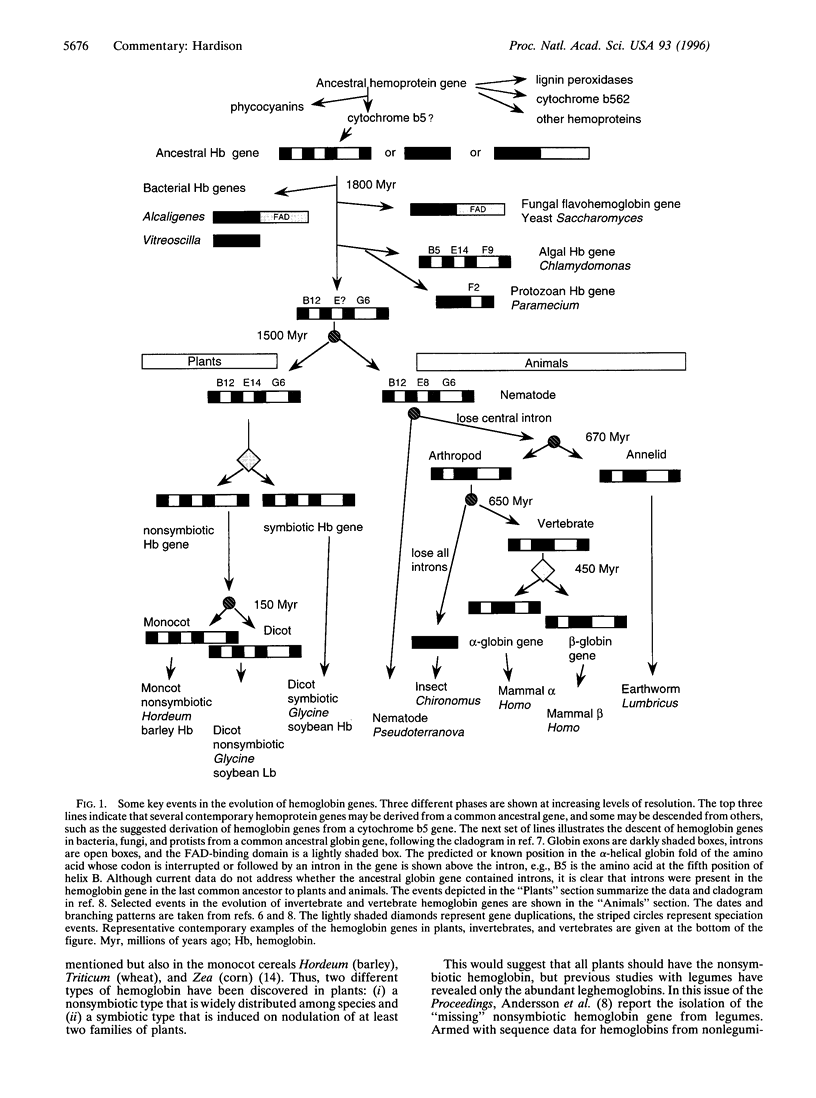
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. R., Jensen E. O., LLewellyn D. J., Dennis E. S., Peacock W. J. A new hemoglobin gene from soybean: a role for hemoglobin in all plants. Proc Natl Acad Sci U S A. 1996 Jun 11;93(12):5682–5687. doi: 10.1073/pnas.93.12.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby C. A., Tjepkema J. D., Trinick M. J. Hemoglobin in a nonleguminous plant, parasponia: possible genetic origin and function in nitrogen fixation. Science. 1983 May 27;220(4600):951–953. doi: 10.1126/science.220.4600.951. [DOI] [PubMed] [Google Scholar]
- Bogusz D., Appleby C. A., Landsmann J., Dennis E. S., Trinick M. J., Peacock W. J. Functioning haemoglobin genes in non-nodulating plants. Nature. 1988 Jan 14;331(6152):178–180. doi: 10.1038/331178a0. [DOI] [PubMed] [Google Scholar]
- Couture M., Chamberland H., St-Pierre B., Lafontaine J., Guertin M. Nuclear genes encoding chloroplast hemoglobins in the unicellular green alga Chlamydomonas eugametos. Mol Gen Genet. 1994 Apr;243(2):185–197. doi: 10.1007/BF00280316. [DOI] [PubMed] [Google Scholar]
- Crawford M. J., Sherman D. R., Goldberg D. E. Regulation of Saccharomyces cerevisiae flavohemoglobin gene expression. J Biol Chem. 1995 Mar 24;270(12):6991–6996. doi: 10.1074/jbc.270.12.6991. [DOI] [PubMed] [Google Scholar]
- Dikshit R. P., Dikshit K. L., Liu Y. X., Webster D. A. The bacterial hemoglobin from Vitreoscilla can support the aerobic growth of Escherichia coli lacking terminal oxidases. Arch Biochem Biophys. 1992 Mar;293(2):241–245. doi: 10.1016/0003-9861(92)90391-9. [DOI] [PubMed] [Google Scholar]
- Dixon B., Pohajdak B. Did the ancestral globin gene of plants and animals contain only two introns? Trends Biochem Sci. 1992 Dec;17(12):486–488. doi: 10.1016/0968-0004(92)90334-6. [DOI] [PubMed] [Google Scholar]
- Dixon B., Walker B., Kimmins W., Pohajdak B. A nematode hemoglobin gene contains an intron previously thought to be unique to plants. J Mol Evol. 1992 Aug;35(2):131–136. doi: 10.1007/BF00183224. [DOI] [PubMed] [Google Scholar]
- Edwards S. L., Raag R., Wariishi H., Gold M. H., Poulos T. L. Crystal structure of lignin peroxidase. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):750–754. doi: 10.1073/pnas.90.2.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermler U., Siddiqui R. A., Cramm R., Friedrich B. Crystal structure of the flavohemoglobin from Alcaligenes eutrophus at 1.75 A resolution. EMBO J. 1995 Dec 15;14(24):6067–6077. doi: 10.1002/j.1460-2075.1995.tb00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Goldberg D. E. The enigmatic oxygen-avid hemoglobin of Ascaris. Bioessays. 1995 Feb;17(2):177–182. doi: 10.1002/bies.950170213. [DOI] [PubMed] [Google Scholar]
- Goodman M., Czelusniak J., Koop B. F., Tagle D. A., Slightom J. L. Globins: a case study in molecular phylogeny. Cold Spring Harb Symp Quant Biol. 1987;52:875–890. doi: 10.1101/sqb.1987.052.01.096. [DOI] [PubMed] [Google Scholar]
- Goodman M., Pedwaydon J., Czelusniak J., Suzuki T., Gotoh T., Moens L., Shishikura F., Walz D., Vinogradov S. An evolutionary tree for invertebrate globin sequences. J Mol Evol. 1988;27(3):236–249. doi: 10.1007/BF02100080. [DOI] [PubMed] [Google Scholar]
- Jhiang S. M., Garey J. R., Riggs A. F. Exon-intron organization in genes of earthworm and vertebrate globins. Science. 1988 Apr 15;240(4850):334–336. doi: 10.1126/science.2832953. [DOI] [PubMed] [Google Scholar]
- Mathews F. S., Bethge P. H., Czerwinski E. W. The structure of cytochrome b562 from Escherichia coli at 2.5 A resolution. J Biol Chem. 1979 Mar 10;254(5):1699–1706. [PubMed] [Google Scholar]
- Potts M., Angeloni S. V., Ebel R. E., Bassam D. Myoglobin in a cyanobacterium. Science. 1992 Jun 19;256(5064):1690–1691. doi: 10.1126/science.256.5064.1690. [DOI] [PubMed] [Google Scholar]
- Runnegar B. Derivation of the globins from type b cytochromes. J Mol Evol. 1984;21(1):33–41. doi: 10.1007/BF02100625. [DOI] [PubMed] [Google Scholar]
- Schirmer T., Bode W., Huber R., Sidler W., Zuber H. X-ray crystallographic structure of the light-harvesting biliprotein C-phycocyanin from the thermophilic cyanobacterium Mastigocladus laminosus and its resemblance to globin structures. J Mol Biol. 1985 Jul 20;184(2):257–277. doi: 10.1016/0022-2836(85)90379-1. [DOI] [PubMed] [Google Scholar]
- Sherman D. R., Kloek A. P., Krishnan B. R., Guinn B., Goldberg D. E. Ascaris hemoglobin gene: plant-like structure reflects the ancestral globin gene. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11696–11700. doi: 10.1073/pnas.89.24.11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus A., Spencer D. F., Zuker M., Logsdon J. M., Jr, Doolittle W. F. Testing the exon theory of genes: the evidence from protein structure. Science. 1994 Jul 8;265(5169):202–207. doi: 10.1126/science.8023140. [DOI] [PubMed] [Google Scholar]
- Taylor E. R., Nie X. Z., MacGregor A. W., Hill R. D. A cereal haemoglobin gene is expressed in seed and root tissues under anaerobic conditions. Plant Mol Biol. 1994 Mar;24(6):853–862. doi: 10.1007/BF00014440. [DOI] [PubMed] [Google Scholar]
- Wittenberg B. A., Wittenberg J. B. Myoglobin-mediated oxygen delivery to mitochondria of isolated cardiac myocytes. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7503–7507. doi: 10.1073/pnas.84.21.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Riggs A. F. Yeast flavohemoglobin is an ancient protein related to globins and a reductase family. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5015–5019. doi: 10.1073/pnas.89.11.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]