Abstract
The NS1 protein of influenza viruses is a major virulence factor and exerts its function through interacting with viral/cellular RNAs and proteins. In this study, we identified heterogeneous nuclear ribonucleoprotein A2/B1 (hnRNP A2/B1) as an interacting partner of NS1 proteins by a proteomic method. Knockdown of hnRNP A2/B1 by small interfering RNA (siRNA) resulted in higher levels of NS vRNA, NS1 mRNA, and NS1 protein in the virus-infected cells. In addition, we demonstrated that hnRNP A2/B1 proteins are associated with NS1 and NS2 mRNAs and that knockdown of hnRNP A2/B1 promotes transport of NS1 mRNA from the nucleus to the cytoplasm in the infected cells. Lastly, we showed that knockdown of hnRNP A2/B1 leads to enhanced virus replication. Our results suggest that hnRNP A2/B1 plays an inhibitory role in the replication of influenza A virus in host cells potentially through suppressing NS1 RNA/protein levels and NS1 mRNA nucleocytoplasmic translocation.
Keywords: Influenza A virus, Proteomics, hnRNP A2/B1, NS1, Protein-protein interaction, mRNA nuclear export
Introduction
Influenza A viruses belong to the family Orthomyxoviridae and harbor an eight segmented, single-stranded, and negative-sense RNA genome, which codes for 11 viral proteins (Hale et al., 2008). Different from most other RNA viruses, influenza viruses replicate in the nucleus of the infected cells (Herz et al., 1981). In the nucleus, the negative-sense virion RNAs (vRNAs) from the input viruses are first transcribed into 5′-capped and 3′-polyadenylated viral mRNAs, which in turn are exported to the cytoplasm where they are translated into viral proteins. Upon import of the viral proteins into the nucleus, vRNAs are synthesized into full-length complementary RNAs (cRNAs), which in turn serve as templates for the synthesis of more vRNAs. The resulting vRNAs are either used as templates for producing more viral mRNAs or encapsidated into ribonucleoprotein structures to be exported to the cytoplasm for virion assembly at the plasma membrane (Amorim et al., 2011).
Influenza viral genome segment 8 codes for NS1 protein from unspliced primary mRNA and NS2 protein from spliced mRNA (Lamb and Lai, 1980; Robb et al., 2010). The NS1 protein is localized in both the cytoplasm and nucleus and plays multiple roles in viral replication cycle (Hale et al., 2008; Li, Yamakita, and Krug, 1998). In the cytoplasm of infected cells, NS1 antagonizes host interferon (IFN) system through targeting protein kinase R (PKR) (Min et al., 2007; Wang et al., 2000), IFN regulatory factor 3 (IRF-3) (Mibayashi et al., 2007), and potentially also IKKγ (Wang et al., 2012). In the nucleus, NS1 inhibits pre-mRNA splicing and mRNA nuclear export through targeting a 30-kDa subunit of the cleavage and polyadenylation specificity factor (CPSF) (Das et al., 2008; Krug et al., 2003; Nemeroff et al., 1998), poly (A)-binding protein II (PABII) (Chen, Li, and Krug, 1999), and/or components of the mRNA export machinery (Satterly et al., 2007).
After being transcribed, cellular pre-mRNAs are known to associate with nuclear proteins to form heterogeneous nuclear ribonucleoprotein (hnRNP) complexes, which function to affect the structure or nucleocytoplasmic transport of mRNAs (Dreyfuss et al., 1993). The hnRNP family includes approximately 20 proteins, ranging from hnRNPs A1 to U, and each hnRNP protein contains RNA binding motifs and auxiliary domains for protein-protein or protein-nucleic acid interactions (Krecic and Swanson, 1999; Pinolroma et al., 1988). Multiple influenza viral proteins have been reported to interact with different hnRNP members, such as hnRNP M, H1 (Jorba et al., 2008), and A1 (Mayer et al., 2007), and the hnRNP family members have been shown to modulate influenza viral replication cycle in virus-infected cells (Ufer, 2012).
Like other viruses, influenza viruses depend on host cellular components, proteins in particular, to complete most (if not all) steps in the viral replication cycle, including viral gene replication/transcription/translation, intracellular trafficking, and virion assembly. This kind of dependence and the intracellular warfare between influenza viruses and host cells create a vast plethora of interactions between viral components and host cellular components in virus-infected cells. Identification of the host cellular factors that play critical roles in viral replication cycle may provide valuable information for designing novel antiviral therapy. In this study, through a two-dimensional gel electrophoresis (2-DE)-based proteomic method, we identified hnRNP A2/B1 as an interacting partner of the influenza viral protein NS1 and found that hnRNP A2/B1 suppresses NS1 RNA/protein levels and NS1 mRNA nucleocytoplasmic export.
Results
Identification of hnRNP A2/B1 as an influenza virus NS1 interacting protein
We used a 2-DE-based proteomic method to identify the proteins that are associated with influenza viral protein NS1 (Zhou, Zhou, and Du, 2012a). Two populations of 293T cells were transiently transfected with plasmids that express Flag alone (control) and Flag-NS1, respectively. After affinity purification of the whole cell lysates from the two populations of cells, the bound proteins eluted from the affinity beads were fractionated with two identical 2-DE gels. The protein spots uniquely appearing in the gel that resolved the proteins purified from the cells that expressed Flag-NS1 were excised, in-gel digested, and the resulting peptides analyzed by mass spectrometry (MS). One of the proteins that were identified by MS was hnRNP A2/B1, which is produced by alternative splicing from a single gene and has been shown to play important roles in RNA processing like RNA transport, translation, splicing, and trafficking (Kamma et al., 1999). hnRNP A2/B1 was identified with high confidence by MS with 18 unique peptides to the proteins and a 58% sequence coverage (Supplementary Fig. S1). Several other proteins were also identified to interact with NS1, and those proteins will be reported in a separate paper.
Validation of the interaction between hnRNP A2/B1 and NS1 by co-immunoprecipitation (co-IP) and mammalian two-hybrid analysis
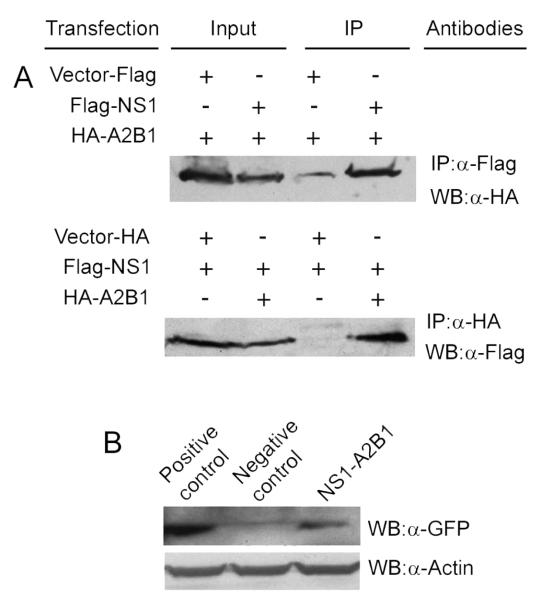
We used co-IPs to validate the interaction between NS1 and hnRNP A2/B1. As shown in Fig. 1A, immobilized anti-Flag antibodies precipitated more HA-hnRNP A2/B1 from the cells co-transfected with the plasmids that express Flag-NS1 and HA-hnRNP A2/B1 than from the cells co-transfected with the plasmids that express Flag alone and HA-hnRNP A2/B1 (top panel). Similarly, in a reciprocal IP, immobilized anti-HA antibodies precipitated a larger amount of Flag-NS1 from the cells co-transfected with the plasmids that express Flag-NS1 and HA-hnRNP A2/B1 than from the cells co-transfected with the plasmids that express HA tag alone and Flag-NS1 (bottom panel). These results strongly suggest that NS1 is specifically associated with hnRNP A2/B1. hnRNP A2/B1 is an RNA binding protein (Han, Tang, and Smith, 2010). In order to test whether the NS1-hnRNP A2/B1 interaction is mediated by RNAs, we used RNase (Sigma) to treat the cell lysates before the IPs. The results demonstrated that the RNase treatment did not change the association of NS1 with hnRNP A2/B1 in the reciprocal co-IPs (data not shown), suggesting the interaction between NS1 and hnRNP A2/B1 is not mediated by RNAs. To further test whether NS1 and hnRNP A2/B1 interact in vivo in cells, we performed a mammalian two-hybrid analysis. In this assay, the interaction between hnRNP A2/B1 and NS1 brings the association of Gal4 DNA-binding domain (BD) and activation domain (AD), which in turn initiates the transcription of a reporter gene GFP (Du et al., 2006). Co-transfection of 293T cells with the Gal4 GFP reporter plasmid and the constructs encoding fusion proteins of BD-NS1 and AD-hnRNP A2/B1 resulted in higher expression of GFP than the negative control, in which BD and AD were fused with two proteins that are known to not interact (Fig. 1B; compare the right lane with the middle lane). As expected, when BD and AD were fused with estrogen receptor α and calmodulin, two known interacting proteins (Li and Sacks, 2007; Li, Joyal, and Sacks, 2001; Zhou, Zhou, and Du, 2012a) respectively, the co-transfection resulted in strong expression of GFP (Fig. 1B; left lane). In short, the results in this section suggest that NS1 interacts with hnRNP A2/B1 in human cells in vivo.
Fig. 1.
Validation of the interaction between hnRNP A2/B1 and NS1. (A) co-IPs. Cell lysates from the cells expressing Flag-NS1 and HA-hnRNP A2/B1, or the cells expressing Flag alone and HA-hnRNP A2/B1 (control), were immunoprecipitated with anti-Flag M2 resin, and the immunoprecipitated proteins were probed with anti-HA antibody in Western blotting. In a reciprocal co-IP, cell lysates from the cells expressing Flag-NS1 and HA-hnRNP A2/B1, or the cells expressing HA alone and Flag-NS1 (control), were precipitated with immobilized anti-HA antibody, and the immunoprecipitated proteins were probed with anti-Flag antibody in Western blotting. (B) Mammalian two-hybrid analysis. 293T cells were co-transfected with plasmids expressing BD-NS1, AD-hnRNP A2/B1, and a Gal4 GFP reporter, and the induction of reporter GFP expression was detected by Western blotting with an anti-GFP antibody. The positive and negative controls were performed by co-transfection of 293T cells with Gal4 GFP reporter plasmid and the plasmids in which the BD and AD were fused with two proteins that are known to interact and not interact, respectively.
hnRNP A2/B1 colocalizes with NS1 in human lung A549 cells
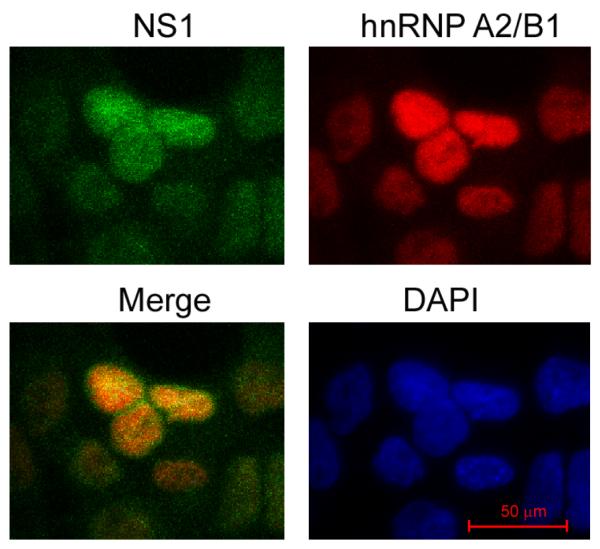
We then performed immunostaining and confocal microscopy analysis using human lung A549 cells to determine whether NS1 and hnRNP A2/B1 physically colocalize in cells (Fig. 2). A549 cells were infected with A/PR/8/34 viruses at a multiplicity of infection (MOI) of 2 and fixed at 2 h postinfection (hpi), followed by incubation with anti-NS1 and anti-hnRNP A2/B1 antibodies, appropriate fluorescence-labeled secondary antibodies, and 4′,6-diamidino-2-phenylindole (DAPI). Confocal microscopy analysis of the stained cells demonstrated that hnRNP A2/B1 protein was predominantly located in the nucleus; while a small portion of NS1 protein in the infected cells was located in the cytoplasm, the majority of it was located in the nucleus at 2 hpi, which was also observed by other research groups (Li, Yamakita, and Krug, 1998; Wolff, O’Neill, and Palese, 1998). The merging of the hnRNP A2/B1- and NS1-stained images suggests that hnRNP A2/B1 and NS1 colocalize in the nucleus of the infected cells at 2 hpi (Fig. 2).
Fig. 2.
Colocalization of hnRNP A2/B1 with NS1 in the nucleus of the virus-infected cells. A549 cells were infected with A/PR/8/34 viruses at an MOI of 2 and stained with DAPI and antibodies directed against hnRNP A2/B1 and NS1 at 2 hpi. The images were acquired by using a confocal laser-scanning microscope. A merged image of the hnRNP A2/B1-staining (in red) and NS1-staining (in green) shows the overlap of hnRNP A2/B1 and NS1 in the nucleus of the infected cells.
hnRNP A2/B1 suppresses NS1 protein levels in the virus-infected cells
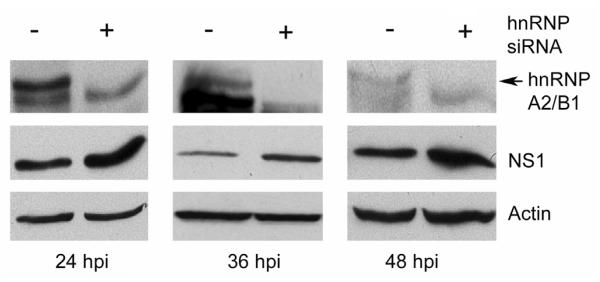
To determine the potential roles of the interaction between hnRNP A2/B1 and NS1, we silenced the expression of endogenous hnRNP A2/B1 by small interfering RNA (siRNA) in A549 cells and then examined the effect of the silencing on NS1 protein levels in the virus-infected cells. The results demonstrated that when the expression of hnRNP A2/B1 was silenced by siRNAs (Fig. 3; top panel), viral NS1 protein levels in the virus-infected cells increased (Fig. 3; middle panel), suggesting that endogenous hnRNP A2/B1 suppresses NS1 protein levels in the virus-infected cells. It is interesting to note that knockdown of hnRNP A1, which is highly homologous to and shares some functions with hnRNP A2/B1 (Han, Tang, and Smith, 2010; Lin et al., 2009), does not affect NS1 protein levels in the influenza A virus-infected cells (Read and Digard, 2010), implying that the effect of hnRNP A2/B1 on NS1 protein levels in the virus-infected cells may be unique.
Fig. 3.
Silencing of endogenous hnRNP A2/B1 results in increases in NS1 proteins in the virus-infected cells. A549 cells were transfected with a randomized siRNA sequence (control) or a siRNA sequence targeting hnRNP A2/B1. Forty-eight h after the transfection, the cells were infected with A/PR/8/34 viruses at an MOI of 0.5 and harvested at the indicated times for examination of the expression of NS1 and hnRNP A2/B1 by Western blotting. Actin was used as a loading control.
hnRNP A2/B1 inhibits the levels of NS vRNA and NS1 mRNA in the virus-infected cells
To elucidate the potential mechanism by which hnRNP A2/B1 suppresses viral NS1 protein levels in the virus-infected cells, we examined the effect of knockdown of hnRNP A2/B1 expression on NS vRNA and NS1 mRNA levels in the virus-infected A549 cells using quantitative real-time PCR (qRT-PCR). Both NS vRNA (Fig. 4A) and NS1 mRNA (Fig. 4B) levels increased significantly (p ≤ 0.05) in the transfected and infected A549 cells when the endogenous hnRNP A2/B1 was knocked down by siRNA. These results suggest that endogenous hnRNP A2/B1 suppresses both NS vRNA and NS1 mRNA levels in the infected cells.
Fig. 4.
hnRNP A2/B1 suppresses the levels of NS vRNA and NS1 mRNA in the virus-infected cells. A549 cells were transfected with a randomized siRNA sequence (control) or a siRNA sequence targeting hnRNP A2/B1. Forty-eight h after the transfection, the cells were infected with A/PR/8/34 viruses at an MOI of 0.5 and harvested at 24 and 36 hpi for examination of NS vRNA (A) and NS1 mRNA (B) by qRT-PCR. Values are the means ± S.E. of three separate sample preparations. * denotes p ≤ 0.05.]
hnRNP A2/B1 binds to NS1 and NS2 mRNAs, and does not affect NS1 mRNA splicing but inhibits NS1 mRNA nuclear export
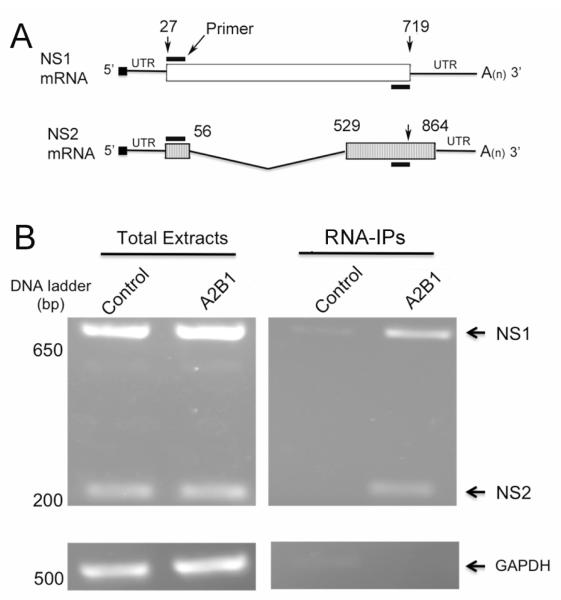
hnRNP proteins are known to regulate splicing (Caputi et al., 1999; Han, Tang, and Smith, 2010) and nuclear export of mRNAs (Reed and Hurt, 2002; Schneider and Wolff, 2009). Thus, we tested whether hnRNP A2/B1 plays a role in regulating the splicing and nuclear export of NS1 mRNA in the virus-infected cells. For this purpose, we first examined whether hnRNP A2/B1 proteins are associated with NS1 or NS2 mRNAs using RNA immunoprecipitation (RNA-IP). 293T cells were transfected with the plasmids that express HA-hnRNP A2/B1 or HA tag alone for control, and then infected with A/PR/8/34 viruses at an MOI of 3. Ten h after the infection, the cells were harvested for IPs with immobilized anti-HA antibodies to pulldown the complexes that were associated with hnRNP A2/B1. After the IPs, proteinase K was used to release the hnRNP A2/B1-associated RNAs from the precipitated complexes, followed by reverse-transcription of mRNAs with the oligo (dT) primer, which left vRNA and cRNA unaffected (Robb et al., 2010), and PCR amplification of the reverse-transcribed cDNAs. Because the primers we used in the PCR amplification were specific for the cDNAs of both NS1 and NS2 mRNAs (Fig. 5A), the amounts (or presence/absence) of both NS1 and NS2 mRNAs in the precipitated complexes could be detected. As shown in Fig. 5B, transfection of the cells with the plasmid that expresses HA-hnRNP A2/B1 did not affect the transcription of either NS1 or NS2 (Fig. 5B, left panel). The immobilized anti-HA antibody precipitated substantially more NS1 and NS2 mRNAs from the cells that express HA-hnRNP A2/B1 than from the control cells (Fig. 5B, right panel), suggesting that both NS1 and NS2 mRNAs are specifically associated with hnRNP A2/B1 proteins in the infected cells.
Fig. 5.
hnRNP A2/B1 proteins are associated with NS1 and NS2 mRNAs. (A) Schematic representation of NS1 mRNA, NS2 pre-mRNA and the positions of the primers used to amplify NS1 and NS2 mRNAs. The coding regions of NS1 and NS2 mRNAs are shown as white and hatched boxes, respectively. The numbers above the coding regions indicate the start and end nucleotide positions in the NS1 and NS2 mRNAs. The NS2 mRNA is alternatively spliced from NS1 mRNA, and the V-shaped line denotes the region that is removed in splicing. With the primers (black bar) indicated in the diagram, the amplicon size for NS1 and NS2 was calculated to be 693 and 221 bps, respectively. (B) hnRNP A2/B1 proteins are associated with NS1 and NS2 mRNAs. 293T cells transiently transfected with the plasmids that express HA-hnRNP A2/B1 or HA tag alone (control) were infected with A/PR/8/34 viruses at an MOI of 3. At 10 hpi, the cells were harvested and lysed, and the resulting whole cell lysates were immunoprecipitated with immobilized anti-HA antibody. The RNAs immunoprecipitated were released from the complexes by incubating with proteinase K, reverse-transcribed, and PCR-amplified with the primers indicated in (A). The resulting DNAs were examined by a 1.3% agarose gel. GAPDH was used as an internal reference.
After confirming that NS1 and NS2 mRNAs are associated with hnRNP A2/B1 proteins in the virus-infected cells (Fig. 5), we examined whether hnRNP A2/B1 affects NS1 pre-mRNA splicing. To test this possibility, we compared the ratios of NS2 to NS1 mRNAs in the infected cells with a normal level of endogenous hnRNP A2/B1 to those in the cells with a depleted hnRNP A2/B1 using reverse transcriptions as described above. The results demonstrated that knockdown of hnRNP A2/B1 by siRNAs does not affect NS1 and NS2 mRNA splicing in infected A549 cells (data not shown).
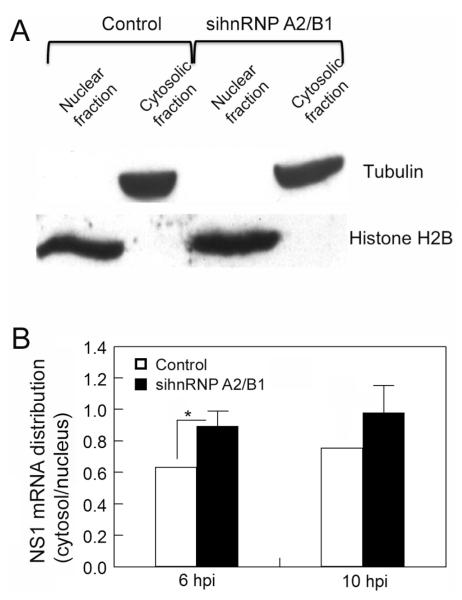
We then examined the potential role of hnRNP A2/B1 in nucleocytoplasmic translocation of NS1 mRNAs. We used a differential-centrifugation-based method (Wang, Zhu, and Levy, 2006) to fractionate cell lysate into cytoplasmic and nuclear parts. Western blot analysis using marker proteins of the cytoplasm and nucleus demonstrated that there were no noticeable cross-contaminations in our cytoplasmic and nuclear fractionations (Fig. 6A). We transfected the A549 cells with the siRNAs oligos targeting hnRNP A2/B1 or a randomized siRNA sequence for control and infected the transfected cells with A/PR/8/34 viruses. The transfected and infected cells were harvested and fractionated, and NS1 mRNAs in the cytoplasmic and nuclear factions were quantified by qRT-PCR. The results demonstrated that when hnRNP A2/B1 expression was silenced by siRNAs, the ratios of the cytosolic to nuclear NS1 mRNAs at 6 and 10 hpi increased (Fig. 6B). These results suggest that endogenous hnRNP A2/B1 inhibits the nuclear export of viral NS1 mRNA in the infected cells.
Fig. 6.
hnRNP A2/B1 inhibits NS1 mRNA nuclear export. (A) Cytoplasmic and nuclear fractionation. A549 cells transfected with a randomized siRNA sequence (control) or a siRNA sequence targeting hnRNP A2/B1 were lysed and fractionated into nuclear and cytoplasmic parts, and the purity of each part was examined by Western blotting using antibodies against the nuclear and cytoplasmic markers (histone H2B and tubulin, respectively). (B) hnRNP A2/B1 inhibits NS1 mRNA nucleocytoplasmic translocation. A549 cells transfected with a randomized siRNA sequence (control) or a siRNA sequence targeting hnRNP A2/B1 were infected with A/PR/8/34 viruses at an MOI of 1. The cells were harvested at the indicated times and fractionated into the nuclear and cytoplasmic parts, and the NS1 mRNA in each part was quantified by qRT-PCR. 18s-rRNA was used as an internal control. Values are the means ± S.E. of three separate sample preparations. * denotes p ≤ 0.05.
hnRNP A2/B1 inhibits influenza virus replication, but virus infection does not affect hnRNP A2/B1 levels in the infected cells
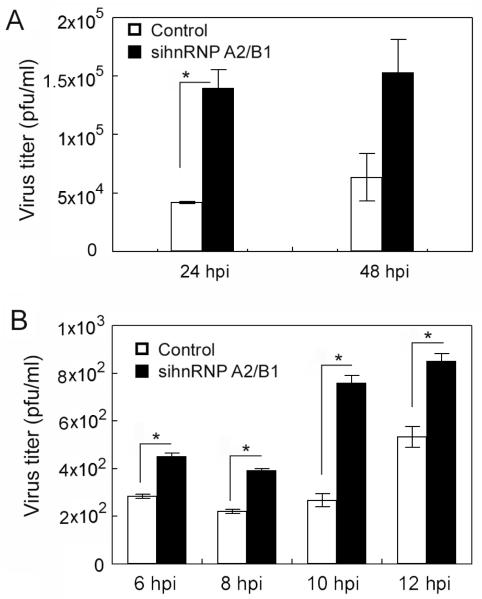
Because hnRNP A2/B1 suppresses influenza NS vRNA, NS1 mRNA, and NS1 protein levels (Figs. 3 and 4), and inhibits NS1 mRNA nuclear export (Fig. 6) in the virus-infected cells, we speculated that hnRNP A2/B1 may affect virus replication. We infected the hnRNP A2/B1-silenced A549 cells with A/PR/8/34 viruses at two different MOIs and harvested the viruses for titer determination at two different time frames. In both cases, when the expression of hnRNP A2/B1 was silenced, the virus titers increased (Fig. 7) (p < 0.05 for all time points except for 48 hpi in Fig. 7A, at which p = 0.077). These results suggest that endogenous hnRNP A2/B1 inhibits influenza virus replication in the infected cells.
Fig. 7.
Silencing of endogenous hnRNP A2/B1 enhances virus replication. A549 cells were transfected with a randomized siRNA sequence (control) or a siRNA sequence targeting hnRNP A2/B1. Forty-eight h after the transfection, the cells were infected with A/PR/8/34 viruses at an MOI of 0.5 (A) or 1 (B). The supernatants were harvested at the indicated times for examinations of virus titers. Values are the means ± S.E. of three separate sample preparations. * denotes p ≤ 0.05.
We also tested whether influenza virus infection would affect the levels of endogenous hnRNP A2/B1 proteins in the virus-infected cells. The results demonstrated that when A549 cells were infected with A/PR/8/34 viruses at an MOI of 0.3 for 24 h, cellular hnRNP A2/B1 protein levels in the infected cells were not changed by virus infection (data not shown).
Discussion
Although the NS1 protein is not part of the influenza virion, it is expressed at high levels in the virus-infected cells (Lazarowitz, Compans, and Choppin, 1971; Liu et al., 2012). NS1 is a multi-functional protein (Hale et al., 2008). One major function of NS1 is to inhibit host immune responses through suppressing the induction of type I-IFNs and IFN-mediated proteins (Billharz et al., 2009; Geiss et al., 2002; Krug et al., 2003; Tisoncik et al., 2011). In addition to its role in inhibiting host immune responses, NS1 also plays important roles in other aspects in the virus-infected cells (Hale et al., 2008). For example, NS1 has been shown to affect mRNA splicing and nuclear export (Garaigorta and Ortin, 2007), protein synthesis (de la Luna et al., 1995; Enami et al., 1994), and cell apoptosis (Ehrhardt et al., 2007). Most, if not all, of the reported functions of NS1 are realized through the physical interactions of NS1 proteins with cellular/viral proteins or RNAs (Hale et al., 2008). In the present study, through a 2-DE-based proteomic approach, we found that NS1 is associated with hnRNP A2/B1, a member of a large family of proteins that are highly divergent in structure and function (Han, Tang, and Smith, 2010; Shyu and Wilkinson, 2000). hnRNP A2 and B1 proteins are produced by alternative splicing of a single gene with the difference of a 12-amino-acid insertion in N-terminal RNA-binding motif in B1 (Burd et al., 1989; Kozu, Henrich, and Schafer, 1995) and have been shown to regulate RNA alternative splicing (Bilodeau et al., 2001; Mayeda et al., 1994), RNA trafficking (Munro et al., 1999; Pinol-Roma, 1992; Shan et al., 2003), and telomere maintenance (Ford, Wright, and Shay, 2002).
Most cellular mRNAs are transported from the nucleus to the cytoplasm through the mRNA export receptors Tap/NXF1 that interact with both mRNAs and components of the nuclear pore complex to direct mRNAs through the nuclear pore complex (Stutz and Izaurralde, 2003). The interaction between mRNA and Tap/NXF1 is mediated by adapter proteins such as Aly (Strasser and Hurt, 2000; Stutz et al., 2000), which is recruited to the mRNA during splicing by export factor UAP56 (Luo et al., 2001). Several lines of evidence suggest that viral mRNA nuclear export in the virus-infected cells is also mediated by the Tap/NXF1 export pathway (Hao et al., 2008; Read and Digard, 2010; Wang et al., 2008), and viruses have evolved strategies to introduce viral mRNAs into the Tap/NXF1 export system through recruiting cellular export factors, such as export factors Aly and UAP56 (Chen et al., 2005; Koffa et al., 2001; Lischka et al., 2006). In the case of influenza viral mRNA nuclear export, it seems that NS1 proteins play an important role in directing viral mRNAs into the nuclear mRNA export machinery via recruiting essential export factors (Schneider and Wolff, 2009). In support of this notion, it has been shown that NS1 proteins from influenza A and B viruses interact with mRNA export factors Tap/NXF1 and UAP56, respectively (Satterly et al., 2007; Schneider et al., 2009), to facilitate nuclear export of viral mRNAs (Schneider and Wolff, 2009). In the present study, we found that nuclear protein hnRNP A2/B1 interacts with both NS1 protein (Fig. 1) and NS1 mRNA (Fig. 5) and inhibits the nuclear export of NS1 mRNA in the influenza virus-infected cells (Fig. 6). A potential mechanism underlying the inhibitory effect of hnRNP A2/B1 on viral NS1 mRNA nuclear export is that the interaction of hnRNP A2/B1 with NS1 protein interferes with NS1 protein’s ability to recruit mRNA export factors such as Tap/NXF1 or other factors to the NS1 mRNAs (Satterly et al., 2007; Schneider and Wolff, 2009). It has been shown that influenza viral NS1 protein is associated with influenza viral mRNAs of NA, M1, and PB1 genes (Wang et al., 2008). If the hypothesis described above is correct, it is highly likely that hnRNP A2/B1 may also play an important role in regulating the nuclear export of other influenza viral mRNAs including (but not limited to) those of NA, M1, and PB1 (Wang et al., 2008).
We also found in the present study that knockdown of hnRNP A2/B1 expression by siRNA resulted in higher levels of NS vRNA, NS1 mRNA (Fig. 4), and NS1 protein (Fig. 3), suggesting that endogenous hnRNP A2/B1 suppresses NS vRNA, NS1 mRNA, and NS1 protein in the virus-infected cells. Because the viral mRNAs exported from the nucleus are translated into viral proteins in the cytoplasm and some of the resulting viral proteins are transported back to the nucleus to facilitate further amplification of vRNAs and viral mRNAs, it is likely that the observed increases in NS vRNA, NS1 mRNA, and NS1 protein (Figs. 3 and 4) were a consequence of the increased nuclear export of NS1 mRNA (Fig. 6) when the expression of hnRNP A2/B1 was silenced. Alternatively, it is also possible that the NS1-hnRNP A2/B1 complex directly inhibits the catalytic activity of viral polymerase, which is responsible for replicating and transcribing viral RNAs, and/or the host RNA polymerase II, which has been shown to affect viral mRNA transcription and viral mRNA nuclear export (Amorim et al., 2007). Furthermore, because hnRNPs, including hnRNP A2/B1, play important roles in regulating protein translation (Lin et al., 2009; Sinnamon and Czaplinski, 2011; Svitkin et al., 1996), it is possible that hnRNP A2/B1 may affect NS1 protein expression through regulating protein translation process. For example, knockdown of hnRNP A2/B1 could lead the virus-infected cells to synthesize NS1 protein at the expense of NS2 production.
The results from the present study revealed a novel role of hnRNP A2/B1 in regulating influenza viral NS1 gene products (proteins and RNAs), major factors that determine the influenza virus virulence and also contribute to the pathogenesis of the viruses. At the same time, our results also raised several questions that need to be addressed in the future. First, our results suggest that hnRNP A2/B1 suppresses NS vRNA and NS1 mRNA levels (Fig. 4) potentially through inhibiting NS gene replication/transcription, or through inhibiting NS1 mRNA nuclear export (Fig. 6). Viral gene replication/transcription and viral mRNA nuclear export are two molecular processes that are closely related to each other in the virus-infected cells. It is not clear at the present time whether hnRNP A2/B1 independently inhibits these two molecular processes or if hnRNP A2/B1 affects one of them and the other is a secondary change. Second, we have shown in the present study that NS1 proteins and NS1/NS2 mRNAs are associated with hnRNP A2/B1 proteins. It remains to be determined whether these kinds of functional associations with hnRNP A2/B1 proteins are specific to NS gene products or also apply to other viral gene products or even some cellular gene products. Third, the molecular mechanism underlying the inhibitory effect of hnRNP A2/B1 on NS1 [and potentially other viral genes (Wang et al., 2008)] mRNA nuclear export in the virus-infected cells requires further investigation. Together with the information obtained in the present study, these results should provide a clear picture of how hnRNP A2/B1 regulates influenza viral gene expression and virus replication in host cells.
Materials and methods
Cell culture and virus infection
Human embryonic kidney (HEK) 293T cells, human lung epithelial A549 cells, and Madin-Darby canine kidney (MDCK) cells (ATCC, Manassas, VA) were cultivated in Dulbecco modified eagle medium (DMEM; Hyclone Laboratories, Logan, UT) supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA) and 1% penicillin and streptomycin. Influenza A/PR/8/34 H1N1 viruses (ATCC) were propagated and titrated in MDCK cells as described previously (Wang et al., 2012).
Plasmid construction and cell transfection
Flag tagged NS1 gene and Flag alone were cloned into pcDNA3.1 as described previously (Wang et al., 2012). For mammalian two-hybrid assay, NS1 and hnRNP A2/B1 cDNAs (GenBank accession no: NM_031243) were inserted into EcoR I and Sal I sites of pM vector with GAL4 BD and pVP16 vector with AD (BD Biosciences, San Jose, CA), respectively. hnRNP A2/B1 gene was also cloned into pCruz HA vector (Santa Cruz, Santa Cruz, CA) by inserting the cDNA into the Not I and Bgl II sites of the vector. All expression plasmids were verified by DNA sequencing. Cell transfection was performed as described previously (Wang et al., 2012).
Affinity purification and 2-DE
Human 293T cells transiently transfected with plasmids expressing Flag alone or Flag-NS1 (approximately 1 × 109 each) were harvested and washed twice with cold phosphate-buffered saline (PBS) 48 h after the transfection. The cells were lysed in 5 packed cell pellet volumes of a lysis buffer (10 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 10 mM NaF, 10 mM β-glycerophosphate, and 1 mM Na3VO4) supplemented with protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN) by douncing with a glass dounce homogenizer (Kontes Glass Co., Vineland, NJ). After centrifugation at 20,000 g for 15 min at 4 °C, the pellets were further extracted once with the lysis buffer and sonication. The combined supernatants from the cells that express Flag-NS1 or the control cells that express Flag alone were incubated separately with 200 μl pre-washed anti-Flag M2 resin (Sigma, St. Louis, MO) for 5 h at 4°C. After washing (4 × 1 ml), the bound proteins were eluted with an elution buffer containing 50 mM Tris-HCl pH 7.5, 0.5 M NaCl, and 250 mM Flag peptide (Sigma), and then concentrated by trichloroacetic acid precipitation. The precipitated proteins from the two groups of cells were separately fractionated by two identical 2-DE gels, as described previously (Zhou, Zhou, and Du, 2012a).
MS analysis and database search
After 2-DE fractionations, the two 2-DE gels that resolved the affinity-purified proteins from the cells that express Flag alone (control) or the cells that express Flag-NS1 were visually inspected. The protein spots uniquely appearing in the gel that resolved the proteins purified from the cells that express Flag-NS1 (but not in the control gel) were excised, in-gel digested, and the resulting peptides analyzed by MS. In-gel digestion, MS analysis, and database search were carried out as described previously (Wang et al., 2012).
Mammalian two-hybrid analysis
Mammalian two-hybrid analysis of protein-protein interactions was performed according to our previous protocol (Du et al., 2006; Wang et al., 2005). Briefly, the coding sequences of NS1 and hnRNP A2/B1 were inserted into the vectors encoding BD and AD, respectively, and the two constructs were co-transfected into the 293T cells with a Gal4 GFP reporter plasmid (2 μg of each plasmid in a 60-mm plate). The positive and negative controls were performed by co-transfection of the 293T cells with Gal4 GFP reporter plasmid and the two expression vectors in which the BD and AD were fused with two proteins that are known to interact (estrogen receptor α and calmodulin (Li and Sacks, 2007; Li, Joyal, and Sacks, 2001; Zhou, Zhou, and Du, 2012a)) and not interact, respectively. The expression of GFP was detected by Western blotting using an anti-GFP antibody.
co-IP
Vectors expressing HA-hnRNP A2/B1 or Flag-NS1 were co-transfected into 293T cells (~1 × 108 cells). For control, the vector expressing Flag alone was used to replace the vector that expresses Flag-NS1 in the co-transfection. Forty-eight h after the transfection, cells were lysed and immunoprecipitated with anti-Flag M2 resin as described above. The NS1-associated proteins were detected by Western blotting using an anti-HA antibody (Santa Cruz). In a reciprocal co-IP, cell transfection, IP, and Western blotting were performed as described above except that 1) for control, the vector expressing HA alone was used to replace the vector that expresses HA-hnRNP A2/B1 in the co-transfection; 2) the IP was performed using immobilized anti-HA antibody (Santa Cruz); 3) the hnRNP A2/B1-associated proteins were detected by Western blotting using an anti-Flag antibody (Sigma).
Immunofluorescence staining and confocal microscopy
The immunofluorescence staining and image acquisition were performed as described in our previous papers (Zhou, Zhou, and Du, 2012a; Zhou, Zhou, and Du, 2012b). Specifically, A549 cells at 90% confluency on a coverslip were infected with A/PR/8/34 viruses at an MOI of 2. At 2 hpi, cells were washed with PBS, fixed by 3.7% formaldehyde in PBS for 15 min, and permeabilized by 0.2% Triton X-100 in PBS for 10 min. The cells were then washed and blocked with 10% normal horse serum in PBS for 1 h, followed by incubation with mouse anti-NS1 antibody (1:1,000) in 5% normal horse serum in PBS overnight at 4°C. After washing, the cells were incubated with DyLight 488 horse anti-mouse IgG antibody (Vector Labs, Burlingame, CA) for 1 h at room temperature. The cells were then washed, blocked with 10% normal goat serum, and incubated with rabbit anti-hnRNP A2/B1 antibody (1:2,500), followed by incubation with Texas Red goat anti-rabbit IgG antibody (Vector Labs) and DAPI. The images were acquired by a NIKON Eclipse 90i confocal fluorescence microscope (Nikon, Tokyo, Japan).
RNA interference (RNAi)
Three siRNA oligos specifically targeting hnRNP A2/B1 [5′-AAGCUUUGAAACCACAGAAGA-3′ (Patry et al., 2003), 5′-AAAGAUCAAGAGGAUUUGGUU-3′ and 5′-GGAACAGUUCCGUAAGCUC-3′ (Iwanaga et al., 2005)] were co-transfected into A549 cells using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA) as described previously (Wang et al., 2012).
qRT-PCR
The analysis was performed according to our previous protocol (Liu et al., 2012; Wang et al., 2012) with the following specifications. A549 cells treated with siRNAs oligos targeting hnRNP A2/B1 or nontargeting siRNAs (negative control) were infected with the A/PR/8/34 viruses. The cells were harvested and total RNA was extracted using an RNeasy Mini kit (Qiagen, Valencia, CA) at appropriate hpi. vRNAs and mRNA were reverse-transcribed (Improm-II Reverse Transcriptase kit; Promega, Madison, WI) with the Uni-12 primer (Hoffmann et al., 2001) and the oligo (dT) primer (Promega), respectively, and the resulting cDNAs were amplified by qRT-PCR with the primers specific for the NS1 gene (Forward: 5′-GACCGGCTGGAGACTCTAAT-3′ and reverse: 5′-CTGGAAGAGAAGGCAATGGT-3′). The relative levels of mRNAs were determined using actin mRNA as an internal control and calculated using the traditional 2−ΔΔCt method (Livak and Schmittgen, 2001). The relative levels of vRNA were determined based on a standard curve generated by serial dilutions of the NS1 expression plasmids used in cell transfection (Shin et al., 2007).
RNA-IP
The vectors expressing HA-hnRNP A2/B1 or HA tag alone as control were transiently transfected into 293T cells. Forty-eight h after the transfection, the cells were infected with A/PR/8/34 viruses at an MOI of 3. Ten h after the infection, the cells were harvested and lysed for IP with immobilized anti-HA antibody as described above. The immunoprecipitated complexes were treated with 150 μg/ml proteinase K for 90 min at 37 °C. After extraction of the total RNA from the immunoprecipitated complexes, the oligo (dT) primer was used to reverse-transcribe mRNAs into cDNAs, and the following primers were then used to PCR-amplify DNA (25 cycles) from the reverse-transcribed cDNAs: NS1 primers (Forward: 5′-ATGGATCCAAACACTGTGTC-3′ and reverse: 5′-TCAAACTTCTGACCTAATTGTTCC-3′); glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primers (Forward: 5′-CGGAGTCAACGGATTTGGCC-3′ and reverse: 5′-GTGGCAGAGATGGCATGGAC-3′). The amplified DNAs were detected with a 1.3% agarose gel and further confirmed by DNA sequencing.
Subcellular fractionation
Nuclear and cytoplasmic fractionations were performed according to a published protocol (Wang, Zhu, and Levy, 2006). Briefly, cells were harvested and swelled on ice in a hypertonic buffer (10 mM Tris-HCl, pH 7.5, 10 mM NaCl, and 3 mM MgCl2) for 3 min, followed by centrifugation at 1,500 g for 3 min. The cell pellet was lysed in four packed cell pellet volumes of buffer A [10 mM Tris-HCl, pH 7.5, 10 mM NaCl, 3 mM MgCl2, 10% (v/v) glycerol, 0.5% (v/v) NP-40, 0.5 mM DTT, and 100 U/ml RNasin or 1× protease inhibitor] by general pipetting. After centrifugation at 4,500 g for 3 min at 4 °C, supernatant was saved as cytoplasmic fraction. The pellet was resuspended in buffer A supplemented with detergents [3.3% (w/v) sodium deoxycholate and 6.6% (v/v) Tween 20] and incubated on ice for 5 min. The insoluble materials were designated as nuclei and collected by centrifugation at 10,000 g for 5 min and washed with buffer A once to remove residual cytoplasmic contamination. The integrity of the isolated nuclei was examined by microscopy after staining with trypan blue. The total RNAs were extracted from the cytoplasmic and nuclear fractions and the isolated RNAs were treated with RNase-free DNase I (Invitrogen) at room temperature for 15 min, followed by incubation at 37 °C for 30 min to remove genomic DNAs. Three micrograms of the resulting RNAs were reverse transcribed into cDNAs as described above. 18S-rRNA was used as an internal control in the qRT-PCR (Zhu and Altmann, 2005).
Plaque assay
Plaque assay was carried out as described previously (Wang et al., 2012).
Statistical Analysis
Statistical analysis was performed using an independent-sample t-test by Systat 13 (SPSS 13).
Supplementary Material
Highlights.
Cellular protein hnRNP A2/B1 interacts with influenza viral protein NS1.
hnRNP A2/B1 suppresses the levels of NS1 protein, vRNA and mRNA in infected cells.
hnRNP A2/B1 protein is associated with NS1 and NS2 mRNAs.
hnRNP A2/B1 inhibits the nuclear export of NS1 mRNAs.
hnRNP A2/B1 inhibits influenza virus replication.
Acknowledgements
We thank Dr. Jonathan Yewdell (National Institute of Allergy and Infectious diseases, NIH, Bethesda, MD) for providing an anti-NS1 antibody, Dr. Benoit Chabot (Université de Sherbrooke, Sherbrooke, Québec, Canada) for an anti-hnRNP A2/B1 antibody, and Dr. Toshi Shioda (Massachusetts General Hospital Cancer Center, Boston, MA) for the Gal4 GFP reporter plasmid. This work was supported by Grants P30 GM103450 and 3P20RR015569-10S2 from the National Institutes of Health (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amorim MJ, Bruce EA, Read EK, Foeglein A, Mahen R, Stuart AD, Digard P. A Rab11- and microtubule-dependent mechanism for cytoplasmic transport of influenza A virus viral RNA. J. Virol. 2011;85:4143–56. doi: 10.1128/JVI.02606-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorim MJ, Read EK, Dalton RM, Medcalf L, Digard P. Nuclear export of influenza A virus mRNAs requires ongoing RNA polymerase II activity. Traffic. 2007;8:1–11. doi: 10.1111/j.1600-0854.2006.00507.x. [DOI] [PubMed] [Google Scholar]
- Billharz R, Zeng H, Proll SC, Korth MJ, Lederer S, Albrecht R, Goodman AG, Rosenzweig E, Tumpey TM, Garcia-Sastre A, Katze MG. The NS1 protein of the 1918 pandemic influenza virus blocks host interferon and lipid metabolism pathways. J. Virol. 2009;83:10557–70. doi: 10.1128/JVI.00330-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilodeau PS, Domsic JK, Mayeda A, Krainer AR, Stoltzfus CM. RNA splicing at human immunodeficiency virus type 1 3 ’ splice site A2 is regulated by binding of hnRNP A/B proteins to an exonic splicing silencer element. J. Virol. 2001;75:8487–8497. doi: 10.1128/JVI.75.18.8487-8497.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd CG, Swanson MS, Gorlach M, Dreyfuss G. Primary Structures of the Heterogeneous Nuclear Ribonucleoprotein A2-Protein, B1-Protein, and C2-Protein - a Diversity of Rna-Binding Proteins Is Generated by Small Peptide Inserts. Proc. Natl. Acad. Sci. U. S. A. 1989;86:9788–9792. doi: 10.1073/pnas.86.24.9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputi M, Mayeda A, Krainer AR, Zahler AM. hnRNP A/B proteins are required for inhibition of HIV-1 pre-mRNA splicing. EMBO J. 1999;18:4060–7. doi: 10.1093/emboj/18.14.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen IH, Li L, Silva L, Sandri-Goldin RM. ICP27 recruits Aly/REF but not TAP/NXF1 to herpes simplex virus type 1 transcription sites although TAP/NXF1 is required for ICP27 export. J. Virol. 2005;79:3949–61. doi: 10.1128/JVI.79.7.3949-3961.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Li Y, Krug RM. Influenza A virus NS1 protein targets poly(A)-binding protein II of the cellular 3′-end processing machinery. EMBO J. 1999;18:2273–83. doi: 10.1093/emboj/18.8.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das K, Ma LC, Xiao R, Radvansky B, Aramini J, Zhao L, Marklund J, Kuo RL, Twu KY, Arnold E, Krug RM, Montelione GT. Structural basis for suppression of a host antiviral response by influenza A virus. Proc. Natl. Acad. Sci. U. S. A. 2008;105:13093–8. doi: 10.1073/pnas.0805213105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Luna S, Fortes P, Beloso A, Ortin J. Influenza virus NS1 protein enhances the rate of translation initiation of viral mRNAs. J. Virol. 1995;69:2427–33. doi: 10.1128/jvi.69.4.2427-2433.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss G, Matunis MJ, Pinol-Roma S, Burd CG. hnRNP proteins and the biogenesis of mRNA. Annu. Rev. Biochem. 1993;62:289–321. doi: 10.1146/annurev.bi.62.070193.001445. [DOI] [PubMed] [Google Scholar]
- Du Y, Gu S, Zhou J, Wang T, Cai H, Macinnes MA, Bradbury EM, Chen X. The dynamic alterations of H2AX complex during DNA repair detected by a proteomic approach reveal the critical roles of Ca(2+)/calmodulin in the ionizing radiation-induced cell cycle arrest. Mol. Cell. Proteomics. 2006;5:1033–44. doi: 10.1074/mcp.M500327-MCP200. [DOI] [PubMed] [Google Scholar]
- Ehrhardt C, Wolff T, Pleschka S, Planz O, Beermann W, Bode JG, Schmolke M, Ludwig S. Influenza A virus NS1 protein activates the PI3K/Akt pathway to mediate antiapoptotic signaling responses. J. Virol. 2007;81:3058–67. doi: 10.1128/JVI.02082-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enami K, Sato TA, Nakada S, Enami M. Influenza virus NS1 protein stimulates translation of the M1 protein. J. Virol. 1994;68:1432–7. doi: 10.1128/jvi.68.3.1432-1437.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford LP, Wright WE, Shay JW. A model for heterogeneous nuclear ribonucleoproteins in telomere and telomerase regulation. Oncogene. 2002;21:580–583. doi: 10.1038/sj.onc.1205086. [DOI] [PubMed] [Google Scholar]
- Garaigorta U, Ortin J. Mutation analysis of a recombinant NS replicon shows that influenza virus NS1 protein blocks the splicing and nucleo-cytoplasmic transport of its own viral mRNA. Nucleic Acids Res. 2007;35:4573–4582. doi: 10.1093/nar/gkm230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss GK, Salvatore M, Tumpey TM, Carter VS, Wang X, Basler CF, Taubenberger JK, Bumgarner RE, Palese P, Katze MG, Garcia-Sastre A. Cellular transcriptional profiling in influenza A virus-infected lung epithelial cells: the role of the nonstructural NS1 protein in the evasion of the host innate defense and its potential contribution to pandemic influenza. Proc. Natl. Acad. Sci. U. S. A. 2002;99:10736–41. doi: 10.1073/pnas.112338099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale BG, Randall RE, Ortin J, Jackson D. The multifunctional NS1 protein of influenza A viruses. J. Gen. Virol. 2008;89:2359–76. doi: 10.1099/vir.0.2008/004606-0. [DOI] [PubMed] [Google Scholar]
- Han SP, Tang YH, Smith R. Functional diversity of the hnRNPs: past, present and perspectives. Biochem. J. 2010;430:379–92. doi: 10.1042/BJ20100396. [DOI] [PubMed] [Google Scholar]
- Hao L, Sakurai A, Watanabe T, Sorensen E, Nidom CA, Newton MA, Ahlquist P, Kawaoka Y. Drosophila RNAi screen identifies host genes important for influenza virus replication. Nature. 2008;454:890–3. doi: 10.1038/nature07151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz C, Stavnezer E, Krug R, Gurney T., Jr. Influenza virus, an RNA virus, synthesizes its messenger RNA in the nucleus of infected cells. Cell. 1981;26:391–400. doi: 10.1016/0092-8674(81)90208-7. [DOI] [PubMed] [Google Scholar]
- Hoffmann E, Stech J, Guan Y, Webster RG, Perez DR. Universal primer set for the full-length amplification of all influenza A viruses. Arch. Virol. 2001;146:2275–2289. doi: 10.1007/s007050170002. [DOI] [PubMed] [Google Scholar]
- Iwanaga K, Sueoka N, Sato A, Hayashi S, Sueoka E. Heterogeneous nuclear ribonucleoprotein B1 protein impairs DNA repair mediated through the inhibition of DNA-dependent protein kinase activity. Biochem. Biophys. Res. Commun. 2005;333:888–895. doi: 10.1016/j.bbrc.2005.05.180. [DOI] [PubMed] [Google Scholar]
- Jorba N, Juarez S, Torreira E, Gastaminza P, Zamarreno N, Albar JP, Ortin J. Analysis of the interaction of influenza virus polymerase complex with human cell factors. Proteomics. 2008;8:2077–2088. doi: 10.1002/pmic.200700508. [DOI] [PubMed] [Google Scholar]
- Kamma H, Horiguchi H, Wan L, Matsui M, Fujiwara M, Fujimoto M, Yazawa T, Dreyfuss G. Molecular characterization of the hnRNP A2/B1 proteins: tissue-specific expression and novel isoforms. Exp. Cell Res. 1999;246:399–411. doi: 10.1006/excr.1998.4323. [DOI] [PubMed] [Google Scholar]
- Koffa MD, Clements JB, Izaurralde E, Wadd S, Wilson SA, Mattaj IW, Kuersten S. Herpes simplex virus ICP27 protein provides viral mRNAs with access to the cellular mRNA export pathway. EMBO J. 2001;20:5769–78. doi: 10.1093/emboj/20.20.5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozu T, Henrich B, Schafer KP. Structure and Expression of the Gene (Hnrpa2b1) Encoding the Human Hnrnp Protein A2/B1. Genomics. 1995;25:365–371. doi: 10.1016/0888-7543(95)80035-k. [DOI] [PubMed] [Google Scholar]
- Krecic AM, Swanson MS. hnRNP complexes: composition, structure, and function. Curr. Opin. Cell Biol. 1999;11:363–371. doi: 10.1016/S0955-0674(99)80051-9. [DOI] [PubMed] [Google Scholar]
- Krug RM, Yuan WM, Noah DL, Latham AG. Intracellular warfare between human influenza viruses and human cells: the roles of the viral NS1 protein. Virology. 2003;309:181–189. doi: 10.1016/s0042-6822(03)00119-3. [DOI] [PubMed] [Google Scholar]
- Lamb RA, Lai CJ. Sequence of interrupted and uninterrupted mRNAs and cloned DNA coding for the two overlapping nonstructural proteins of influenza virus. Cell. 1980;21:475–85. doi: 10.1016/0092-8674(80)90484-5. [DOI] [PubMed] [Google Scholar]
- Lazarowitz SG, Compans RW, Choppin PW. Influenza virus structural and nonstructural proteins in infected cells and their plasma membranes. Virology. 1971;46:830–43. doi: 10.1016/0042-6822(71)90084-5. [DOI] [PubMed] [Google Scholar]
- Li L, Sacks DB. Functional interactions between calmodulin and estrogen receptor-alpha. Cell Signal. 2007;19:439–43. doi: 10.1016/j.cellsig.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Li YZ, Yamakita Y, Krug RM. Regulation of a nuclear export signal by an adjacent inhibitory sequence: The effector domain of the influenza virus NS1 protein. Proc. Natl. Acad. Sci. U. S. A. 1998;95:4864–4869. doi: 10.1073/pnas.95.9.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Joyal JL, Sacks DB. Calmodulin enhances the stability of the estrogen receptor. J. Biol. Chem. 2001;276:17354–60. doi: 10.1074/jbc.M010238200. [DOI] [PubMed] [Google Scholar]
- Lin JY, Shih SR, Pan M, Li C, Lue CF, Stollar V, Li ML. hnRNP A1 interacts with the 5′ untranslated regions of enterovirus 71 and Sindbis virus RNA and is required for viral replication. J. Virol. 2009;83:6106–14. doi: 10.1128/JVI.02476-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lischka P, Toth Z, Thomas M, Mueller R, Stamminger T. The UL69 transactivator protein of human cytomegalovirus interacts with DEXD/H-Box RNA helicase UAP56 to promote cytoplasmic accumulation of unspliced RNA. Mol. Cell. Biol. 2006;26:1631–43. doi: 10.1128/MCB.26.5.1631-1643.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhou J, Wang Y, Mason RJ, Funk CJ, Du Y. Proteome alterations in primary human alveolar macrophages in response to influenza A virus infection. J. Proteome Res. 2012;11:4091–101. doi: 10.1021/pr3001332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(−Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luo ML, Zhou Z, Magni K, Christoforides C, Rappsilber J, Mann M, Reed R. Pre-mRNA splicing and mRNA export linked by direct interactions between UAP56 and Aly. Nature. 2001;413:644–7. doi: 10.1038/35098106. [DOI] [PubMed] [Google Scholar]
- Mayeda A, Munroe SH, Caceres JF, Krainer AR. Function of conserved domains of hnRNP A1 and other hnRNP A/B proteins. EMBO J. 1994;13:5483–95. doi: 10.1002/j.1460-2075.1994.tb06883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer D, Molawi K, Martinez-Sobrido L, Ghanem A, Thomas S, Baginsky S, Grossmann J, Garcia-Sastre A, Schwemmle M. Identification of cellular interaction partners of the influenza virus ribonucleoprotein complex and polymerase complex using proteomic-based approaches. J Proteome Res. 2007;6:672–682. doi: 10.1021/pr060432u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mibayashi M, Martinez-Sobrido L, Loo YM, Cardenas WB, Gale M, Jr., Garcia-Sastre A. Inhibition of retinoic acid-inducible gene I-mediated induction of beta interferon by the NS1 protein of influenza A virus. J. Virol. 2007;81:514–24. doi: 10.1128/JVI.01265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min JY, Li SD, Sen GC, Krug RM. A site on the influenza A virus NS1 protein mediates both inhibition of PKR activation and temporal regulation of viral RNA synthesis. Virology. 2007;363:236–243. doi: 10.1016/j.virol.2007.01.038. [DOI] [PubMed] [Google Scholar]
- Munro TP, Magee RJ, Kidd GJ, Carson JH, Barbarese E, Smith LM, Smith R. Mutational analysis of a heterogeneous nuclear ribonucleoprotein A2 response element for RNA trafficking. J. Biol. Chem. 1999;274:34389–34395. doi: 10.1074/jbc.274.48.34389. [DOI] [PubMed] [Google Scholar]
- Nemeroff ME, Barabino SM, Li Y, Keller W, Krug RM. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3′end formation of cellular pre-mRNAs. Mol. Cell. 1998;1:991–1000. doi: 10.1016/s1097-2765(00)80099-4. [DOI] [PubMed] [Google Scholar]
- Patry C, Bouchard L, Labrecque P, Gendron D, Lemieux B, Toutant J, Lapointe E, Wellinger R, Chabot B. Small interfering RNA-mediated reduction in heterogeneous nuclear ribonucleoparticule A1/A2 proteins induces apoptosis in human cancer cells but not in normal mortal cell lines. Cancer Res. 2003;63:7679–7688. [PubMed] [Google Scholar]
- Pinol-Roma S, Dreyfuss G. Shuttling of pre-mRNA binding proteins between nucleus and cytoplasm. Nature. 1992;355:730–732. doi: 10.1038/355730a0. [DOI] [PubMed] [Google Scholar]
- Pinolroma S, Choi YD, Matunis MJ, Dreyfuss G. Immunopurification of Heterogeneous Nuclear Ribonucleoprotein-Particles Reveals an Assortment of Rna-Binding Proteins. Genes Dev. 1988;2:215–227. doi: 10.1101/gad.2.2.215. [DOI] [PubMed] [Google Scholar]
- Read EK, Digard P. Individual influenza A virus mRNAs show differential dependence on cellular NXF1/TAP for their nuclear export. J. Gen. Virol. 2010;91:1290–301. doi: 10.1099/vir.0.018564-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R, Hurt E. A conserved mRNA export machinery coupled to pre-mRNA splicing. Cell. 2002;108:523–31. doi: 10.1016/s0092-8674(02)00627-x. [DOI] [PubMed] [Google Scholar]
- Robb NC, Jackson D, Vreede FT, Fodor E. Splicing of influenza A virus NS1 mRNA is independent of the viral NS1 protein. J. Gen. Virol. 2010;91:2331–2340. doi: 10.1099/vir.0.022004-0. [DOI] [PubMed] [Google Scholar]
- Satterly N, Tsai PL, van Deursen J, Nussenzveig DR, Wang Y, Faria PA, Levay A, Levy DE, Fontoura BM. Influenza virus targets the mRNA export machinery and the nuclear pore complex. Proc. Natl. Acad. Sci. U. S. A. 2007;104:1853–8. doi: 10.1073/pnas.0610977104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J, Dauber B, Melen K, Julkunen I, Wolff T. Analysis of influenza B Virus NS1 protein trafficking reveals a novel interaction with nuclear speckle domains. J. Virol. 2009;83:701–11. doi: 10.1128/JVI.01858-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J, Wolff T. Nuclear functions of the influenza A and B viruses NS1 proteins: do they play a role in viral mRNA export? Vaccine. 2009;27:6312–6. doi: 10.1016/j.vaccine.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Shan JG, Munro TP, Barbarese E, Carson JH, Smith R. A molecular mechanism for mRNA trafficking in neuronal dendrites. J. Neurosci. 2003;23:8859–8866. doi: 10.1523/JNEUROSCI.23-26-08859.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YK, Liu Q, Tikoo SK, Babiuk LA, Zhou Y. Effect of the phosphatidylinositol 3-kinase/Akt pathway on influenza A virus propagation. J. Gen. Virol. 2007;88:942–50. doi: 10.1099/vir.0.82483-0. [DOI] [PubMed] [Google Scholar]
- Shyu AB, Wilkinson MF. The double lives of shuttling mRNA binding proteins. Cell. 2000;102:135–138. doi: 10.1016/s0092-8674(00)00018-0. [DOI] [PubMed] [Google Scholar]
- Sinnamon JR, Czaplinski K. mRNA trafficking and local translation: the Yin and Yang of regulating mRNA localization in neurons. Acta Biochim. Biophys. Sin. (Shanghai) 2011;43:663–70. doi: 10.1093/abbs/gmr058. [DOI] [PubMed] [Google Scholar]
- Strasser K, Hurt E. Yra1p, a conserved nuclear RNA-binding protein, interacts directly with Mex67p and is required for mRNA export. EMBO J. 2000;19:410–20. doi: 10.1093/emboj/19.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz F, Bachi A, Doerks T, Braun IC, Seraphin B, Wilm M, Bork P, Izaurralde E. REF, an evolutionary conserved family of hnRNP-like proteins, interacts with TAP/Mex67p and participates in mRNA nuclear export. RNA. 2000;6:638–50. doi: 10.1017/s1355838200000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz F, Izaurralde E. The interplay of nuclear mRNP assembly, mRNA surveillance and export. Trends Cell Biol. 2003;13:319–27. doi: 10.1016/s0962-8924(03)00106-5. [DOI] [PubMed] [Google Scholar]
- Svitkin YV, Ovchinnikov LP, Dreyfuss G, Sonenberg N. General RNA binding proteins render translation cap dependent. EMBO J. 1996;15:7147–55. [PMC free article] [PubMed] [Google Scholar]
- Tisoncik JR, Billharz R, Burmakina S, Belisle SE, Proll SC, Korth MJ, Garcia-Sastre A, Katze MG. The NS1 protein of influenza A virus suppresses interferon-regulated activation of antigen-presentation and immune-proteasome pathways. J. Gen. Virol. 2011;92:2093–104. doi: 10.1099/vir.0.032060-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ufer C. The biology of the RNA binding protein guanine-rich sequence binding factor 1. Curr. Protein Pept. Sci. 2012;13:347–57. doi: 10.2174/138920312801619457. [DOI] [PubMed] [Google Scholar]
- Wang T, Gu S, Ronni T, Du Y, Chen X. In vivo dual-tagging proteomic approach in studying signaling pathways in immune response. J. Proteome Res. 2005;4:941–949. doi: 10.1021/pr050031z. [DOI] [PubMed] [Google Scholar]
- Wang W, Cui ZQ, Han H, Zhang ZP, Wei HP, Zhou YF, Chen Z, Zhang XE. Imaging and characterizing influenza A virus mRNA transport in living cells. Nucleic Acids Res. 2008;36:4913–28. doi: 10.1093/nar/gkn475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li M, Zheng H, Muster T, Palese P, Beg AA, Garcia-Sastre A. Influenza A virus NS1 protein prevents activation of NF-kappaB and induction of alpha/beta interferon. J. Virol. 2000;74:11566–73. doi: 10.1128/jvi.74.24.11566-11573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhou J, Ruan C, Du Y. Inhibition of Type I Interferon Production via Suppressing IKK-Gamma Expression: A New Strategy for Counteracting Host Antiviral Defense by Influenza A Viruses? J. Proteome Res. 2012;11:217–223. doi: 10.1021/pr200894t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YM, Zhu W, Levy DE. Nuclear and cytoplasmic mRNA quantification by SYBR green based real-time RT-PCR. Methods. 2006;39:356–362. doi: 10.1016/j.ymeth.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Wolff T, O’Neill RE, Palese P. NS1-binding protein (NS1-BP): a novel human protein that interacts with the influenza A virus nonstructural NS1 protein is relocalized in the nuclei of infected cells. J. Virol. 1998;72:7170–7180. doi: 10.1128/jvi.72.9.7170-7180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Zhou J, Du Y. Estrogen receptor alpha interacts with mitochondrial protein HADHB and affects beta-oxidation activity. Mol. Cell. Proteomics. 2012a;11:M111 011056. doi: 10.1074/mcp.M111.011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Zhou J, Du Y. Estrogen receptor beta interacts and colocalizes with HADHB in mitochondria. Biochem. Biophys. Res. Commun. 2012b;427:305–8. doi: 10.1016/j.bbrc.2012.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LJ, Altmann SW. mRNA and 18S-RNA coapplication-reverse transcription for quantitative gene expression analysis. Anal. Biochem. 2005;345:102–109. doi: 10.1016/j.ab.2005.07.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.