Abstract
Pancreatic cancer is a lethal disease and currently available therapies have significant limitations. Pancreatic cancer is thus an ideal setting for the development of novel treatment modalities such as immunotherapy. However, relevant obstacles must be overcome for immunotherapeutic regimens against pancreatic cancer to be successful. Vaccine therapy relies on the administration of biological preparations that include an antigen that (at least ideally) is specifically expressed by malignant cells, boosting the natural ability of the immune system to react against neoplastic cells. There are a number of ways to deliver anticancer vaccines. Potent vaccines stimulate antigen presentation by dendritic cells, hence driving the expansion of antigen-specific effector and memory T cells. Unlike vaccines given as a prophylaxis against infectious diseases, anticancer vaccines require the concurrent administration of agents that interfere with the natural predisposition of tumors to drive immunosuppression. The safety and efficacy of vaccines against pancreatic cancer are nowadays being tested in early phase clinical trials.
Keywords: pancreatic cancer, immunotherapy, cancer vaccine, clinical trials, immune checkpoint
Introduction
PDA is the fourth leading cause of cancer-related death in the United States, with an estimated 43 920 new cases and 37 390 deaths in 2012.1 Surgical resection is the only known curative treatment for PDA,2 and patients who develop recurrence usually present between 9 and 12 mo after resection.3 The median survival of PDA patients upon surgery is 15–20 mo, with a 5-y survival rate of approximately 20%.3,4 Along similar lines, the median survival of patients with locally advanced, unresectable disease is very poor, i.e., 10–12 mo.2,5 There are only a few chemotherapeutic agents that have shown to be effective against PDA to date, including gemcitabine with or without abraxane (Von Hoff et al., American Society of Clinical Oncology Annual Meeting Abstract, 2013) as well as a combination of 5-FU, leucovorin, oxaliplatin and irinotecan (the so-called FOLFIRINOX regimen).6,7 The survival of patients treated with these regimens is marginal and hence we are in urgent need of novel therapeutic approaches against PDA. As immunotherapies act differently than chemotherapy or radiation therapy, they represent a promising alternative treatment modality for this deadly disease.
Mechanistic Basis for Vaccine-Based Immunotherapy
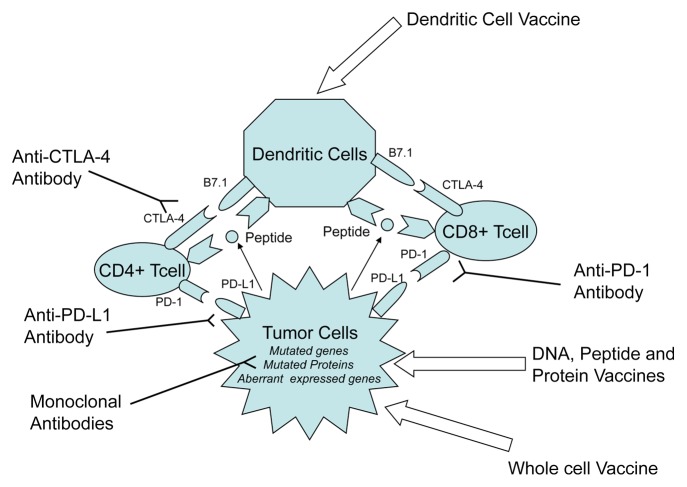
Cancer cells are derived from their normal counterparts owing to genetic and epigenetic alterations that de facto underpin their malignant phenotype. These genetic and epigenetic changes lead to the expression of TAAs, i.e., proteins that are generally not expressed by non-transformed calls. Vaccine-based anticancer immunotherapy aims to harness the natural ability of the immune system to recognize and react against new antigens, in particular TAAs. Thus, the goal of anticancer vaccination is to activate and expand tumor-specific T cells as a means of eliciting novel or boosting pre-existent anticancer immune responses. To induce a robust antitumor immune response, TAAs must be presented to T cells in the context of major MHC molecules. Professional APCs such as DCs are highly efficient T-cell activators. Indeed, the intracellular antigen processing machinery that is unique to DCs enables them to efficiently process TAAs and present them on both MHC class I and II molecules, resulting in the activation of tumor-specific CD8+ and CD4+ T cells (Fig. 1).8 These T cells harbor unique T-cell receptors that recognize specific TAA epitopes bound to MHC molecules. This interaction provides T cells with a stimulatory signal known as “Signal 1.” Signal 1 alone is insufficient for the robust activation of T cells, rather resulting in anergy or apoptosis, which allow tumors to evade recognition by the immune system. The binding of co-stimulatory molecules, such as CD80 (best known as B7–1) and CD86 (best known as B7–2), which are expressed by APCs, to CD28, which is found on the T-cell surface, results in “Signal 2,” a critical component in T-cell activation.
Figure 1. Strategies for anticancer vaccination. Anticancer immunotherapy aims at harnessing the natural ability of the immune system to recognize and react against potentially immunogenic TAAs. DNA-, peptide-, or protein-based vaccines rely on identified immunodominant TAA epitopes to stimulate antitumor T-cell responses. DC-based vaccines attempt to exploit the pronounced ability of DCs to operate as antigen-presenting cells by isolating them, loading them with TAAs or tumor-derived mRNA ex vivo, and subsequently re-infusing them in patients. Whole cancer cell-based vaccines circumvent the for targeting specific TAAs because they rely on irradiated malignant cells as a whole. Immunotherapeutic strategies that inhibit immune checkpoints such as those mediated by CTLA4 and PD-1 reduce the barriers that vaccines must overcome to trigger therapeutically relevant anticancer immune responses.
Most malignant cells lack the necessary surface molecules, that is, B7-1 (CD80) and B7-2 (CD86), to complete signal 2.9 By contrast, cell surface molecules such as CTLA-4, PD-1, and PD-L1 (B7-H1) and PD-L2 (B7-DC) are expressed to activate immune checkpoint signaling pathways and thereby down-regulate T-cell activation. A successful anticancer vaccine thus must convey robust immunostimulatory signals while overcoming all the barriers raised by malignant cells against immune activation.
Vaccine Therapy Against Pancreatic Cancer
Antigen specific vaccines
The natural starting point for vaccines against PDA was represented by tumor markers such as CEA, MUC1 as well as by proteins that play an early and prominent role in PDA initiation, including KRAS and telomerase.10 Peptide and protein-based vaccines were the first form of anti-PDA vaccinations investigated, attempting to use immunodominant tumor epitopes to stimulate antitumor T-cell responses.
KRAS-targeting vaccines
The first peptide-based vaccine investigated in clinical trials involving pancreatic cancer patients targeted KRAS, which is mutated in more than 90% of PDA patients, showing that this immunotherapeutic approach is safe.11 In a Phase I/II study, the administration of synthetic KRAS-derived peptides to unresectable pancreatic cancer patients resulted in an immune response in 2 out of 5 individuals.12 In another Phase I/II clinical trial, synthetic peptides derived from mutant KRAS were administered together with GM-CSF to 48 PDA patients (10 surgically resected and 38 with advanced disease) on an outpatient basis.13 Peptide-specific immune responses were induced in 25 of 43 (58%) evaluable patients, indicating that this protocol is potent enough to elicit immune responses even in patients with end-stage disease. Patients with advanced PDA manifesting an immune response to vaccination exhibited a prolonged survival as compared with immunological non-responders (median survival 148 d vs. 61 d; p = 0.0002). In an independent study, patients with resected pancreatic cancers harboring KRAS mutations at codon 12 were vaccinated once monthly for 3 mo with a 21-mer epitope encompassing the patient specific mutation.14 About 200 μg of the vaccine were injected intradermally on day 7 of a 10-d course involving intradermal GM-CSF. Of 62 screened patients, 24 were vaccinated. Median recurrence-free survival time was 8.6 mo and median overall survival time was 20.3 mo. Thus, KRAS-targeting vaccines proved to be well tolerated by patients with resectable PDA. Although these preparations demonstrated some immunogenicity, however, their clinical efficacy remains unproven.
Telomerase-targeting vaccines
Telomerase, which is reactivated in more than 85% of PDA cells, has also been used to develop peptide-based vaccines against pancreatic cancer. The telomerase-targeting vaccine (GV1001) consist a 16-aa peptide derived from human TERT that binds to multiple MHC molecules. In a Phase I/II clinical study, which GV1001 was well tolerated by PDA patients and prolonged survival.15 Forty-eight patients with non-resectable PDA received intradermal injections of GV1001 (at three dose levels) in combination with GM-CSF for 10 weeks. Immune responses were observed in 24 of 38 evaluable patients, with the highest proportion of immunological responders belonging to the intermediate-dose group. The median survival of patients receiving intermediate doses of GV1001 was 8.6 mo, which was significantly longer than that of subjects in the low- and high-dose groups. One-year survival among evaluable patients of the intermediate-dose group was 25%. Two Phase III studies tested GV1001 in patients with nonresectable PDA, namely, the PrimoVax and TeloVac trials. The PrimoVax trial examined the efficacy of GV1001 monotherapy vs. gemcitabine but was terminated owing to a lack of survival advantage.16 The TeloVac study investigated the efficacy of GV1001 in sequential combination with gemcitabine vs. gemcitabine alone in subjects with locally advanced and metastatic adenocarcinoma of the pancreas.16 It should be noted that the vaccine cycles overlapped with the gemcitabine cycles in the combinational arm of this study, raising concerns on the effectiveness of vaccination in the setting of chemotherapy-induced immunosuppression. The results of the TeloVac study have recently been reported, demonstrating no survival benefit for the combination of GV1001 and gemcitabine as compared with gemcitabine alone.16
Gastrin-based vaccines
Gastrin and cholecystokinin B receptor (CCKBR, best known as CCK-2) are upregulated and co-expressed in both pancreatic cell lines and human PDA specimens and have been implicated in autocrine, paracrine, and endocrine growth pathways.17,18 In a multi-institutional, double-blinded, placebo-controlled clinical trial, the administration of a gastrin-based vaccine to chemotherapy-refractory advanced-stage PDA patients resulted in a nearly 2-fold increase in median overall survival, as compared with placebo (151 vs. 82 d, respectively; p = 0.03).18 Gastrin-based vaccines appear therefore to be well tolerated by and could represent a new therapeutic option for pancreatic cancer.
Survivin-targeting vaccines
Survivin is a member of the inhibitor of apoptosis family and is known to exert robust antiapoptotic effects. Survivin is expressed to high levels by a majority of human carcinomas, including pancreas cancer, but not by non-transformed adult tissues.19 A 72-y-old patient suffering from gemcitabine-refractory PDA experienced a complete remission (with a duration of 8 mo) upon receiving a survivin-targeting that consisted of a modified HLA-A2+-restricted survivin epitope (residues 96–104) in Montanide.20 Immunological monitoring revealed a strong vaccine-induced immunoreactivity against survivin, and when the patient was weaned from vaccination, he developed recurrent disease. In another study, a survivin-derived peptide (AYACNTSTL) was used in combination with IFNα to vaccinate six patients who had advanced pancreatic cancers. Tetramer and ELISPOT assays revealed that more than half of the patients had manifested immunological responses to vaccination, which were often accompanied by clinical benefits.21 Nonetheless, this vaccine was tested in a limited number of individuals and its application would be limited to HLA-A2+ patients.
HSP-peptide complex-based vaccines
HSPs exist ubiquitously across all species and their function as chaperones can be harness to stabilize peptides for ex vivo and in vivo delivery to APCs. HSP-peptide complexes can be presented on MHC class I molecules on the cell surface. Tumor-derived HSP-peptide complexes have been shown to induce antitumor immune responses in preclinical studies. HSP96-peptides complexes produced from resected tumor tissues were the first to be employed in anticancer vaccines. In a Phase I clinical trial, 10 resected PDA patients who did not receive adjuvant chemoradiation were vaccinated with autologous HSP96-peptide complexes weekly, for a total number of 4 vaccination, exhibiting a median overall survival of 2.2 y.22 Although this pilot study demonstrated the feasibility of preparing HSP96-peptide complexes from resected tumors, it would be a technical challenge to produce such an autologous complex for a large number of patients.
Recombinant virus-based CEA- and MUC1-targeting vaccines
Recombinant vaccines are based on bacterial and viral antigen carriers that increasing DC activation and improve antigen presentation. Clinical trials testing recombinant CEA- and MUC1-targeting vaccines in PDA patients have shown little efficacy. TRICOM is a poxvirus-based vaccine encoding a combination three distinct T-cell co-stimulatory molecules: B7–1, ICAM1 and CD58 (best known as LFA-3). In a Phase I study, viral vaccines targeting CEA and MUC1 were tested in 10 advanced pancreatic cancer patients.23 The vaccination regimen consisted of a vaccinia virus expressing CEA and MUC1 (PANVAC-V) coupled to a fowlpox virus expressing the same antigens and co-stimulatory molecules (PANVAC-F). Patients were primed with PANVAC-V followed by three booster vaccinations with PANVAC-F. GM-CSF was used as a local adjuvant after each vaccination and for 3 consecutive days thereafter. The median overall survival of vaccinated patients was 6.3 mo and a significant increase in overall survival was noted among those individuals patients who developed CEA- and/or MUC-1-specific immune responses as compared with those who did not (15.1 vs. 3.9 mo, respectively; p = 0.002). In a Phase III, randomized clinical trial involving 255 patients with metastatic PDA, PANVAC-V was compared with standard gemcitabine-based chemotherapy. Regrettably, the vaccine failed to ameliorate overall survival in this setting.24
Listeria-based vaccines
The ability of L. monocytogenes to stimulate robust, multi-functional, cell-mediated adaptive immune responses is mainly based on its intracellular life cycle25,26 and its ability to target DCs in vivo.27 However, as L. monocytogenes is a food-borne pathogen, it cannot be employed as such as an anticancer vaccine. Thus, a vaccine-compatible L. monocytogenes strain (ΔactA/ΔinlB) that is safe yet retains the potency of wild-type bacteria has been developed and evaluated in 3 separate Phase I clinical studies in patients with malignant and infectious diseases.28 The ΔactA/ΔinlB strain is a genetically defined, live-attenuated L. monocytogenes variant in which the genes encoding 2 virulence determinants have been deleted. This results in a greater than 1,000-fold reduction in toxicity as compared with wild-type L. monocytogenes, but fails to affect the immunostimulatory activity of the fully virulent wild-type pathogen.29 In one study, patients bearing hepatic metastases from mesothelioma, ovarian cancer, non-small cell lung carcinoma and PDA were given this L. monocytogenes strain further engineered to express mesothelin, a cell surface molecule overexpressed by a large majority of PDAs, mesotheliomas, ovarian cancers, and non-small cell lung carcinomas.30 Thirty-seven percent of these patients survived 15 mo or more. Half of them patients were those harboring metastatic PDAs, and immunological analyses revealed that they had developed listeriolysin O- and mesothelin-specific T-cell responses. In light of these findings, a Phase II study of the mesothelin-coding listerial vaccine in metastatic PDA patients has been conducted, as described below.
Dendritic cell-based vaccines
As mentioned above, DCs are widely considered as the most potent APCs as they are very efficient at priming naïve T cells to generate memory T cells and B cells that mediate robust antigen-specific immune responses. Several groups have attempted to harness these characteristics by isolating DCs, loading them with TAAs as well as with TAA-coding or tumor-derived mRNA ex vivo, and subsequently re-infusing them in patients. The safety and efficacy of DC-based vaccines in PDA patients have been tested in two clinical trials only. The first of these studies is a Phase I/II clinical trial in which 12 pancreatic and biliary cancer patients were treated upon tumor resection with DCs loaded ex vivo with a MUC1-derived peptide.31 These patients have been followed for > 4 y after vaccination, and 4 of them are alive, all without evidence of recurrence. In the second study, a DC-based vaccine alone or combined with LAK cells was administered together with gemcitabine and/or S-1 to 49 patients with inoperable pancreatic cancer.32 Of these patients, 2 manifested a complete remission, 5 a partial remission, and 10 had stable disease. The median survival of these individuals was 360 d, which appeared to be longer than what could be achieved with gemcitabine and/or S-1. Thus, the combination of DC-based immunotherapy and chemotherapy was well tolerated by advanced PDA patients and warrants further investigation.
Whole cancer cell-based vaccines
Whole cancer cell-based vaccines circumvent the need for targeting a selected TAA as they rely on irradiated tumor cells that by definition express a whole panel of TAAs. In this setting, allogeneic preparations overcome the technical difficulties that may be posed by the producing of autologous vaccines, calling for the isolation of a sufficient amount of malignant tissue from patients. Whole cell-based vaccines also provide a means to immunize lymphocytes and sera against TAAs in a non-biased way, resulting in the generation of reagent that may be used to identify immunologically relevant TAAs to be used for the design antigen-specific vaccination strategies. Two allogeneic whole cell-based anticancer vaccines are currently being investigate for their safety and antineoplastic effects in PDA patients.
Allogeneic GM-CSF-secreting vaccines
The first whole cell-based anticancer vaccine developed for the treatment of pancreatic cancer (pancreatic GVAX) comprised two allogeneic human pancreatic cancer cell lines, both of which were engineered to express GM-CSF. This vaccine and several analogs directed against other malignancies were developed based on preclinical studies demonstrating that the elicitation of effective antitumor immune responses by anticancer vaccines required the secretion of high levels of GM-CSF at the site of vaccination for several days.33 GM-CSF-expressing cancer cells indeed prime the immune system very efficiently as they boost the capacity of DCs to present TAAs, which in this setting are several. Jaffee and colleagues have conducted multiple Phase I/II clinical trials to test the safety and efficacy of irradiated, allogeneic GM-CSF-secreting pancreatic cancer cell lines in patients with resected PDAs or metastatic PDA.34,35 In the context of 2 completed Phase I/II clinical trials36,37 and one ongoing study16 (ClinicalTrials.gov identifier: NCT00727441), resectable PDA patients underwent vaccination after surgery, before the initiation of adjuvant chemotherapy or radiation therapy, and received 4 additional vaccine doses only once they had completed chemotherapy and radiation therapy. Such a sequential design was intended to avoid as much as possible the immunosuppressive effects of chemoradiation on vaccine-elicited immune responses. In the Phase I study, 3 out of 8 patients who received the highest 2 doses of vaccine still remains disease free, now for more than 15 y.34 In the Phase II study, 60 patients with resected PDA were treated with the vaccine in combination with standard chemoradiation in a similar schedule as in the Phase I study.36 The median disease-free survival is 17.3 mo with median overall survival of 24.8 mo. The post-vaccination induction of mesothelin-specific CD8+ T cells in HLA-A1+ and HLA-A2+ patients correlates with their disease-free survivals. Comparing the Kaplan-Meier curved of subjects treated with the vaccine plus adjuvant chemoradiation in this clinical trial and a historical cohort of PDA patients treated at the Johns Hopkins Hospital with adjuvant chemoradiation alone suggests that the vaccine may provide clinical benefits over chemoradiation in the first 2 y after surgery. However, there was no significant difference in the median overall survival of the 2 cohorts, suggesting that the immune responses elicited by the vaccine may have weaned off after stopping vaccination. In light of these results, 2 new studies are currently being conducted in which PDA patients who remain disease-free in response to the pancreatic GVAX vaccine are treated with boost vaccinations every 6 mo after they have completed the first cycle of immunization. The pancreatic GVAX vaccine has also been tested in patients with metastatic PDA.35 Two patient cohorts were enrolled in this open-label Phase II study: cohort A, including 30 patients who received a maximum of six doses of GVAX at 21-d intervals; and cohort B, including 20 patients who were treated with intravenous cyclophosphamide at a low dose (250 mg/m2) one day prior to the administration of GVAX. The median survival of cohort A and cohort B was 2.3 and 4.7 mo, respectively. These findings supported the initiation of additional studies to evaluate the effects of low-dose cyclophosphamide, which was shown to deplete Tregs in preclinical settings, in combination with anticancer vaccines.35 Mesothelin-specific T-cell responses were shown to be of higher avidity in patients receiving cyclophosphamide/GVAX as compared with subjects treated with GVAX only, and these responses correlated with prolonged patient survival. This observation stimulated the launch of a multi-institutional study that has recently been completed. In this setting, 90 patients with metastatic PDAs were randomized in a 2:1 ratio to receive 2 doses of cyclophosphamide/GVAX as a priming vaccine, followed by 4 doses of CRS-207 (arm A) or 6 doses of cyclophosphamide/GVAX (arm B) as a boost vaccination every 3 weeks. Clinically stable patients were offered additional 20-week courses.37 The median overall survival was 6 mo in arm A vs. 3.4 mo in arm B (p = 0.0114). This prime-boost approach therefore constitutes a vaccination platform that warrants further investigation.
Algenpantucel-L
Algenpantucel-L (also known as hyperacute-pancreatic cancer vaccine) consists in 2 irradiated, live, human allogeneic pancreatic cancer cell lines that express murine α-1,3-galactosyltransferase, which is responsible for the synthesis of α-galactosylated epitopes on cell surface proteins. Hardacre et al. presented the results of an open-label, multi-institutional Phase II clinical trial investigating Algenpantucel-L in combination with standard adjuvant chemoradiotherapy for the treatment of resected PDA patients.38 The first cycle of treatment consisted of Algenpantucel-L on days 1 and 8. One week after the second vaccination, gemcitabine was administered weekly for 3 weeks, on days 1, 8, and 15, in conjunction with Algenpantucel-L on days 1 and 15 of cycle 2. Radiotherapy was initiated 1 to 2 weeks after the completion of cycle 2. Vaccination continued along with radiation therapy on days 1, 15, 29, and 43. After a median follow-up of 21 mo, the 12-mo disease-free survival was 62%, and the 12-mo overall survival was 86%. At present, the addition of Algenpantucel-L to standard adjuvant therapy for the treatment of resected PDA patients is being investigated in a Phase III clinical study.
Perspectives
Although clinical trials testing anticancer vaccines in pancreatic cancer patients have generated promising results, most of these studies have failed to demonstrate a robust efficacy and a statistically significant improvement in patient survival. Nonetheless, important lessons have been learned through the experience accumulated in the course of these clinical trials. In some of them, novel TAAs that elicit antitumor immune responses, and hence can be harnessed for the development of novel vaccines, have been identified. Moreover, most of these clinical studies identified a number of critical aspects that must be carefully considered for the design the next generation of cancer vaccines.
Overcoming tolerance to TAAs
Most often, cancer patients have developed a state of immunological tolerance against TAAs. Indeed, although mutated oncogenes may produce neo-antigens, the expression of these potentially antigenic epitopes occurs at a specific stage of tumorigenesis.39 KRAS is mutated in the early stage of pancreatic oncogenesis, implying that the tolerance to mutated KRAS may be established long before invasive PDA develops. KRAS-targeting vaccines may therefore have a potential for the prevention of PDA, but not for the treatment of established pancreatic neoplasms. Along similar lines, vaccines that target other proteins harboring tumor-associated mutations would have difficulty in overcoming the state of immunological tolerance that develops relative to these proteins in patients with established tumors. Recent advances in high throughput genome sequencing may provide the opportunity to develop patient-specific vaccines that target multiple, as opposed to just one, cancer-associated mutant proteins.40
Optimizing the combination of anticancer vaccines with chemotherapy and radiation therapy
Essentially all the clinical trials performed so far to compare anticancer vaccines with standard chemotherapy failed to demonstrate the superiority of the former. Apparently, vaccine therapy would not be able to replace chemotherapy and radiation therapy. Moreover, although recent clinical studied have investigated the sequential administration of anticancer vaccines and chemoradiation, the immunosuppressive effects of these standard antineoplastic regimens may compromise the efficacy of immunotherapy. Therefore, it will be critical to identify an optimal way to combine anticancer vaccination with chemotherapy and/or radiation therapy. It will also be interesting to test vaccines as a maintenance therapy for patients who are grossly disease-free upon, or whose disease is at least stabilized, upon chemotherapy and/or radiation therapy. Prime-boost vaccination strategies, in particular those that use listerial vaccines for boosting, are a promising approach for maintenance therapy.
Combining anticancer vaccines with immune checkpoint inhibitors
The immunotherapy of pancreatic cancer will greatly benefit from the identification of PDA-specific TAAs. However, as mentioned above, cancer cells often exploit immune checkpoints to evade detection by cytotoxic T cells. Reciprocally, although immune checkpoint blockers are effective as single agents against specific malignancies,41-43 this is not the case of pancreatic cancer, which elicits limited adaptive immune responses owing to a high degree of immunological tolerance at baseline.43-45 Indeed, the efficiency of immune checkpoint-targeting agents is dependent on adaptive immune responses.46 Thus, it is conceivable to combine immune checkpoint blockers with anticancer vaccines, presumably resulting in the elicitation of robust antigen-specific adaptive immune responses. This notion is supported by the results of a recent clinical study investigating the clinical profile of ipilimumab plus GVAX as compared with ipilimumab alone in previously treated locally advanced or metastatic PDA patients.47,48
Breaking immunosuppression within the tumor microenvironment
A large proportion of the immunological infiltrate of PDA lesions exerts pro-inflammatory functions. However, these pro-inflammatory components are insufficient to elicit adequate antitumor immune responses. Cytokines such as TGFβ, IL-6 and IL-10, just to name a few, are produced by the pro-inflammatory infiltrate of PDA lesions and can stimulate tumor growth. Once the tumor is established, its microenvironment is skewed toward a highly immunosuppressive state characterized by a high frequency of tumor-associated macrophages with an M2 phenotype, increased neutrophils with an N2 phenotype, TH2 immune responses, and abundant Tregs, which further contribute to immune evasion.45 In the presence of such an immunosuppressive microenvironment, immune effector cells are unable to mediate cytotoxic functions even when they have been fully activated in the periphery. Therefore, for anticancer vaccines to elicit therapeutically relevant tumor-specific immune responses, new strategies must be designed that convert the highly immunosuppressive microenvironment of pancreatic tumors into an immunostimulatory one.45 All clinical trials are summarized in Table 1.
Table 1. Clinical trials of vaccine therapy in pancreas cancer.
|
Vaccine type |
Investigator |
Phase |
Stage |
Vaccine |
Clinical and Immunology Outcomes |
|
|---|---|---|---|---|---|---|
|
Peptide vaccines |
KRAS-targeting vaccines |
Gjertsen (1995)12 |
I/II |
5 patients with histologically confirmed PDA |
Mutated K-ras peptide |
2 immune responders showed longer survival |
|
Gjertsen (2001)13 |
I/II |
48 patients, 10 surgically resected PDA, 38 with advanced PDA |
Mutated K-ras peptide with GM-CSF |
148 days in responders vs. 61 days in nonresponders |
||
|
Abou-Alfa (2011)14 |
- |
24 patients resected PDA |
Mutated K-ras peptide |
Median recurrence free survival 8.6 months; Median overall survival 20.3 months |
||
| Telomerase-targeting vaccines |
Bernhardt et al (2006)15 |
I/II |
48 patients with unresectable PDA |
Telomerase peptide (GV1001) with GM-CSF |
Median overall survival 8.6 months in intermediate dose group |
|
|
Gastrine based vaccine |
Gilliam et al (2012)18 |
- |
154 patients with advanced PDA, unwilling or unable to take chemotherapy |
Gastrin peptide vaccine (G17DT) versus placebo |
151 days G17DT vs. 82 days placebo p=0.03 |
|
|
HSP-peptide complex-based vaccines |
Maki et al (2007)22 |
I |
10 patients with resected PDA |
HSPCC-96 |
Median overall survival was 2.2 years |
|
|
Recombinant virus-based |
MUC-1 and CEA in poxvirus |
Kaufman et al (2007)23 |
I |
10 patients with advanced stage PDA |
TRICOM, MUC-1 and CEA in poxvirus with GM-CSF |
15.1 months in responders vs. 3.9 months in nonresponders (p=0.002) |
|
Listeria-based vaccines |
Live attenuated Listeria vaccine |
Le et al (2012)30 |
I |
28 patients with mesothelioma, lung, pancreas, or ovarian cancer liver metastasis | Live attenuated Listeria vaccine (ANZ-100) vs Live attenuated mesothelin expressingListeria vaccine (CRS-207) |
37% of patients in CRS-207 arm live after 15 months |
|
Dentritic cell vaccines |
MUC-1 pulsed autologous DC vaccine |
Lepisto et al (2008)31 |
I/II |
12 patients with resected pancreatic and biliary cancer |
MUC-1 pulsed autologous DC vaccine |
Median overall survival 26 months |
|
DC-based vaccine plus LAK |
Kimura et al (2012)32 |
- |
49 patients with inoperable PDA (Stage III,, IVA, IVB) |
DC-based vaccine plus LAK with gemcitabine or S-1 |
Median overall survival of patients receiving DC vaccine and chemotherapy plus LAK cell therapy was longer than those receiving DC vaccine in combination with chemotherapy but no LAK cells |
|
|
Whole cell vaccines |
GM-CSF vaccine |
Jaffee et al (2001)34 |
I |
14 patients with resected PDA |
GM-CSF vaccine with chemoradiotherapy |
3 patients disease free at leas 25 months after diagnosis |
|
Laheru et al (2008)35 |
II |
50 patients with advanced PDA |
GM-CSF vaccine (arm A) Cy/GM-CSF vaccine(arm B) |
Median overall survival in arm A : 2.3 months Median overall survival in arm B: 4.3 months |
||
|
Lutz et al (2012)36 |
II |
60 patients with resected PDA |
GM-CSF vaccine with chemotherapy (5FU) and radiotherapy |
Median overall survival : 24.8 months |
||
|
Le at al (2013)37 |
II |
60 patients with metastatic PDA |
2 doses of Cy/ GM-CSF vaccine followed by 4 doses CRS-207 (arm A) 6 doses ofCy/ GM-CSF vaccine (arm B) |
Median overall survival was 6 months in Arm A vs. 3.4 months in Arm B (p=0.0114). |
||
|
Algenpantucel-L |
Hardacre et al (2010)38 |
II |
62 patients with resected PDA |
Algenpantucel-L with chemotherapy (gemcitabine and 5 FU)+ radiotherapy |
12-month disease-free survival was 62 %, and the 12-month overall survival was 86 %. |
|
|
Immune-modulating agents and vaccine combination therapys |
Ipilimumab + Whole cell vaccines |
Le et al (2012)48 |
Ib |
30 patients with, local advanced, treatment refractory or metastatic PDA |
Ipilimumab alone vsIpilimumab plus Cy/GM-CSF vaccine |
Median overall survival in Ipilimumab alone : 3.3 months Median overall survival in Ipilimumab alone : 5.5 months |
Disclosure of Potential Conflicts of Interest
Under a licensing agreement between Aduro Biotech and the Johns Hopkins University, the University is entitled to milestone payments and royalty on sales of the GM-CSF secreting pancreatic tumor vaccine product described in this manuscript.
Acknowledgments
This work was supported by the NIH K23 CA148964–01 (L.Z.), Johns Hopkins School of Medicine Clinical Scientist Award (L.Z.), an American Society of Clinical Oncology Young Investigator Award (L.Z.), Viragh Foundation and the Skip Viragh Pancreatic Cancer Center at Johns Hopkins (E.M.J. nd L.Z), The National Pancreas Foundation (L.Z.), Lefkofsky Family Foundation (L.Z.), the NCI SPORE in Gastrointestinal Cancers P50 CA062924 (E.M.J. and L.Z.), Lustgarten Foundation (L.Z.), and the Sol Goldman Pancreatic Cancer Center (L.Z. and B.H.E.), E.M.J. is the first recipient of the Dana and Albert “Cubby” Broccoli Endowed Professorship.
Glossary
Abbreviations:
- 5-FU
5-fluorouracil
- APC
antigen-presenting cell
- CEA
carcinoembryonic antigen
- CTLA4
cytotoxic T-lymphocyte-associated protein 4
- DC
dendritic cell
- GM-CSF
granulocyte macrophage colony-stimulating factor
- HLA
human leukocyte antigen
- HSP
heat shock protein
- ICAM1
intercellular adhesion molecule 1
- IL
interleukin
- IFN
interferon
- LAK
lymphokine-activated killer
- mAb
monoclonal antibody
- MHC
major histocompatibility complex
- MUC1
mucin-1
- PD-1
programmed cell death protein 1
- PD-L
programmed cell death ligant
- PDA
pancreatic ductal adenocarcinoma
- TAA
tumor-associated antigen
- TERT
telomerase reverse transcriptase
- TGF
transforming growth factor
- Treg
regulatory T cell
Citation: Salman B, Zhou D, Jaffee EM, Edil BH, Zheng L. Vaccine therapy for pancreatic cancer. OncoImmunology 2013; 2:e26662; 10.4161/onci.26662
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26662
References
- 1.American Cancer Society. Cancer Facts & Figures 2012. Atlanta: American Cancer Society; 2012. [Google Scholar]
- 2.Cress RD, Yin D, Clarke L, Bold R, Holly EA. Survival among patients with adenocarcinoma of the pancreas: a population-based study (United States) Cancer Causes Control. 2006;17:403–9. doi: 10.1007/s10552-005-0539-4. [DOI] [PubMed] [Google Scholar]
- 3.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, Hodgin MB, Sauter PK, Hruban RH, Riall TS, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006;10:1199–210, discussion 1210-1. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Garcea G, Dennison AR, Pattenden CJ, Neal CP, Sutton CD, Berry DP. Survival following curative resection for pancreatic ductal adenocarcinoma. A systematic review of the literature. JOP. 2008;9:99–132. [PubMed] [Google Scholar]
- 5.Roy R, Maraveyas A. Chemoradiation in pancreatic adenocarcinoma: a literature review. Oncologist. 2010;15:259–69. doi: 10.1634/theoncologist.2009-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, et al. Groupe Tumeurs Digestives of Unicancer. PRODIGE Intergroup FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 7.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–77. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 8.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–74. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 9.Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol. 2003;3:939–51. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]
- 10.Gaudernack G. Prospects for vaccine therapy for pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:299–314. doi: 10.1016/j.bpg.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Gjertsen MK, Bakka A, Breivik J, Saeterdal I, Gedde-Dahl T, 3rd, Stokke KT, 3rd, Sølheim BG, Egge TS, Søreide O, Thorsby E, et al. Ex vivo ras peptide vaccination in patients with advanced pancreatic cancer: results of a phase I/II study. Int J Cancer. 1996;65:450–3. doi: 10.1002/(SICI)1097-0215(19960208)65:4<450::AID-IJC10>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 12.Gjertsen MK, Bakka A, Breivik J, Saeterdal I, Solheim BG, Søreide O, Thorsby E, Gaudernack G. Vaccination with mutant ras peptides and induction of T-cell responsiveness in pancreatic carcinoma patients carrying the corresponding RAS mutation. Lancet. 1995;346:1399–400. doi: 10.1016/S0140-6736(95)92408-6. [DOI] [PubMed] [Google Scholar]
- 13.Gjertsen MK, Buanes T, Rosseland AR, Bakka A, Gladhaug I, Søreide O, Eriksen JA, Møller M, Baksaas I, Lothe RA, et al. Intradermal ras peptide vaccination with granulocyte-macrophage colony-stimulating factor as adjuvant: Clinical and immunological responses in patients with pancreatic adenocarcinoma. Int J Cancer. 2001;92:441–50. doi: 10.1002/ijc.1205. [DOI] [PubMed] [Google Scholar]
- 14.Abou-Alfa GK, Chapman PB, Feilchenfeldt J, Brennan MF, Capanu M, Gansukh B, Jacobs G, Levin A, Neville D, Kelsen DP, et al. Targeting mutated K-ras in pancreatic adenocarcinoma using an adjuvant vaccine. Am J Clin Oncol. 2011;34:321–5. doi: 10.1097/COC.0b013e3181e84b1f. [DOI] [PubMed] [Google Scholar]
- 15.Bernhardt SL, Gjertsen MK, Trachsel S, Møller M, Eriksen JA, Meo M, Buanes T, Gaudernack G. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: A dose escalating phase I/II study. Br J Cancer. 2006;95:1474–82. doi: 10.1038/sj.bjc.6603437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gunturu KS, Rossi GR, Saif MW. Immunotherapy updates in pancreatic cancer: are we there yet? Ther Adv Med Oncol. 2013;5:81–9. doi: 10.1177/1758834012462463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monstein HJ, Ohlsson B, Axelson J. Differential expression of gastrin, cholecystokinin-A and cholecystokinin-B receptor mRNA in human pancreatic cancer cell lines. Scand J Gastroenterol. 2001;36:738–43. doi: 10.1080/003655201300192003. [DOI] [PubMed] [Google Scholar]
- 18.Gilliam AD, Broome P, Topuzov EG, Garin AM, Pulay I, Humphreys J, et al. An international multicenterrandomized controlled trial of G17DT in patients with pancreatic cancer. Pancrea. 2012;41:374–9. doi: 10.1097/MPA.0b013e31822ade7e. [DOI] [PubMed] [Google Scholar]
- 19.Satoh K, Kaneko K, Hirota M, Masamune A, Satoh A, Shimosegawa T. Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer. 2001;92:271–8. doi: 10.1002/1097-0142(20010715)92:2<271::AID-CNCR1319>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Wobser M, Keikavoussi P, Kunzmann V, Weininger M, Andersen MH, Becker JC. Complete remission of liver metastasis of pancreatic cancer under vaccination with a HLA-A2 restricted peptide derived from the universal tumor antigen survivin. Cancer Immunol Immunother. 2006;55:1294–8. doi: 10.1007/s00262-005-0102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kameshima H, Tsuruma T, Kutomi G, Shima H, Iwayama Y, Kimura Y, Imamura M, Torigoe T, Takahashi A, Hirohashi Y, et al. Immunotherapeutic benefit of α-interferon (IFNα) in survivin2B-derived peptide vaccination for advanced pancreatic cancer patients. Cancer Sci. 2013;104:124–9. doi: 10.1111/cas.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maki RG, Livingston PO, Lewis JJ, Janetzki S, Klimstra D, Desantis D, Srivastava PK, Brennan MF. A phase I pilot study of autologous heat shock protein vaccine HSPPC-96 in patients with resected pancreatic adenocarcinoma. Dig Dis Sci. 2007;52:1964–72. doi: 10.1007/s10620-006-9205-2. [DOI] [PubMed] [Google Scholar]
- 23.Kaufman HL, Kim-Schulze S, Manson K, DeRaffele G, Mitcham J, Seo KS, Kim DW, Marshall J. Poxvirus-based vaccine therapy for patients with advanced pancreatic cancer. J Transl Med. 2007;5:60. doi: 10.1186/1479-5876-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arlen PM, Gulley JL, Madan RA, Hodge JW, Schlom J. Preclinical and clinical studies of recombinant poxvirus vaccines for carcinoma therapy. Crit Rev Immunol. 2007;27:451–62. doi: 10.1615/CritRevImmunol.v27.i5.40. [DOI] [PubMed] [Google Scholar]
- 25.Tilney LG, Portnoy DA. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bahjat KS, Liu W, Lemmens EE, Schoenberger SP, Portnoy DA, Dubensky TW, Jr., Brockstedt DG. Cytosolic entry controls CD8+-T-cell potency during bacterial infection. Infect Immun. 2006;74:6387–97. doi: 10.1128/IAI.01088-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neuenhahn M, Kerksiek KM, Nauerth M, Suhre MH, Schiemann M, Gebhardt FE, Stemberger C, Panthel K, Schröder S, Chakraborty T, et al. CD8alpha+ dendritic cells are required for efficient entry of Listeria monocytogenes into the spleen. Immunity. 2006;25:619–30. doi: 10.1016/j.immuni.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 28.Brockstedt DG, Dubensky TW. Promises and challenges for the development of Listeria monocytogenes-based immunotherapies. Expert Rev Vaccines. 2008;7:1069–84. doi: 10.1586/14760584.7.7.1069. [DOI] [PubMed] [Google Scholar]
- 29.Brockstedt DG, Giedlin MA, Leong ML, Bahjat KS, Gao Y, Luckett W, Liu W, Cook DN, Portnoy DA, Dubensky TW., Jr. Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proc Natl Acad Sci U S A. 2004;101:13832–7. doi: 10.1073/pnas.0406035101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le DT, Brockstedt DG, Nir-Paz R, Hampl J, Mathur S, Nemunaitis J, Sterman DH, Hassan R, Lutz E, Moyer B, et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Cancer Res. 2012;18:858–68. doi: 10.1158/1078-0432.CCR-11-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lepisto AJ, Moser AJ, Zeh H, Lee K, Bartlett D, McKolanis JR, Geller BA, Schmotzer A, Potter DP, Whiteside T, et al. A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther. 2008;6(B):955–64. [PMC free article] [PubMed] [Google Scholar]
- 32.Kimura Y, Tsukada J, Tomoda T, Takahashi H, Imai K, Shimamura K, Sunamura M, Yonemitsu Y, Shimodaira S, Koido S, et al. Clinical and immunologic evaluation of dendritic cell-based immunotherapy in combination with gemcitabine and/or S-1 in patients with advanced pancreatic carcinoma. Pancreas. 2012;41:195–205. doi: 10.1097/MPA.0b013e31822398c6. [DOI] [PubMed] [Google Scholar]
- 33.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90:3539–43. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, Goemann M, Coleman J, Grochow L, Donehower RC, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001;19:145–56. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 35.Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, Tartakovsky I, Nemunaitis J, Le D, Sugar E, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455–63. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, Sugar E, Piantadosi S, Cameron JL, Solt S, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328–35. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le DT, Wang-Gillam A, Picozzi VJ, Greten TF, Crocenzi TS, Gregory M, et al. Interim safety and efficacy analysis of a phase II, randomized study of GVAX pancreas and CRS-207 immunotherapy in patients with metastatic pancreatic cancer. J Clin Oncol. 2013;31(suppl):abstr 4040. [Google Scholar]
- 38.Hardacre JM, Mulcahy M, Small W, Talamonti M, Obel J, Krishnamurthi S, Rocha-Lima CS, Safran H, Lenz HJ, Chiorean EG. Addition of algenpantucel-L immunotherapy to standard adjuvant therapy for pancreatic cancer: a phase 2 study. J Gastrointest Surg. 2013;17:94–100, discussion 100-1. doi: 10.1007/s11605-012-2064-6. [DOI] [PubMed] [Google Scholar]
- 39.Goggins M, Kern SE, Offerhaus JA, Hruban RH. Progress in cancer genetics: lessons from pancreatic cancer. Ann Oncol. 1999;10(Suppl 4):4–8. doi: 10.1093/annonc/10.suppl_4.S4. [DOI] [PubMed] [Google Scholar]
- 40.Segal NH, Parsons DW, Peggs KS, Velculescu V, Kinzler KW, Vogelstein B, Allison JP. Epitope landscape in breast and colorectal cancer. Cancer Res. 2008;68:889–92. doi: 10.1158/0008-5472.CAN-07-3095. [DOI] [PubMed] [Google Scholar]
- 41.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng L, Xue J, Jaffee EM, Habtezion A. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology. 2013;144:1230–40. doi: 10.1053/j.gastro.2012.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–33. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–12. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Le TL, Lutz E, Huang L, Onners B, Uram J, Solt S, et al. Phase Ib study of ipilimumab alone or in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene (vaccine) in pancreatic cancer. ASCO Meeting Abstracts Jan 30, 2012: 211 [Google Scholar]
- 48. Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, Diaz LA, Jr, Donehower RC, Jaffee EM, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36:382–9. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]