyra F, Cress WD, Brass AL, Rosenberg ES, Walker BD, Yu XG, Lichterfeld M. 2011. CD4+ T cells from elite controllers resist HIV-1 infection by selective upregulation of p21. J. Clin. Invest. 121:1549–1560. 10.1172/JCI44539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sáez-Cirión A, Hamimi C, Bergamaschi A, David A, Versmisse P, Melard A, Boufassa F, Barre-Sinoussi F, Lambotte O, Rouzioux C, Pancino G, ANRS CO18 Cohort 2011. Restriction of HIV-1 replication in macrophages and CD4+ T cells from HIV controllers. Blood 118:955–964. 10.1182/blood-2010-12-327106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ackerman ME, Crispin M, Yu X, Baruah K, Boesch AW, Harvey DJ, Dugast AS, Heizen EL, Ercan A, Choi I, Streeck H, Nigrovic PA, Bailey-Kellogg C, Scanlan C, Alter G. 2013. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J. Clin. Invest. 123:2183–2192. 10.1172/JCI65708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lambotte O, Ferrari G, Moog C, Yates NL, Liao HX, Parks RJ, Hicks CB, Owzar K, Tomaras GD, Montefiori DC, Haynes BF, Delfraissy JF. 2009. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS 23:897–906. 10.1097/QAD.0b013e328329f97d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sáez-Cirión A, Pancino G. 2013. HIV controllers: a genetically determined or inducible phenotype? Immunol. Rev. 254:281–294. 10.1111/imr.12076 [DOI] [PubMed] [Google Scholar]
- 55.Goujard C, Chaix ML, Lambotte O, Deveau C, Sinet M, Guergnon J, Courgnaud V, Rouzioux C, Delfraissy JF, Venet A, Meyer L, Agence Nationale de Recherche sur le Sida PRIMO Study Group 2009. Spontaneous control of viral replication during primary HIV infection: when is “HIV controller” status established? Clin. Infect. Dis. 49:982–986. 10.1086/605504 [DOI] [PubMed] [Google Scholar]
- 56.Lacabaratz-Porret C, Urrutia A, Doisne JM, Goujard C, Deveau C, Dalod M, Meyer L, Rouzioux C, Delfraissy JF, Venet A, Sinet M. 2003. Impact of antiretroviral therapy and changes in virus load on human immunodeficiency virus (HIV)-specific T cell responses in primary HIV infection. J. Infect. Dis. 187:748–757. 10.1086/368333 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.