Abstract
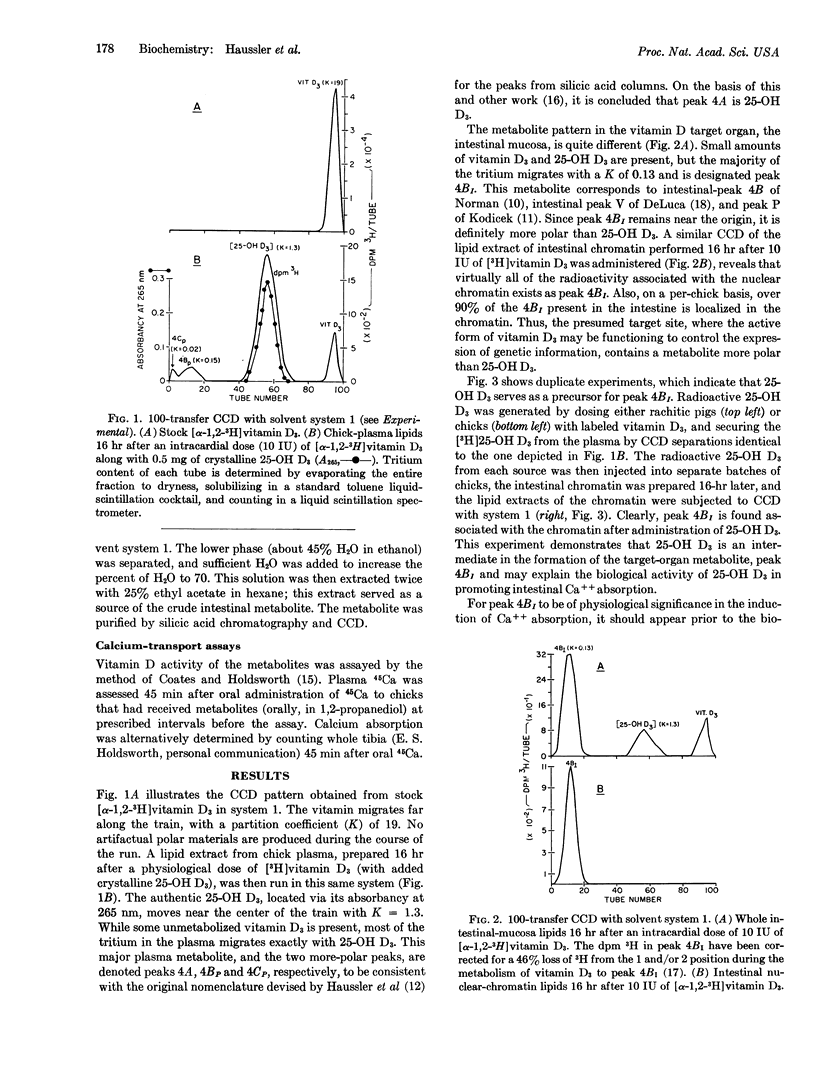
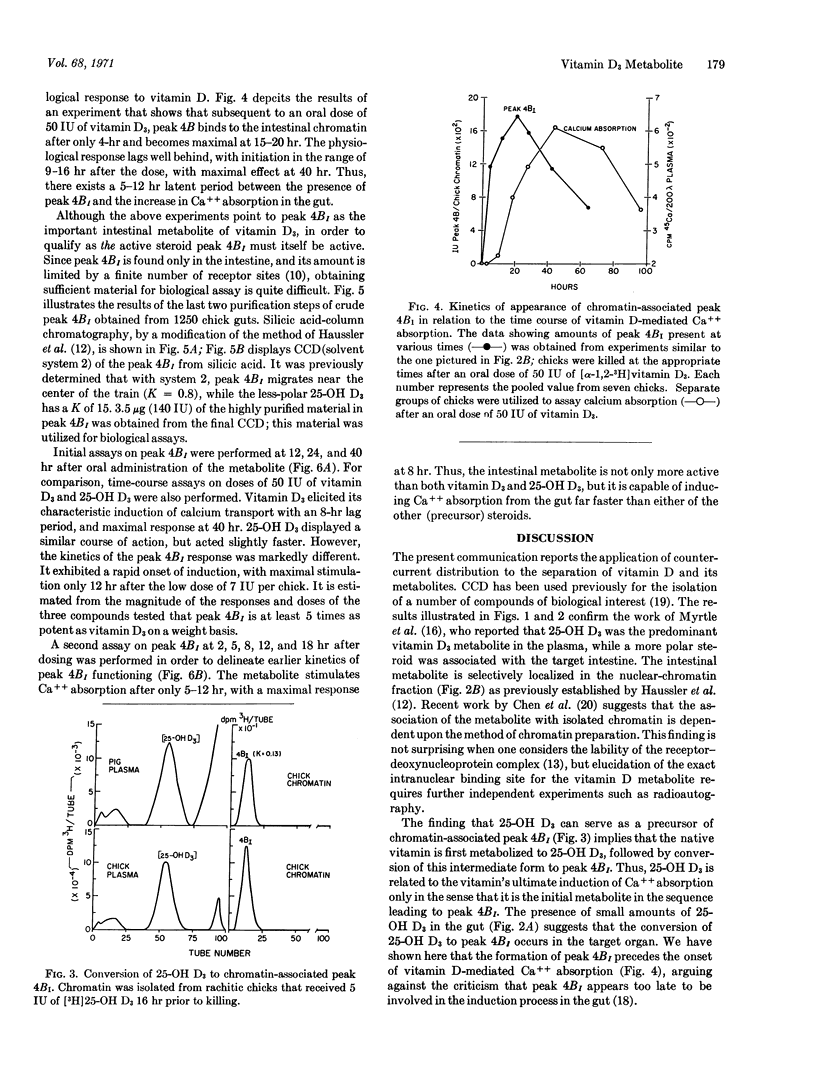
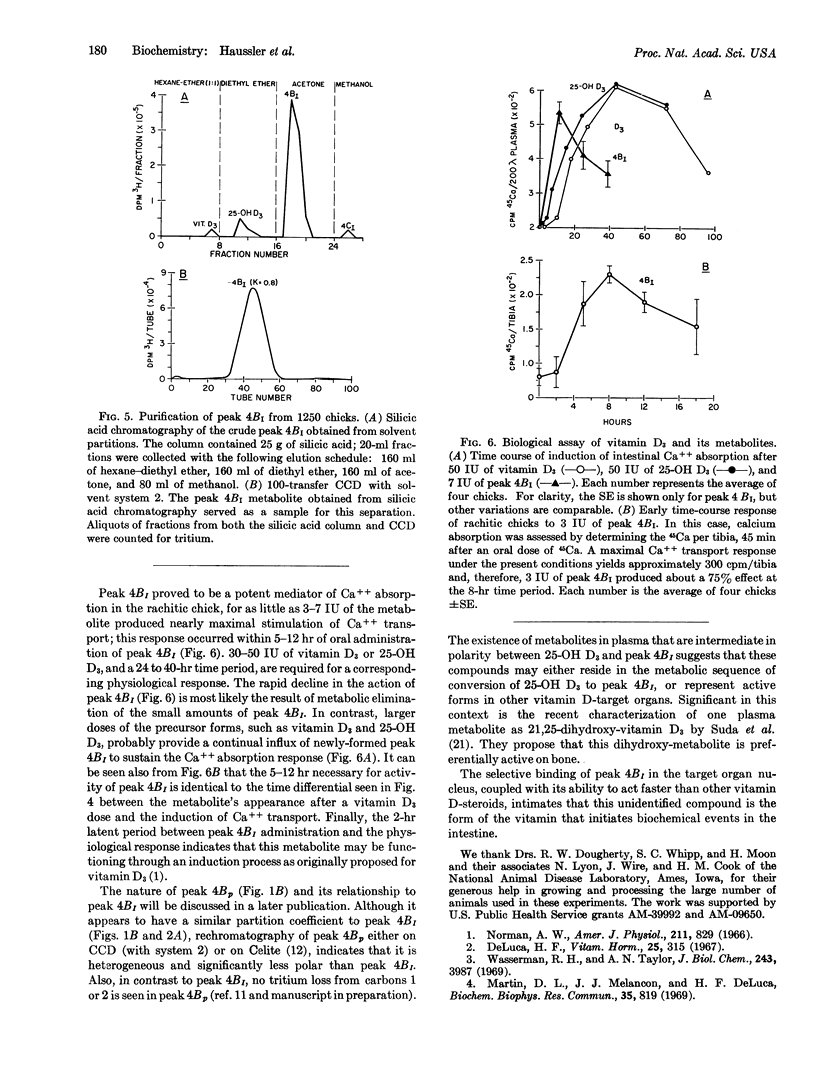
A vitamin D3 metabolite in intestine more polar than 25-hydroxy-vitamin D3(25-OH D3) has been detected by countercurrent distribution. The intestinal metabolite is found also after administration of labeled hydroxy D3, indicating that it arises from vitamin D3 via the intermediate 25-hydroxy derivative. The more polar metabolite is localized in the nuclear-chromatin fraction and appears in the gut before the physiological response to vitamin D3.
3.5 μg of the intestinal metabolite was isolated from 1250 chickens; the resulting purified material proved to be a potent mediator of calcium absorption. On a weight basis, it was at least 5 times as effective as vitamin D3, and acted 3 times faster than either hydroxy D3 or D3 in stimulating intestinal calcium transport in the rachitic chick. It is proposed that this as yet uncharacterized steroid represents the active form of vitamin D3 in the intestine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Blunt J. W., DeLuca H. F., Schnoes H. K. 25-hydroxycholecalciferol. A biologically active metabolite of vitamin D3. Biochemistry. 1968 Oct;7(10):3317–3322. doi: 10.1021/bi00850a001. [DOI] [PubMed] [Google Scholar]
- Blunt J. W., Tanaka Y., DeLuca H. F. Biological activity of 25-hydroxycholecalciferol, a metabolite of vitamin D3. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1503–1506. doi: 10.1073/pnas.61.4.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COATES M. E., HOLDSWORTH E. S. Vitamin D3 and absorption of calcium in the chick. Br J Nutr. 1961;15:131–147. doi: 10.1079/bjn19610014. [DOI] [PubMed] [Google Scholar]
- Chen T. C., Weber J. C., DeLuca H. F. On the subcellular location of vitamin D metabolites in intestine. J Biol Chem. 1970 Aug 10;245(15):3776–3780. [PubMed] [Google Scholar]
- DeLuca H. F. Mechanism of action and metabolic fate of vitamin D. Vitam Horm. 1967;25:315–367. doi: 10.1016/s0083-6729(08)60039-4. [DOI] [PubMed] [Google Scholar]
- Haussler M. R., Myrtle J. F., Norman A. W. The association of a metabolite of vitamin D3 with intestinal mucosa chromatin in vivo. J Biol Chem. 1968 Aug 10;243(15):4055–4064. [PubMed] [Google Scholar]
- Haussler M. R., Norman A. W. Chromosomal receptor for a vitamin D metabolite. Proc Natl Acad Sci U S A. 1969 Jan;62(1):155–162. doi: 10.1073/pnas.62.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING T. P., CRAIG L. C. Countercurrent distribution. Methods Biochem Anal. 1962;10:201–228. doi: 10.1002/9780470110270.ch7. [DOI] [PubMed] [Google Scholar]
- Lawson D. E., Wilson P. W., Kodicek E. Metabolism of vitamin D. A new cholecalciferol metabolite, involving loss of hydrogen at C-1, in chick intestinal nuclei. Biochem J. 1969 Nov;115(2):269–277. doi: 10.1042/bj1150269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J., DeLuca H. F. Biologically active metabolite of vitamin D3 from bone, liver, and blood serum. J Lipid Res. 1966 Nov;7(6):739–744. [PubMed] [Google Scholar]
- Martin D. L., Melancon M. J., Jr, DeLuca H. F. Vitamin D stimulated, calcium-dependent adenosine triphosphatase from brush borders of rat small intestine. Biochem Biophys Res Commun. 1969 Jun 27;35(6):819–823. doi: 10.1016/0006-291x(69)90697-4. [DOI] [PubMed] [Google Scholar]
- Myrtle J. F., Haussler M. R., Norman A. W. Evidence for the biologically active form of cholecalciferol in the intestine. J Biol Chem. 1970 Mar 10;245(5):1190–1196. [PubMed] [Google Scholar]
- Norman A. W. Actinomycin D effect on lag in vitamin D-mediated calcium absorption in the chick. Am J Physiol. 1966 Sep;211(3):829–834. doi: 10.1152/ajplegacy.1966.211.3.829. [DOI] [PubMed] [Google Scholar]
- Norman A. W., Haussler M. R., Adams T. H., Myrtle J. F., Roberts P., Hibberd K. A. Basic studies on the mechanism of action of vitamin D. Am J Clin Nutr. 1969 Apr;22(4):396–411. doi: 10.1093/ajcn/22.4.396. [DOI] [PubMed] [Google Scholar]
- Ponchon G., DeLuca H. F. Metabolism and biological activity of vitamin D. Calcif Tissue Res. 1970;(Suppl):43–44. doi: 10.1007/BF02152345. [DOI] [PubMed] [Google Scholar]
- Suda T., DeLuca H. F., Schnoes H. K., Ponchon G., Tanaka Y., Holick M. F. 21,25-dihydroxycholecalciferol. A metabolite of vitamin D3 preferentially active on bone. Biochemistry. 1970 Jul 7;9(14):2917–2922. doi: 10.1021/bi00816a025. [DOI] [PubMed] [Google Scholar]
- Wasserman R. H., Taylor A. N. Vitamin D-dependent calcium-binding protein. Response to some physiological and nutritional variables. J Biol Chem. 1968 Jul 25;243(14):3987–3993. [PubMed] [Google Scholar]


