Abstract
Tumors are sustained by complex networks of interactions between malignant cells, stromal cells and tumor-infiltrating immune cells. These networks differ from patient to patient in terms of nature, composition and organization as well as with regard to the precise localization of tumor-infiltrating cells. Of note, the heterogeneity of the immunological component of the tumor microenvironment, as opposed to its mere abundance, has been shown to influence disease outcome. However, a key question remains: where does the activation of tumor-specific T cells take place? The recently described, tumor-associated lymph node-like entities termed “tertiary lymphoid structures” exhibit a structural organization that is reminiscent of secondary lymphoid organs, and thus may imprint the local immune contexture. Here, we discuss how cancer-associated tertiary lymphoid structures impact on the tumor micro-architecture, immune microenvironment, and ultimately, patient survival.
Keywords: adaptive immune response, migration, tertiary lymphoid structure, tumor immunology, vasculature
Introduction
A tumor is a complex network of interacting cellular compartments, comprising malignant cells as well as endothelial, stromal, and immune cells. This so-called “tumor interactome,” which differs from patient to patient, is intimately associated with tumor progression and disease outcome.1
Tumors have been shown to establish an immunosuppressive microenvironment that robustly inhibits, if not completely neutralizes, immune effector cells. This is instrumental for malignant cells to replicate uncontrolled and spread throughout the entire body. Several lines of evidence from mouse and human studies have reported that the immune system can control oncogenesis and tumor progression in spite of such an immunosuppressive pressure, at least under selected circumstances.2,3 More particularly, an association between an abundant infiltration of neoplastic lesions by T cells with improved clinical outcome has been reported in patients affected by multiple types of solid neoplasms.2 However, the mechanisms that govern the shaping of an efficient immune contexture within the tumor remain poorly understood. Moreover, the site(s) at which the activation of tumor-specific effector cells takes place remain(s) unknown. The dogma states that professional antigen-presenting cells (APCs) sample and process antigens within the tumor, then migrate through lymphatic and/or blood vessels to tumor-draining lymph nodes, where they present processed peptides to antigen-specific T and B cells.4 However, an increasing body of evidence has demonstrated that adaptive immune responses that efficiently protect the host against infectious agents can be initiated independently of secondary lymphoid organs (SLOs).5,6 In this setting, antigen presentation appears to take place in ectopic lymph node-like structures that have been named tertiary lymphoid structures (TLSs). TLSs share many structural characteristics with SLOs. In particular, T and B lymphocytes are segregated into 2 distinct and adjacent regions surrounded by specialized blood vessels called high endothelial venules (HEVs), which allows the extravasation of lymphocytes from the blood to the tissue. The T-cell rich area comprises clusters of T cells and mature dendritic cells (DCs). A B-cell follicle is also present, encompassing a ring of naïve B cells around a germinal center that mainly contains B cells, but also T cells, follicular DCs, and macrophages.
The immunological functions of TLSs in cancer have been poorly investigated. Here, we will review novel insights on the impact of cancer-associated TLSs on the micro-architecture of the tumor microenvironment, i.e., its vasculature and global immune contexture, and how the presence of TLSs ultimately affects patient survival.
The Tumor Microenvironment: a Permissive Milieu for Lymphoid Neogenesis?
TLSs have first been described in humans in many non-neoplastic inflammatory contexts, such as autoimmune diseases, viral and bacterial infections, graft rejection, and several idiopathic diseases.5-7 The inducible nature of TLSs was first highlighted by Kratz and colleagues in a transgenic mouse model expressing lymphotoxin (LT)α in pancreatic islets (RIP-LTα model). These authors proposed the term “lymphoid neogenesis” for the process governing the development of TLSs.8 In contrast to Kratz and colleagues, Moyron-Quiroz et al. observed the genesis of TLSs in a LTα-deficient mice,9,10 although these structures appeared less organized than in wild-type animals, primarily consisting of B cells but containing few T cells, no follicular DCs, and no HEVs. These data suggest that LTα participates in the organization of TLSs but it is not an absolute requirement for lymphoid neogenesis. In the RIP-LTα model, LTα induced the expression of multiple lymphoid chemokines, including chemokine (C-C motif) ligand 19 (CCL19), CCL21, and chemokine (C-X-C motif) ligand 13 (CXCL13).11 In addition, the ectopic expression of CCL19, CCL21, and CXCL13 promoted the local recruitment of T and B cells into TLS,7,12 and the absence of one of these chemokines was sufficient to abrogate the development of TLSs in the lungs of mice infected by the influenza virus.13 Thus, even if each chemokine can act as a TLS inducer, their concomitant expression seems mandatory for a complete program of lymphoid neogenesis. Subsequently, lymphoid chemokines were shown to sustain lymphoid neogenesis by participating in a self-amplificatory loop involving their own expression and that of LTα, as clearly demonstrated in SLOs.
It is well known that lymphoid tissue inducer (LTi) cells play a critical role in the initial phases of lymphoid organogenesis. Thus, many studies have investigated the role of LTi cells in TLS neogenesis. It seems that TLS induction can be initiated independently from LTi cells, as TLSs can emerge in their absence.7,14 Thus, lymphoid neogenesis is likely to involve additional players that can substitute for LTi cells. Some immune cells have indeed the capacity to activate local resident mesenchymal stromal cells through LTα and tumor necrosis factor α (TNFα) receptors. Moreover, the expression of LTα1β2 by T cells can be induced by CCL21 and CCL19, whereas B cells can be stimulated to secrete LTα1β2 by CXCL13.15-17 Nevertheless, the precise stimulus initiating the development of TLSs, in particular cancer-associated TLSs, has not been clearly identified to date.
Several studies showed that TLSs are induced in a chronic inflammatory environment and their presence is associated with the exacerbation of local immune responses. Although the tumor microenvironment presents many similarities with such a setting (including the chronicity of the inflammation and the presence of functional APCs), it differs by at least one major aspect, its immunosuppressive nature.18,19 One could suspect that this immunosuppressive microenvironment would represent a major obstacle for the emergence of TLSs and/or for their functionality.
The presence of intratumoral lymphoid aggregates with varying levels of organization (i.e., comprising or not a B-cell follicle and/or HEVs) has been described in patients affected by primary colorectal cancer,20-24 breast carcinoma,24-30 melanoma,31-34 mucosal-associated lymphoid tissue (MALT) lymphoma,35,36 ovarian carcinoma,24 and lung cancer24,37-39 (Table 1). TLSs have been observed even in the context of metastatic disease, especially in colorectal carcinoma,40 renal cell carcinoma,40 and melanoma patients,31,34 demonstrating that lymphoid neogenesis can occur and/or be maintained at all disease stages. However, distinct levels of TLS organization have been observed in metastatic melanoma31 and primary lung cancer (unpublished data from our laboratory). Such differences may reflect different stages in the program of lymphoid neogenesis, as previously suggested in non-transformed inflammatory settings.10,41 Finally, the density of TLSs is highly heterogeneous even among tumors of a given type. This heterogeneity might also reflect the diversity of individuals tumor microenvironments, being more or less permissive to lymphoid neogenesis.
Table 1. Studies reporting the presence of tertiary lymphoid structures in human neoplasms.
| Cancers | Cellular composition of lymphoid agregates/TLS | Studied cases | Stage of the disease | References |
|---|---|---|---|---|
| Breast carcinoma | T cells (including CD4+ T cells), mature DCs | 32 patients | carcinoma in situ to grade III | Bell et al., 1999 |
| T cells, B cells (GC B cells and naive B cells), FDCs | 3 patients | grade II to III | Coronella et al., 2002 | |
| T cells, B cells, PCs, FDCs | 4 patients | ND | Nzula et al., 2003 | |
| lymphocytes (hematoxylin counterstaining) | 191 patients | grade II to III | Gobert et al., 2009 | |
| T cells, B cells, HEVs | 146 patients | grade I to III | Martinet et al., 2011 | |
| T cells (Tfh, CD4+ T cells and few CD8+ T cells), B cells (GC B cells), FDCs | 70 patients | grade I to III | Gu-Trantien et al., 2013 | |
| T cells, B cells, mature DCs, HEVs | 146 patients | grade I to III | Martinet et al., 2013 | |
| Colorectal carcinoma | T cells (including CD4+ T cells, memory T cells, few CD8+ T cells), B cells, mature DCs | 17 patients | ND | Suzuki et al., 2002 |
| T cells, mature DCs | 40 patients | grade I to IV | McMullen et al., 2010 | |
| T cells, B cells, FDCs | ND | ND | Bergomas et al., 2011 | |
| T cells, B cells (including B cell precursors), FDCs | 21 patients | grade 0 to IVA | Coppola et al., 2011 | |
| T cells, B cells, HEVs | 5 patients | ND | Martinet et al., 2011 | |
| T cells, B cells, mature DCs | 25 patients | ND | Remark et al., 2013 | |
| Colorectal carcinoma liver metastasis | mature DCs | 70 patients | ND | Miyagawa et al., 2004 |
| Colorectal carcinoma lung metastasis | T cells, B cells, mature DCs | 140 patients | stage IV | Remark et al., 2013 |
| Lung carcinoma | T cells (including CD4+ T cells and few CD8+ T cells), B cells (including GC B cells), mature DCs, FDCs | 74 patients | stage I to II | Dieu-Nosjean et al., 2008 |
| no NK cells | 86 patients | stage I to III | Platonova et al., 2011 | |
| T cells (including memory T cells and few naïve T cells), mature DCs, HEVs | 15 patients | stage I to III | de Chaisemartin et al., 2011 | |
| T cells, B cells, HEVs | 5 patients | ND | Martinet et al., 2011 | |
| Melanoma | memory T cells, mature DCs | 82 patients | stage IA to IIIA | Ladányi et al., 2007 |
| T cells (including CD4+ and CD8+ T cells, rare FoxP3+ cells), B cells, mature DCs | 21 patients | stage IV | Messina et al., 2012 | |
| T cells, B cells, HEVs | 18 patients | ND | Martinet et al., 2012 | |
| T cells (including CD8+ T cells), B cells (including AID+ GC B cells), mature DCs, FDCs, HEVs | 29 patients | stage IIIB to IV | Cipponi et al., 2012 | |
| Mucosal-Associated Lymphoid Tissue lymphoma | T cells, B cells (including naïve B cells, AID+ GC B cells, marginal zone B cells, malignant B cells), FDCs | 18 patients | low grade | Bombardieri et al., 2007 |
| T cells, B cells (including naïve B cells, AID+ GC B cells, marginal zone B cells, malignant B cells), FDCs | 20 patients | ND | Barone et al., 2008 | |
| Ovary carcinoma | T cells, B cells, HEVs | 18 patients | ND | Martinet et al., 2011 |
| Renal cell carcinoma | T cells, B cells, mature DCs | 24 patients | ND | Remark et al., 2013 |
| Renal cell carcinoma lung metastasis | T cells, B cells, mature DCs | 52 patients | stage IV | Remark et al., 2013 |
Abbreviations: DC, dendritic cell; FDC, follicular DC; GC, germinal center; HEV, high endothelial venule; ND, not determined; TLS, tertiary lymphoid structure.
TLSs and HEVs Crosstalk in a Finely Regulated Manner Within the Tumor Microenvironment
In many tumors, one of the major hurdles against the efficacy of chemo- and immunotherapy (including anticancer vaccines) is the limited accessibility of immune cells, notably T cells, to the neoplastic lesion. Several mechanisms have been proposed to underlie this phenomenon including the disruption of the architecture and structure of intratumoral blood vessels. The presence of an abnormal vasculature, associated with reduced vessel density, has been reported in multiple cancers, suggesting some sort of disturbance in the neoangiogenetic program. Two main types of blood vessels co-exist in tumors, CD34+ (or CD31+) blood vessels and peripheral node addressin (PNAd)+ HEVs, each of which can differentiate into the other under physiological and pathophysiological conditions. One study suggested that no correlation exists between the densities of blood vessels and that of HEVs in breast carcinoma, suggesting that their developmental programs can proceed independently from each other.24 Strikingly, the intratumoral density of these 2 different types of vessels has an opposite prognostic value in cancer patients. Indeed, high densities of CD34+ (or CD31+) blood vessels are associated with tumor growth and poor clinical outcome in breast,42 colorectal,43 and lung44-46 cancer patients. These data support a model according to which neoangiogenesis is stimulated by the tumor microenvironment, promoting the formation of vascular emboli and support the metastatic spread of neoplastic cells to distant sites. In contrast, the presence of HEVs, which have been observed in melanoma31,33 and breast cancer24,30 lesions, correlated with long-term patient survival.24,33,47 Moreover, the density of HEVs closely correlated with that of tumor-infiltrating T and B cells,24,33,47 and HEVs were shown to co-localize with cancer-associated TLSs.24,30,31,33,38 In lung cancer, the PNAd ligand CD62L, is expressed by most lymphocytes found within TLSs (with the exception of germinal center B cells), but not by lymphocytes present in the other areas of the neoplastic lesion.38 HEVs are vessels specialized in the extravasation of lymphocytes from the bloodstream to SLOs. Given the strong analogies between SLOs and TLSs, we suggest that HEVs may actively participate in the recruitment of circulating lymphocytes to intratumoral TLSs. Recently, Girard and colleagues demonstrated that DCs play a key role in the regulation of the entry of lymphocyte into SLOs as they maintain a fully mature HEV endothelium in a LTβ receptor-dependent manner.48,49 Mature DCs are exclusively detected in the T cell-rich areas of TLSs, adjacent to HEVs.30,31,33,38 Thus, it is tempting to speculate, but remains to be formally demonstrated, that DCs may regulate lymphocyte trafficking not only in SLOs but also in cancer-associated TLSs.
The mechanisms governing the extravasation of lymphocytes into SLOs are well characterized, and involve 3 main families of molecules: adhesion molecules, chemokines, and integrins. In lung cancer patients, a specific set of adhesion molecules expressed by HEVs have been selectively associated with the presence of TLS: intercellular adhesion molecule (ICAM)2, ICAM3, vascular cell adhesion molecule 1 (VCAM1), mucosal vascular addressin cell adhesion molecule 1 (MAdCAM1) and PNAd.38 In mouse and rat models, intratumoral endothelial cells were shown not to express ICAM1 and VCAM1, as opposed to endothelial cells in distant tissues,50,51 in accordance with the downregulation of VCAM1 on the surface of endothelial cells cultured in presence of neoplastic cells.52 HEVs expressing ICAM1, VCAM1, MAdCAM1, and PNAd have been also observed in the close proximity of lymphoid aggregates in LT-induced chronic inflammatory lesions.8 Moreover, the expression of ICAM2, VCAM1, and PNAd has also been observed in lymphoid aggregates found in non-inflamed human lungs,53,54 suggesting that TLSs could represent a favorable microenvironment for the differentiation and maintenance of blood vessels into functional HEVs. ICAM2 is involved in the arrest of circulating T cells along the HEV endothelium of SLOs,55 and may have the same function within TLSs. VCAM1 is induced upon inflammation, and contributes to the migration of circulating memory T cells to tissues.56 Thus, VCAM1 may represent a key molecule for the recruitment of memory T cells, which are the main T-cell components of lung cancer-associated TLSs.38 In contrast, MAdCAM1 is a gut-homing molecule that regulates T-cell migration,57 and has not previously shown to contribute to TLS. This suggests that HEVs from lung cancer-associated TLSs exhibit an adhesion molecule profile that differ from that of TLSs surging in non-neoplastic settings.
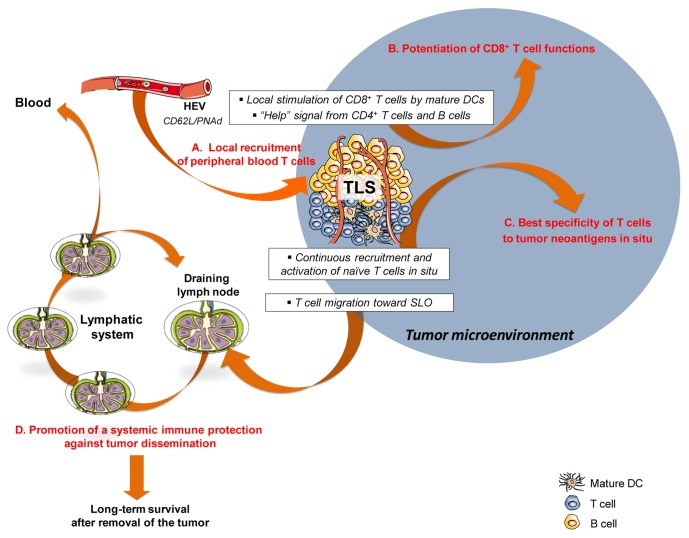
Altogether, these observations corroborate the hypothesis that TLSs may represent a privileged site for the maturation and maintenance of functional HEVs, unlike the other areas of the tumor microenvironment. TLS-serving HEVs may provide a gateway for the recruitment of circulating T cells into the tumor, and thus represent an interesting target for anticancer immunotherapy (Fig. 1A).
Figure 1. Role of tertiary lymphoid structures in the initiation of local and systemic protective immune responses against primary neoplastic lesions and metastases. DC, dendritic cell; HEV, high endothelial venule; SLO, secondary lymphoid organ; TLS, tertiary lymphoid structure.
Chemokines, an Orchestra Conductor of TLS Organization
The migration of lymphocytes into SLOs is dependent on the expression of various lymphoid chemokines, including CCL19, CCL21, and CXCL13.12,14,58 The overexpression of these chemokines has been documented in the TLSs of lung and breast carcinoma patients.27,38 Moreover, in several murine models, the injection of cancer cells engineered to express either CCL19 and/or CCL21 has been shown to induce a robust infiltration of developing neoplastic lesions by T cells and DCs.59-63 This has been associated with a reduction in tumor growth and, at least in some cases, with the induction of a systemic antitumor immune response linked to the control of metastatic dissemination.59,64 In human lung cancers, CCL19+ cells are predominantly mature DCs, which are selectively present in TLS.38 In the lung of mice infected by the influenza virus, the depletion of DCs correlated with a dramatic decrease in TLS density, and the few remaining TLSs were significantly disorganized.65,66 The maintenance of TLSs may be partly mediated by DCs via the recruitment of CCR7+ cells, including newly activated DCs and naïve and central-memory T cells, as we observed in lung cancer-associated TLSs.38 Recently, DCs have also been shown to play a critical role in the induction of lymphangiogenesis within TLSs in a LT- and CCL21-dependent manner.67 As observed in various inflammatory diseases, lung cancer-associated lymphatic vessels express CCL21. Thus, it is tempting to speculate that CCL21+ lymphatic vessels in the proximity of TLS may represent a major path for immune cells (e.g., activated DCs and central-memory T cells) to reach tumor-draining lymph nodes and establish a systemic protection against metastatic dissemination.
In human lung cancers, CXCL13 was detected on follicular DCs that homed specifically to the germinal centers of B-cell areas within TLSs.38 Therein, follicular DCs co-localized with a subset of TLS T cells expressing CD4 and chemokine (C-X-C motif) receptor 5 (CXCR5), hence displaying a phenotype of follicular helper T cells.38 Interestingly, CXCL13+ follicular helper T cells have recently been detected within breast carcinoma-associated TLSs, and their density was associated with long-term patient survival.27 The density of CXCR5+ follicular B cells also appears to correlated with a favorable clinical outcome among lung cancer patients (Dieu-Nosjean et al., unpublished data) suggesting that the expression of CXCL13 in cancer-associated TLSs may promote the local initiation of a protective humoral immune response through the recruitment of CXCR5+ cells into the germinal center.
CCL17 and CCL22 are also overexpressed in lung cancer-associated TLSs, in agreement with the higher proportion of CCR4+ T cells among TLS-associated T cells as compared with T cells found in other areas of the neoplastic lesion.38 CCL17 and CCL22 operate by a shared receptor (CCR4), which is expressed by many T-cell subpopulations including TH2, TH17, TH22 T cells, regulatory T cells (Tregs), and effector T cells under inflammatory conditions.68 Nonetheless, the role of the CCL17-CCL22/CCR4 in cancer remains controversial. In some murine models, the intratumoral injection of CCR4 ligands induces tumor regression with a concomitant recruitment of effector T cells,69-72 whereas in others studies a deleterious effect associated with a strong influx of Tregs has been reported.71,73,74 However, it should be noted that the prognostic value of tumor-infiltrating Tregs in cancer patients is also a matter of debate.2 This is likely due to the limited specificity of biomarkers for human Tregs, such as the transcription factor forkhead box P3 (FOXP3), which is also an indicator of the T-cell activation status. Gobert et al. have shown that Tregs are present in the stroma of breast tumors as well as within intratumoral lymphoid aggregates.29 The migration of Tregs to lymphoid structures is mediated by the CCL22/CCR4 axis. Tregs can be activated by mature DCs, and their high density in lymphoid aggregates is correlated with short-term survival of breast cancer patients. Murine models have illustrated that a high density of intratumoral Tregs correlates with low densities of HEVs, suggesting that HEV neogenesis is inhibited by Tregs.75,76 However, in melanoma patients, no correlation was observed between the density of HEVs and that of Tregs.33 This apparent discrepancy suggest that further investigations are required in order to better define the role of TLS-associated Tregs in oncogenesis and tumor progression.
Interleukin (IL)-16 is also expressed by T and B cells infiltrating lung cancer-associated TLSs.38 In an asthma model, IL-16 has been shown to operate as a chemoattractant for various CD4+ cells, including CD4+ T cells, DCs and myeloid cells.77,78 IL-16 can also prime CD4+ T cells to respond to IL-2 by proliferating, becoming activated and/or differentiating into memory cells.79 Thus, the role of IL-16 within TLSs may be of particular importance for the functionality of tumor-infiltrating T lymphocytes.
Thus, the expression of a defined set of chemokines by cancer-associated TLSs strongly suggests that some immune cell populations could be selectively recruited into TLSs with a putative key impact on the cell-to-cell cross-talk and global intratumoral immune contexture.
The Presence of TLS Impacts on the Local Immune Microenvironment
The selective recruitment of immune cells, especially naïve and central-memory CD62L+ T cells, to TLSs led us to hypothesize that TLSs may represent a privileged site for the differentiation and activation of tumor-infiltrating lymphocytes. To obtain further insights into these issues, we have compared the characteristics of the local immune microenvironment with the density of TLSs in patients with lung cancer (Goc et al., manuscript submitted). Patients were stratified into two groups according to the presence of a high or a low density of mature DCs, a cell population that is selectively detected within TLSs and hence is used as a specific TLS marker. Tumor bearing high densities of mature DCs (DChi tumors) contained increased amounts of CD4+ and CD8+ T cells (encompassing naïve, central-memory and effector-memory cells) as well as of activated T cells, as compared with tumors scarcely infiltrated by mature DCs (DClo tumors). Elevated TLS densities also correlated with the overexpression as well as the coordination of genes linked to TH1 polarization, cytotoxicity, activation, and immune effector functions. In contrast, genes involved in TH2 polarization, inflammation, immunosuppression, and angiogenesis were not differentially expressed by DChi and DClo tumors. These data indicate that high TLS densities are associated with a specific intratumoral contexture that is characterized by the coordination of adaptive cellular immune responses (Fig. 1B). This suggests that TLS might directly impact on the intratumoral immune contexture in lung cancer patients. Similar findings have been reported in lung metastases of colorectal carcinoma but not renal cell carcinoma, in agreement with the differential density of TLSs found in these cancer types.40 Of note, the protective effects of a TH1 and cytotoxic immune signature have been widely demonstrated in mice,80 and correlate with a favorable disease outcome in patients affected by most solid cancers (exception made for renal cell carcinoma and ocular melanoma2). However, the possibility that such immune signature may be associated with the density of TLSs has never been reported.
One of the remaining key questions is whether TLSs are directly involved in the induction of antitumor immune responses, or, vice versa, whether they relay locally an intense immune reaction initiated in SLOs. Several lines of evidence support the former possibility. First, it has been demonstrated that antitumor immune responses can develop within neoplastic lesions in mice lacking SLOs. This demonstrates that effective T-cell priming can be achieved within intratumoral TLSs independently of SLOs.81,82 Second, the presence of TLSs has been correlated with the generation of tumor-specific T cells, suggesting that TLSs could act as a functional site for the induction of protective antitumor immunity.82,83 Similar findings have also been obtained in models of infectious diseases,9,66 indicating that TLSs may represent an active site for the induction of adaptive immune responses in multiple settings.
In summary, it seems that the presence of a favorable (or at least a permissive) tumor microenvironment allows for the creation of TLSs, which is critical for the local coordination and polarization of protective antitumor immune responses.
TLS as a Novel Biomarker in Cancer
The majority of prognostic studies has highlighted a positive impact of intratumoral T cells on the survival of patients affected by solid tumors.2,84 The high reproducibility of these data strongly support the idea that one should take into account immune criteria for the prognostic staging of neoplastic lesions.85 Several studies have indeed reported a favorable clinical outcome for lung cancers patients whose neoplastic lesions contain a high density of CD8+ T cells (Refs. 86,87 and Goc et al., submitted manuscript). Moreover, a high density of mature DCs (a specific marker of TLS) has been correlated with the long-term survival of patients affected by early-stage, advanced and metastatic lung cancer (Refs. 37,40 and Goc et al., submitted manuscript).
Thus the survival of lung cancer patients appears to be positively influenced by the abundance of tumor-infiltrating mature DCs and CD8+ T cells. This raises the question of evaluating the clinical impact of these tumor-infiltrating cells in combination. The stratification of lung cancer patients (at all disease stages) according to low or high densities of intratumoral mature DCs and CD8+ T cells (DClo/CD8lo, DClo/CD8hi, DChi/CD8lo, and DChi/CD8hi patients) provided several interesting hints (Goc et al., submitted manuscript). First, patients with a high density of mature DCs, regardless of the density of CD8+ T cells (DChi/CD8lo and DChi/CD8hi groups), were at lower risk of death than their DClo counterparts, indicating that elevated amounts of intratumoral mature DCs are sufficient to favorably impact on clinical outcome. This said, it should be noted that most of DChi tumors are also highly infiltrated by CD8+ T cells. In this context, the scarcity of DChi/CD8lo tumors suggests a causal link between the presence of TLSs and that of tumor-infiltrating CD8+ T cells. This observation further supports the hypothesis that intratumoral TLSs represent an active site for the recruitment, activation, proliferation, and priming of tumor-infiltrating T cells. Second, patients with low intratumoral amounts of both mature DCs and CD8+ T cells (DClo/CD8lo group) turned out to have a poor clinical outcome as compared with the three other groupsm of patients (DClo/CD8hi, DChi/CD8lo, and DChi/CD8hi patients). This demonstrates that the DC/CD8 score allows for the identification of a subgroup of patients with a very high risk of death. Finally, patients with a high density of tumor-infiltrating CD8+ T cells but a low density of mature DCs (DClo/CD8hi individuals) exhibited an intermediate risk of death. These data suggest that a high density of tumor-infiltrating CD8+ T cells in the absence of a robust tumor infiltration by mature DCs is not sufficient to predict favorable disease outcome among lung cancer patients. The presence of mature DCs appears therefore to be required to license the positive prognostic value of tumor-infiltrating CD8+ T cells. In absence of TLSs, high density of intratumoral CD8+ T cells may be less efficient in maintaining long-term protective antitumor immunity. Similar results have been obtained with regard to tumor-infiltrating B cells and CD8+ T cells in ovarian cancer patients.88,89
The presence of TLSs may imprint the behavior of tumor-infiltrating CD8+ T cells. This assumption is in agreement with the involvement of TLSs in the local activation of CD8+ T cells, which has been demonstrated in different murine models.66,83,90 Several mechanisms might explain the survival benefit related to elevated densities of cancer-associated TLSs. Mature DCs, which may have engulfed a large spectrum of tumor-associated antigens (TAAs), are very efficient at presenting processed peptides to antigen-specific T cells (Fig. 1B). In addition, the recruitment of naïve T cells into TLSs is highly favorable for the continuous activation of new T-cell clones specific for newly arising TAAs (Fig. 1C). Moreover, the local stimulation of intratumoral T cells by mature DCs might potentiate their effector functions and limit their sensitivity to anergy and/or exhaustion.91,92 Finally, CD4+ T cells, which represent the major T-cell subset of TLSs, could deliver important help signals to CD8+ T cells. In a murine model of pancreatic cancer, it has been illustrated that the “help” coming from CD4+ T cells can also limit the induction of tolerance and the depletion of CD8+ T cells, promote the survival of effector-memory T cells and optimize their effector activity.89,93
It should be noted that the improved prognosis associated with a high density of tumor-infiltrating mature DCs was observed among lung cancer patients who underwent surgical tumor resection, and hence who were deprived of all tumor-infiltrating immune cells. This suggests that the prognostic value of intratumoral mature DCs is most likely related to the development of systemic antitumor immunity, in turn originating from local adaptive immune responses orchestrated within TLSs. As such, the presence of lymphatic vessels close to TLSs may participate in the emigration of some immune cell populations, including central-memory T cells, to tumor-draining lymph nodes, a key pathway for the establishment of robust systemic immunity against micro-metastasis (Fig. 1D).
Conclusions
The presence of TLSs has been documented in many solid tumors, in both mice and humans. Interestingly, TLSs manifest a huge variability in terms of density and cellular organization, indicating that the nature of tumor microenvironment is critical for lymphoid neogenesis. In this context, one of the challenges for the future is to precisely determine the conditions that provide an optimal environment for the induction and maintenance of cancer-associated TLSs.
In addition, the presence of particular vessels, namely, HEVs, around TLSs strongly suggests that this microenvironment represents an ideal gateway for the entry of circulating lymphocytes into TLSs, a step that is critical for the initiation of adaptive immune responses, as demonstrated in SLOs. Moreover, TLSs can imprint the local immune contexture, hence influencing disease outcome in cancer patients. Thus, the density of CD8+ T cells predicts long-term survival among lung cancer patients only in presence of TLS-associated DCs. These data need to be confirmed in patients affected by other solid tumors. Nonetheless, the presence of TLSs provides per se a survival benefit to cancer patients. We speculate that lymphoid neogenesis might represent an interesting target for eliciting and/or boosting antitumor immunity in cancer patients. Moreover, TLSs may be used in the future as a novel biomarker for the identification of cancer patients with a high risk of relapse. A deeper comprehension of the cellular and molecular mechanisms whereby TLSs confer long-term protection to cancer patients is essential to develop efficient strategies for their therapeutic manipulation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Pr. J. Bienenstock and Dr. J.-L. Teillaud for critical reading of the manuscript. We thank Dr. Helen Angell for English critics of this manuscript.
Glossary
Abbreviations:
- ADC
adenocarcinoma
- APC
antigen-presenting cell
- DC
dendritic cell
- HEV
high endothelial venule
- NSCLC
non-small-cell lung cancer
- LT
lymphotoxin
- LTi
lymphoid tissue inducer
- OS
overall survival
- SCC
squamous cell carcinoma
- SLO
secondary lymphoid organ
- TIL
tumor-infiltrating lymphocyte
- TLS
tertiary lymphoid structure
- Treg
regulatory T cell
Citation: Goc J, Fridman WH, Sautès-Fridman C, Dieu-Nosjean MC. Characteristics of tertiary lymphoid structures in primary cancers. OncoImmunology 2013; 2:e26836; 10.4161/onci.26836
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26836
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 3.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–7. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 4.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–9. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7:344–53. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- 6.Neyt K, Perros F, GeurtsvanKessel CH, Hammad H, Lambrecht BN. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol. 2012;33:297–305. doi: 10.1016/j.it.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–17. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 8.Kratz A, Campos-Neto A, Hanson MS, Ruddle NH. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J Exp Med. 1996;183:1461–72. doi: 10.1084/jem.183.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, Randall TD. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10:927–34. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 10.Moyron-Quiroz JE, Rangel-Moreno J, Hartson L, Kusser K, Tighe MP, Klonowski KD, Lefrançois L, Cauley LS, Harmsen AG, Lund FE, et al. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity. 2006;25:643–54. doi: 10.1016/j.immuni.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Hjelmström P, Fjell J, Nakagawa T, Sacca R, Cuff CA, Ruddle NH. Lymphoid tissue homing chemokines are expressed in chronic inflammation. Am J Pathol. 2000;156:1133–8. doi: 10.1016/S0002-9440(10)64981-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carragher DM, Rangel-Moreno J, Randall TD. Ectopic lymphoid tissues and local immunity. Semin Immunol. 2008;20:26–42. doi: 10.1016/j.smim.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rangel-Moreno J, Moyron-Quiroz JE, Hartson L, Kusser K, Randall TD. Pulmonary expression of CXC chemokine ligand 13, CC chemokine ligand 19, and CC chemokine ligand 21 is essential for local immunity to influenza. Proc Natl Acad Sci U S A. 2007;104:10577–82. doi: 10.1073/pnas.0700591104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cupedo T, Mebius RE. Role of chemokines in the development of secondary and tertiary lymphoid tissues. Semin Immunol. 2003;15:243–8. doi: 10.1016/j.smim.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Ansel KM, Ngo VN, Hyman PL, Luther SA, Förster R, Sedgwick JD, Browning JL, Lipp M, Cyster JG. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–14. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 16.Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity. 2000;12:471–81. doi: 10.1016/S1074-7613(00)80199-5. [DOI] [PubMed] [Google Scholar]
- 17.Luther SA, Bidgol A, Hargreaves DC, Schmidt A, Xu Y, Paniyadi J, Matloubian M, Cyster JG. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol. 2002;169:424–33. doi: 10.4049/jimmunol.169.1.424. [DOI] [PubMed] [Google Scholar]
- 18.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–60. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 19.Kerkar SP, Restifo NP. Cellular constituents of immune escape within the tumor microenvironment. Cancer Res. 2012;72:3125–30. doi: 10.1158/0008-5472.CAN-11-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergomas F, Grizzi F, Doni A, Pesce S, Laghi L, Allavena P, Mantovani A, Marchesi F. Tertiary Intratumor Lymphoid Tissue in Colo-Rectal Cancer. Cancers. 2011;4:1–10. doi: 10.3390/cancers4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMullen TPW, Lai R, Dabbagh L, Wallace TM, de Gara CJ. Survival in rectal cancer is predicted by T cell infiltration of tumour-associated lymphoid nodules. Clin Exp Immunol. 2010;161:81–8. doi: 10.1111/j.1365-2249.2010.04147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki A, Masuda A, Nagata H, Kameoka S, Kikawada Y, Yamakawa M, Kasajima T. Mature dendritic cells make clusters with T cells in the invasive margin of colorectal carcinoma. J Pathol. 2002;196:37–43. doi: 10.1002/path.1018. [DOI] [PubMed] [Google Scholar]
- 23.Coppola D, Nebozhyn M, Khalil F, Dai H, Yeatman T, Loboda A, Mulé JJ. Unique ectopic lymph node-like structures present in human primary colorectal carcinoma are identified by immune gene array profiling. Am J Pathol. 2011;179:37–45. doi: 10.1016/j.ajpath.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinet L, Garrido I, Filleron T, Le Guellec S, Bellard E, Fournie J-J, Rochaix P, Girard J-P. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 2011;71:5678–87. doi: 10.1158/0008-5472.CAN-11-0431. [DOI] [PubMed] [Google Scholar]
- 25.Bell D, Chomarat P, Broyles D, Netto G, Harb GM, Lebecque S, Valladeau J, Davoust J, Palucka KA, Banchereau J. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med. 1999;190:1417–26. doi: 10.1084/jem.190.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coronella JA, Spier C, Welch M, Trevor KT, Stopeck AT, Villar H, Hersh EM. Antigen-driven oligoclonal expansion of tumor-infiltrating B cells in infiltrating ductal carcinoma of the breast. J Immunol. 2002;169:1829–36. doi: 10.4049/jimmunol.169.4.1829. [DOI] [PubMed] [Google Scholar]
- 27.Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, Ravoet M, Le Buanec H, Sibille C, Manfouo-Foutsop G, et al. CD4⁺ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. 2013;123:2873–92. doi: 10.1172/JCI67428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nzula S, Going JJ, Stott DI. Antigen-driven clonal proliferation, somatic hypermutation, and selection of B lymphocytes infiltrating human ductal breast carcinomas. Cancer Res. 2003;63:3275–80. [PubMed] [Google Scholar]
- 29.Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, Biota C, Doffin AC, Durand I, Olive D, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69:2000–9. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]
- 30.Martinet L, Filleron T, Le Guellec S, Rochaix P, Garrido I, Girard J-P. High endothelial venule blood vessels for tumor-infiltrating lymphocytes are associated with lymphotoxin β-producing dendritic cells in human breast cancer. J Immunol. 2013;191:2001–8. doi: 10.4049/jimmunol.1300872. [DOI] [PubMed] [Google Scholar]
- 31.Cipponi A, Mercier M, Seremet T, Baurain J-F, Théate I, van den Oord J, Stas M, Boon T, Coulie PG, van Baren N. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res. 2012;72:3997–4007. doi: 10.1158/0008-5472.CAN-12-1377. [DOI] [PubMed] [Google Scholar]
- 32.Ladányi A, Kiss J, Somlai B, Gilde K, Fejős Z, Mohos A, Gaudi I, Tímár J. Density of DC-LAMP(+) mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol Immunother. 2007;56:1459–69. doi: 10.1007/s00262-007-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinet L, Le Guellec S, Filleron T, Lamant L, Meyer N, Rochaix P, Garrido I, Girard J-P. High endothelial venules (HEVs) in human melanoma lesions: Major gateways for tumor-infiltrating lymphocytes. Oncoimmunology. 2012;1:829–39. doi: 10.4161/onci.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Messina JL, Fenstermacher DA, Eschrich S, Qu X, Berglund AE, Lloyd MC, Schell MJ, Sondak VK, Weber JS, Mulé JJ. 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? Sci Rep. 2012;2:765. doi: 10.1038/srep00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barone F, Bombardieri M, Rosado MM, Morgan PR, Challacombe SJ, De Vita S, Carsetti R, Spencer J, Valesini G, Pitzalis C. CXCL13, CCL21, and CXCL12 expression in salivary glands of patients with Sjogren’s syndrome and MALT lymphoma: association with reactive and malignant areas of lymphoid organization. J Immunol. 2008;180:5130–40. doi: 10.4049/jimmunol.180.7.5130. [DOI] [PubMed] [Google Scholar]
- 36.Bombardieri M, Barone F, Humby F, Kelly S, McGurk M, Morgan P, Challacombe S, De Vita S, Valesini G, Spencer J, et al. Activation-induced cytidine deaminase expression in follicular dendritic cell networks and interfollicular large B cells supports functionality of ectopic lymphoid neogenesis in autoimmune sialoadenitis and MALT lymphoma in Sjögren’s syndrome. J Immunol. 2007;179:4929–38. doi: 10.4049/jimmunol.179.7.4929. [DOI] [PubMed] [Google Scholar]
- 37.Dieu-Nosjean M-C, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26:4410–7. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 38.de Chaisemartin L, Goc J, Damotte D, Validire P, Magdeleinat P, Alifano M, Cremer I, Fridman W-H, Sautès-Fridman C, Dieu-Nosjean M-C. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res. 2011;71:6391–9. doi: 10.1158/0008-5472.CAN-11-0952. [DOI] [PubMed] [Google Scholar]
- 39.Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, André P, Dieu-Nosjean M-C, Alifano M, Régnard J-F, et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 2011;71:5412–22. doi: 10.1158/0008-5472.CAN-10-4179. [DOI] [PubMed] [Google Scholar]
- 40.Remark R, Alifano M, Cremer I, Lupo A, Dieu-Nosjean M-C, Riquet M, Crozet L, Ouakrim H, Goc J, Cazes A, et al. Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: influence of tumor origin. Clin Cancer Res Off J Am Assoc Cancer Res 2013 [DOI] [PubMed] [Google Scholar]
- 41.Thaunat O, Patey N, Caligiuri G, Gautreau C, Mamani-Matsuda M, Mekki Y, Dieu-Nosjean M-C, Eberl G, Ecochard R, Michel J-B, et al. Chronic rejection triggers the development of an aggressive intragraft immune response through recapitulation of lymphoid organogenesis. J Immunol. 2010;185:717–28. doi: 10.4049/jimmunol.0903589. [DOI] [PubMed] [Google Scholar]
- 42.Uzzan B, Nicolas P, Cucherat M, Perret G-Y. Microvessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta-analysis. Cancer Res. 2004;64:2941–55. doi: 10.1158/0008-5472.CAN-03-1957. [DOI] [PubMed] [Google Scholar]
- 43.Des Guetz G, Uzzan B, Nicolas P, Cucherat M, Morere J-F, Benamouzig R, Breau J-L, Perret G-Y. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer. 2006;94:1823–32. doi: 10.1038/sj.bjc.6603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donnem T, Al-Saad S, Al-Shibli K, Delghandi MP, Persson M, Nilsen MN, Busund L-T, Bremnes RM. Inverse prognostic impact of angiogenic marker expression in tumor cells versus stromal cells in non small cell lung cancer. Clin Cancer Res. 2007;13:6649–57. doi: 10.1158/1078-0432.CCR-07-0414. [DOI] [PubMed] [Google Scholar]
- 45.Kadota K, Huang CL, Liu D, Ueno M, Kushida Y, Haba R, Yokomise H. The clinical significance of lymphangiogenesis and angiogenesis in non-small cell lung cancer patients. Eur J Cancer. 2008;44:1057–67. doi: 10.1016/j.ejca.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 46.Mineo TC, Ambrogi V, Baldi A, Rabitti C, Bollero P, Vincenzi B, Tonini G. Prognostic impact of VEGF, CD31, CD34, and CD105 expression and tumour vessel invasion after radical surgery for IB-IIA non-small cell lung cancer. J Clin Pathol. 2004;57:591–7. doi: 10.1136/jcp.2003.013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martinet L, Garrido I, Girard J-P. Tumor high endothelial venules (HEVs) predict lymphocyte infiltration and favorable prognosis in breast cancer. Oncoimmunology. 2012;1:789–90. doi: 10.4161/onci.19787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moussion C, Girard J-P. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature. 2011;479:542–6. doi: 10.1038/nature10540. [DOI] [PubMed] [Google Scholar]
- 49.Girard J-P, Moussion C, Förster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol. 2012;12:762–73. doi: 10.1038/nri3298. [DOI] [PubMed] [Google Scholar]
- 50.Chen Q, Wang W-C, Evans SS. Tumor microvasculature as a barrier to antitumor immunity. Cancer Immunol Immunother. 2003;52:670–9. doi: 10.1007/s00262-003-0425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu NZ, Klitzman B, Dodge R, Dewhirst MW. Diminished leukocyte-endothelium interaction in tumor microvessels. Cancer Res. 1992;52:4265–8. [PubMed] [Google Scholar]
- 52.Piali L, Fichtel A, Terpe HJ, Imhof BA, Gisler RH. Endothelial vascular cell adhesion molecule 1 expression is suppressed by melanoma and carcinoma. J Exp Med. 1995;181:811–6. doi: 10.1084/jem.181.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawamata N, Xu B, Nishijima H, Aoyama K, Kusumoto M, Takeuchi T, Tei C, Michie SA, Matsuyama T. Expression of endothelia and lymphocyte adhesion molecules in bronchus-associated lymphoid tissue (BALT) in adult human lung. Respir Res. 2009;10:97. doi: 10.1186/1465-9921-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu B, Wagner N, Pham LN, Magno V, Shan Z, Butcher EC, Michie SA. Lymphocyte homing to bronchus-associated lymphoid tissue (BALT) is mediated by L-selectin/PNAd, alpha4beta1 integrin/VCAM-1, and LFA-1 adhesion pathways. J Exp Med. 2003;197:1255–67. doi: 10.1084/jem.20010685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miyasaka M, Tanaka T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat Rev Immunol. 2004;4:360–70. doi: 10.1038/nri1354. [DOI] [PubMed] [Google Scholar]
- 56.DeNucci CC, Mitchell JS, Shimizu Y. Integrin function in T-cell homing to lymphoid and nonlymphoid sites: getting there and staying there. Crit Rev Immunol. 2009;29:87–109. doi: 10.1615/CritRevImmunol.v29.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Agace WW. T-cell recruitment to the intestinal mucosa. Trends Immunol. 2008;29:514–22. doi: 10.1016/j.it.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 58.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 59.Hamanishi J, Mandai M, Matsumura N, Baba T, Yamaguchi K, Fujii S, Konishi I. Activated local immunity by CC chemokine ligand 19-transduced embryonic endothelial progenitor cells suppresses metastasis of murine ovarian cancer. Stem Cells. 2010;28:164–73. doi: 10.1002/stem.256. [DOI] [PubMed] [Google Scholar]
- 60.Liang CM, Zhong CP, Sun RX, Liu BB, Huang C, Qin J, Zhou S, Shan J, Liu YK, Ye SL. Local expression of secondary lymphoid tissue chemokine delivered by adeno-associated virus within the tumor bed stimulates strong anti-liver tumor immunity. J Virol. 2007;81:9502–11. doi: 10.1128/JVI.00208-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vicari AP, Ait-Yahia S, Chemin K, Mueller A, Zlotnik A, Caux C. Antitumor effects of the mouse chemokine 6Ckine/SLC through angiostatic and immunological mechanisms. J Immunol. 2000;165:1992–2000. doi: 10.4049/jimmunol.165.4.1992. [DOI] [PubMed] [Google Scholar]
- 62.Yang S-C, Hillinger S, Riedl K, Zhang L, Zhu L, Huang M, Atianzar K, Kuo BY, Gardner B, Batra RK, et al. Intratumoral administration of dendritic cells overexpressing CCL21 generates systemic antitumor responses and confers tumor immunity. Clin Cancer Res. 2004;10:2891–901. doi: 10.1158/1078-0432.CCR-03-0380. [DOI] [PubMed] [Google Scholar]
- 63.Yang S-C, Batra RK, Hillinger S, Reckamp KL, Strieter RM, Dubinett SM, Sharma S. Intrapulmonary administration of CCL21 gene-modified dendritic cells reduces tumor burden in spontaneous murine bronchoalveolar cell carcinoma. Cancer Res. 2006;66:3205–13. doi: 10.1158/0008-5472.CAN-05-3619. [DOI] [PubMed] [Google Scholar]
- 64.Yousefieh N, Hahto SM, Stephens AL, Ciavarra RP. Regulated expression of CCL21 in the prostate tumor microenvironment inhibits tumor growth and metastasis in an orthotopic model of prostate cancer. Cancer Microenviron. 2009;2:59–67. doi: 10.1007/s12307-009-0021-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.GeurtsvanKessel CH, Willart MA, Bergen IM, van Rijt LS, Muskens F, Elewaut D, Osterhaus AD, Hendriks R, Rimmelzwaan GF, Lambrecht BN. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J Exp Med. 2009;206:2339–49. doi: 10.1084/jem.20090410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Halle S, Dujardin HC, Bakocevic N, Fleige H, Danzer H, Willenzon S, Suezer Y, Hämmerling G, Garbi N, Sutter G, et al. Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells. J Exp Med. 2009;206:2593–601. doi: 10.1084/jem.20091472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muniz LR, Pacer ME, Lira SA, Furtado GC. A critical role for dendritic cells in the formation of lymphatic vessels within tertiary lymphoid structures. J Immunol. 2011;187:828–34. doi: 10.4049/jimmunol.1004233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zielinski CE, Corti D, Mele F, Pinto D, Lanzavecchia A, Sallusto F. Dissecting the human immunologic memory for pathogens. Immunol Rev. 2011;240:40–51. doi: 10.1111/j.1600-065X.2010.01000.x. [DOI] [PubMed] [Google Scholar]
- 69.Inoue H, Iga M, Xin M, Asahi S, Nakamura T, Kurita R, Nakayama M, Nakazaki Y, Takayama K, Nakanishi Y, et al. TARC and RANTES enhance antitumor immunity induced by the GM-CSF-transduced tumor vaccine in a mouse tumor model. Cancer Immunol Immunother. 2008;57:1399–411. doi: 10.1007/s00262-008-0476-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kanagawa N, Niwa M, Hatanaka Y, Tani Y, Nakagawa S, Fujita T, Yamamoto A, Okada N. CC-chemokine ligand 17 gene therapy induces tumor regression through augmentation of tumor-infiltrating immune cells in a murine model of preexisting CT26 colon carcinoma. Int J Cancer. 2007;121:2013–22. doi: 10.1002/ijc.22908. [DOI] [PubMed] [Google Scholar]
- 71.Kang S, Xie J, Ma S, Liao W, Zhang J, Luo R. Targeted knock down of CCL22 and CCL17 by siRNA during DC differentiation and maturation affects the recruitment of T subsets. Immunobiology. 2010;215:153–62. doi: 10.1016/j.imbio.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 72.Okada N, Sasaki A, Niwa M, Okada Y, Hatanaka Y, Tani Y, Mizuguchi H, Nakagawa S, Fujita T, Yamamoto A. Tumor suppressive efficacy through augmentation of tumor-infiltrating immune cells by intratumoral injection of chemokine-expressing adenoviral vector. Cancer Gene Ther. 2006;13:393–405. doi: 10.1038/sj.cgt.7700903. [DOI] [PubMed] [Google Scholar]
- 73.Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, Fujii H. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int J Cancer. 2008;122:2286–93. doi: 10.1002/ijc.23392. [DOI] [PubMed] [Google Scholar]
- 74.Qin X-J, Shi H-Z, Deng J-M, Liang Q-L, Jiang J, Ye Z-J. CCL22 recruits CD4-positive CD25-positive regulatory T cells into malignant pleural effusion. Clin Cancer Res. 2009;15:2231–7. doi: 10.1158/1078-0432.CCR-08-2641. [DOI] [PubMed] [Google Scholar]
- 75.Hindley JP, Jones E, Smart K, Bridgeman H, Lauder SN, Ondondo B, Cutting S, Ladell K, Wynn KK, Withers D, et al. T-cell trafficking facilitated by high endothelial venules is required for tumor control after regulatory T-cell depletion. Cancer Res. 2012;72:5473–82. doi: 10.1158/0008-5472.CAN-12-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Quezada SA, Peggs KS, Simpson TR, Shen Y, Littman DR, Allison JP. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J Exp Med. 2008;205:2125–38. doi: 10.1084/jem.20080099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaser A, Dunzendorfer S, Offner FA, Ludwiczek O, Enrich B, Koch RO, Cruikshank WW, Wiedermann CJ, Tilg HB. B lymphocyte-derived IL-16 attracts dendritic cells and Th cells. J Immunol. 2000;165:2474–80. doi: 10.4049/jimmunol.165.5.2474. [DOI] [PubMed] [Google Scholar]
- 78.Krug N, Cruikshank WW, Tschernig T, Erpenbeck VJ, Balke K, Hohlfeld JM, Center DM, Fabel H. Interleukin 16 and T-cell chemoattractant activity in bronchoalveolar lavage 24 hours after allergen challenge in asthma. Am J Respir Crit Care Med. 2000;162:105–11. doi: 10.1164/ajrccm.162.1.9908055. [DOI] [PubMed] [Google Scholar]
- 79.Parada NA, Center DM, Kornfeld H, Rodriguez WL, Cook J, Vallen M, Cruikshank WW. Synergistic activation of CD4+ T cells by IL-16 and IL-2. J Immunol. 1998;160:2115–20. [PubMed] [Google Scholar]
- 80.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 81.Kirk CJ, Hartigan-O’Connor D, Mulé JJ. The dynamics of the T-cell antitumor response: chemokine-secreting dendritic cells can prime tumor-reactive T cells extranodally. Cancer Res. 2001;61:8794–802. [PubMed] [Google Scholar]
- 82.Schrama D, Voigt H, Eggert AO, Xiang R, Zhou H, Schumacher TNM, Andersen MH, thor Straten P, Reisfeld RA, Becker JC. Immunological tumor destruction in a murine melanoma model by targeted LTalpha independent of secondary lymphoid tissue. Cancer Immunol Immunother. 2008;57:85–95. doi: 10.1007/s00262-007-0352-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schrama D, thor Straten P, Fischer WH, McLellan AD, Bröcker EB, Reisfeld RA, Becker JC. Targeting of lymphotoxin-alpha to the tumor elicits an efficient immune response associated with induction of peripheral lymphoid-like tissue. Immunity. 2001;14:111–21. doi: 10.1016/S1074-7613(01)00094-2. [DOI] [PubMed] [Google Scholar]
- 84.Senovilla L, Vacchelli E, Galon J, Adjemian S, Eggermont A, Fridman WH, Sautès-Fridman C, Ma Y, Tartour E, Zitvogel L, et al. Trial watch: Prognostic and predictive value of the immune infiltrate in cancer. Oncoimmunology. 2012;1:1323–43. doi: 10.4161/onci.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Angell H, Galon J. From the immune contexture to the Immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol. 2013;25:261–7. doi: 10.1016/j.coi.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 86.Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund L-T. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14:5220–7. doi: 10.1158/1078-0432.CCR-08-0133. [DOI] [PubMed] [Google Scholar]
- 87.Suzuki K, Kachala SS, Kadota K, Shen R, Mo Q, Beer DG, Rusch VW, Travis WD, Adusumilli PS. Prognostic immune markers in non-small cell lung cancer. Clin Cancer Res. 2011;17:5247–56. doi: 10.1158/1078-0432.CCR-10-2805. [DOI] [PubMed] [Google Scholar]
- 88.Nielsen JS, Sahota RA, Milne K, Kost SE, Nesslinger NJ, Watson PH, Nelson BH. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res. 2012;18:3281–92. doi: 10.1158/1078-0432.CCR-12-0234. [DOI] [PubMed] [Google Scholar]
- 89.Nielsen JS, Nelson BH. Tumor-infiltrating B cells and T cells: Working together to promote patient survival. Oncoimmunology. 2012;1:1623–5. doi: 10.4161/onci.21650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thompson ED, Enriquez HL, Fu Y-X, Engelhard VH. Tumor masses support naive T cell infiltration, activation, and differentiation into effectors. J Exp Med. 2010;207:1791–804. doi: 10.1084/jem.20092454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 92.Aubert RD, Kamphorst AO, Sarkar S, Vezys V, Ha S-J, Barber DL, Ye L, Sharpe AH, Freeman GJ, Ahmed R. Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection. Proc Natl Acad Sci U S A. 2011;108:21182–7. doi: 10.1073/pnas.1118450109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res. 2010;70:8368–77. doi: 10.1158/0008-5472.CAN-10-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]