Abstract
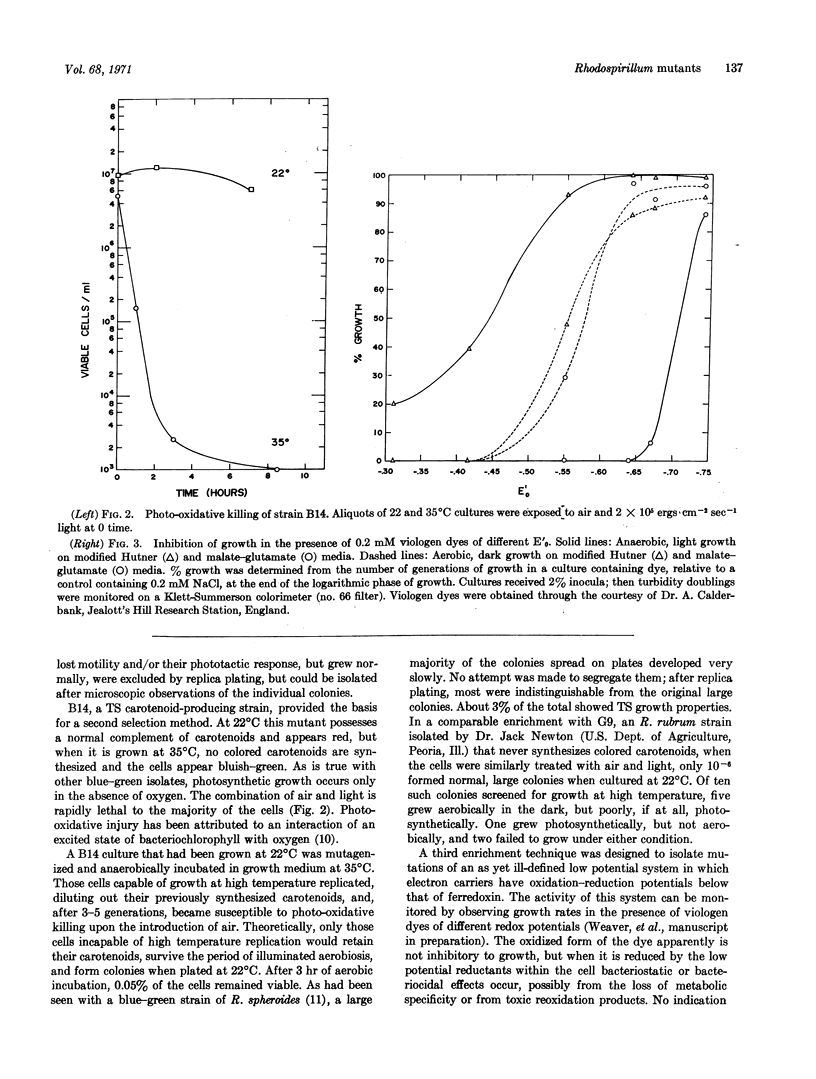
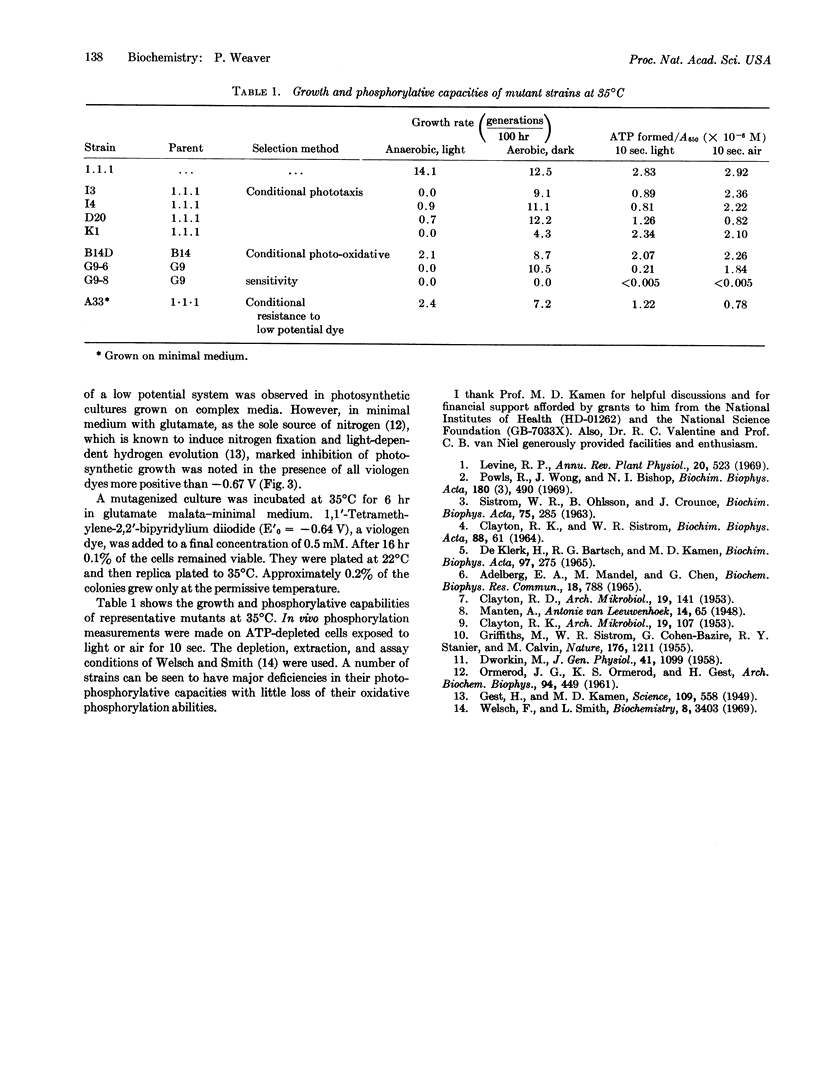
Temperature-sensitive mutants of Rhodospirillum rubum have been isolated by enrichment techniques selecting for conditionally aberrant electron flow in various portions of the electron transport scheme. The temperature sensitivity of a class of these strains is shown to preferentially affect the photosynthetic mode of growth and energy production over the aerobic mode.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLAYTON R. K. Studies in the phototaxis of Rhodospirillum rubrum. I. Action spectrum, growth in green light, and Weber law adherence. Arch Mikrobiol. 1953;19(2):107–124. doi: 10.1007/BF00446395. [DOI] [PubMed] [Google Scholar]
- CLAYTON R. K. Studies in the phototaxis of Rhodospirillum rubrum. III. Quantitative relations between stimulus and response. Arch Mikrobiol. 1953;19(2):141–165. doi: 10.1007/BF00446397. [DOI] [PubMed] [Google Scholar]
- DEKLERK H., BARTSCH R. G., KAMEN M. D. ATYPICAL SOLUBLE HAEM PROTEINS FROM A STRAIN OF RHODOPSEUDOMONAS PALUSTRIS SP. Biochim Biophys Acta. 1965 Feb 15;97:275–280. doi: 10.1016/0304-4165(65)90092-9. [DOI] [PubMed] [Google Scholar]
- DWORKIN M. Endogenous photosensitization in a carotenoidless mutant of Rhodopseudomonas speroides. J Gen Physiol. 1958 Jul 20;41(6):1099–1112. doi: 10.1085/jgp.41.6.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIFFITHS M., SISTROM W. R., COHENBAZIRE G., STANIER R. Y., CALVIN M. Function of carotenoids in photosynthesis. Nature. 1955 Dec 24;176(4495):1211–1215. doi: 10.1038/1761211a0. [DOI] [PubMed] [Google Scholar]
- Gest H., Kamen M. D. Photoproduction of Molecular Hydrogen by Rhodospirillum rubrum. Science. 1949 Jun 3;109(2840):558–559. doi: 10.1126/science.109.2840.558. [DOI] [PubMed] [Google Scholar]
- ORMEROD J. G., ORMEROD K. S., GEST H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch Biochem Biophys. 1961 Sep;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- Powls R., Wong J., Bishop N. I. Electron transfer components of wild-type and photosynthetic mutant strains of Scenedesmus obliquus D3. Biochim Biophys Acta. 1969 Aug 5;180(3):490–499. doi: 10.1016/0005-2728(69)90027-9. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R., CLAYTON R. K. STUDIES ON A MUTANT OF RHODOPSEUDOMONAS SPHEROIDES UNABLE TO GROW PHOTOSYNTHETICALLY. Biochim Biophys Acta. 1964 Jul 29;88:61–73. doi: 10.1016/0926-6577(64)90154-8. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R., OHLSSON B. M., CROUNCE J. ABSENCE OF LIGHT-INDUCED ABSORBANCY CHANGES IN A MUTANT OF RHODOPSEUDOMONAS SPHEROIDES UNABLE TO GROW PHOTOSYNTHETICALLY. Biochim Biophys Acta. 1963 Sep 24;75:285–286. doi: 10.1016/0006-3002(63)90612-7. [DOI] [PubMed] [Google Scholar]
- Welsch F., Smith L. Kinetics of synthesis and utilization of adenosine triphosphate by intact cells of Rhodospirillum rubrum. Biochemistry. 1969 Aug;8(8):3403–3408. doi: 10.1021/bi00836a039. [DOI] [PubMed] [Google Scholar]


