Abstract
We have recently described an assay for imaging interstitial collagen degradation in vivo, which allows for the identification of cell types and molecules involved in collagen turnover in the course of pathological and physiological tissue remodeling. The assay revealed a dominant role of receptor-mediated intracellular collagen degradation by M2-polarized macrophages in extracellular matrix turnover.
Keywords: Endo180, M2-polarized macrophages, Mrc1, Mrc2, cathepsin, collagen endocytosis, collagenase, in vivo imaging, interstitial collagen degradation, lysosome, mannose receptor, matrix metalloproteinase, tumor invasion, urokinase plasminogen activator receptor-associated protein
Macrophages play multiple essential roles, not only as components of the innate immune system, but also as they contribute to the preservation of tissue homeostasis and orchestrate tissue remodeling and repair. Although originating from a common monocyte precursor, macrophages may attain a variety of phenotypes to fulfill these diverse functions. In this respect, tissue remodeling and repair is believed to critically depend on a subpopulation of macrophages variably known as M2-polarized, alternatively activated or wound healing macrophages, which in tissues can be identified as they express elevated levels of mannose receptor and other markers. This macrophage subpopulation (hereafter referred to as M2-polarized macrophages) produces high levels of anti-inflammatory cytokines and promotes tissue repair by expressing molecules involved in the synthesis of extracellular matrix components and by secreting mitogenic factors.1
Tumor progression is generally associated with a devastating loss of interstitial collagen from adjacent tissues, leading to structural and functional organ breakdown. Although paramount to the morbidity and mortality of cancer, the tumor-associated degradation of interstitial collagen remains poorly understood at the cellular and molecular level, mainly due to the lack of assays for imaging collagen turnover in vivo. In most human carcinomas, collagenolytic enzymes and receptors that mediate collagen endocytosis are predominantly expressed by non-malignant cells of the tumor stroma. Collagenolytic enzymes include secreted and membrane-associated proteases of the matrix metalloproteinase (MMP) and cysteine cathepsin family, while collagen endocytosis receptors encompass collagen-binding β1-integrins and members of the mannose receptor family. Frequently, these molecules are expressed by human carcinoma cells that have adapted to grow ex vivo, and the relative contribution of stromal and carcinoma cells to interstitial collagen degradation in human cancers is the subject of controversy.2-6
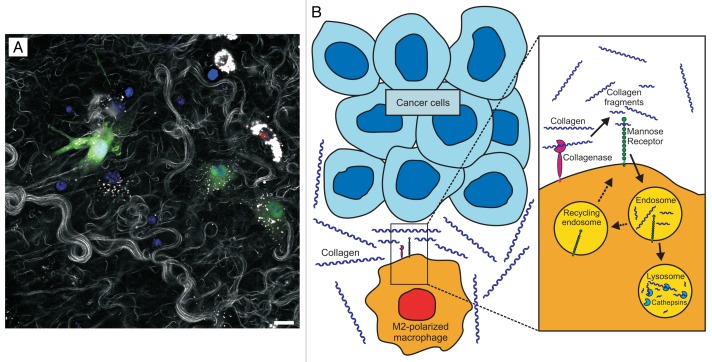
We have recently described the first assay for directly imaging the turnover of interstitial collagen in vivo, a technical development that may prove useful in alleviating the considerable gaps in our knowledge of tumor-associated collagen degradation (Fig. 1).7 Our assay takes advantage of the ability of fluorescently-labeled interstitial collagen fibrils introduced into the connective tissue of living mice to self-assemble into collagen fiber networks, coupled to the possibility to visualize the fate of these networks provided by multi-photon and confocal microscopy. When employed in mice with cell lineage-specific fluorescent labels or mice with targeted gene ablations, this approach allows for the evaluation of the role of specific cell types and molecules in interstitial collagen turnover in vivo. When the exogenous collagen is placed into the dermis, the inoculation trauma and excess collagen combines to induce a matrix catabolic environment that favors collagen degradation and resembles the wound healing microenvironment. This is evidenced by the recruitment of inflammatory cells to the injection site, by the increased expression of extracellular matrix-degrading enzymes, and by the much shortened half-life of injected interstitial collagen, as compared with dermal interstitial collagen in homeostatic conditions. By using this novel assay, the turnover of interstitial collagen in vivo was found to involve the cooperation between extracellular matrix metalloproteinase collagenases, 2 endocytic receptors, the urokinase plasminogen receptor-associated protein, the mannose receptor, and lysosomal cathepsins. The mechanistic dissection of this catabolic sequence revealed it to include the initial coarse fragmentation of collagen fibers into smaller fragments by collagenases, followed by the receptor-mediated cellular uptake of these fragments and their routing to complete lysosomal degradation. Several cell types appear to engage in this turnover process, including tissue-resident fibroblasts and infiltrating chemokine (C-X3-C) receptor 1 (Cx3cr1)+ macrophages. The dominant cells executing collagen turnover in vivo, however, turned out to be M2-polarized macrophages. Indeed, although M2-polarized macrophages only constituted 15% of cells, they accounted for 60% of all cellular collagen uptake. Of note, in our model, M2-polarized macrophages internalized collagen in a mannose receptor-dependent manner.
Figure 1. Proposed role of M2-polarized macrophages in tumor-associated collagen degradation. (A) Imaging collagen degradation in vivo. The fate of fluorescent fibrillar collagen introduced into the dermis of live mice was imaged using confocal microscopy. The co-localization of intracellular collagen fragments (white dots) with a lysosomal marker (red) demonstrates that extracellular collagen fibers (white strands) are degraded, internalized and routed to lysosomal degradation. A medium level of collagen uptake by green fluorescent protein (GFP)-expressing fibroblasts (green) and a very high level of collagen uptake by M2-polarized macrophages (two cells in upper right corner of the image) are observed. Scale bar = 50 µm. (B) Proposed role for M2-polarized macrophages in interstitial collagen degradation in the course of tumor invasion. M2-polarized macrophages are recruited to the tumor stroma, where they mediate interstitial collagen degradation through the concerted action of membrane-associated matrix metalloproteinases (collagenases), which fragment large collagen fibers, and the mannose receptor, which internalizes partially digested collagen and directs it to complete cathepsin-mediated degradation within lysosomes.
The unexpected key contribution of M2-polarized macrophages to collagen turnover, in hindsight, is entirely consistent with the wound healing-promoting properties of this macrophage subpopulation, and in particular with its typically elevated expression of the mannose receptor. Although the mannose receptor was first shown to mediate the carbohydrate-dependent phagocytosis of specific microbial pathogens, it is also a high affinity receptor for collagen that efficiently targets collagen to endocytosis and lysosomal degradation in ex vivo model systems.8
The identification of a dominant function of M2-polarized macrophages in collagen turnover adds an exciting new property to this multifunctional macrophage population. This may have important implications for understanding tumor-associated interstitial collagen degradation. The unique property of M2-polarized macrophages to suppress inflammatory responses and promote cell growth appears to be exploited by malignant cells, because (1) M2-polarized macrophages (in this context termed “tumor-associated macrophages”) are a recurrent constituent of the mature stroma of human carcinomas, and (2) M2-polarized macrophages promote tumor progression in animal models of mammary and pancreatic adenocarcinoma.9,10 We propose that a catabolic sequence involving the matrix metalloproteinase-mediated fragmentation of interstitial collagen, the mannose receptor-dependent endocytosis of collagen fragments, and their cysteine cathepsin-mediated lysosomal degradation represents an additional important mechanism by which M2-polarized macrophages exert tumor-promoting effects (Fig. 1).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Madsen DH, Bugge TH. Imaging collagen degradation in vivo highlights a key role for M2-polarized macrophages in extracellular matrix degradation. OncoImmunology 2013; 2:e27127; 10.4161/onci.27127
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/27127
References
- 1.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okada A, Bellocq JP, Rouyer N, Chenard MP, Rio MC, Chambon P, Basset P. Membrane-type matrix metalloproteinase (MT-MMP) gene is expressed in stromal cells of human colon, breast, and head and neck carcinomas. Proc Natl Acad Sci U S A. 1995;92:2730–4. doi: 10.1073/pnas.92.7.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bugge TH, Behrendt N. Cooperation between proteolysis and endocytosis in collagen turnover. In: Parks WC, Mecham RP, eds. Extracellular matrix degradation. Heidelberg: Springer, 2011:53-74. [Google Scholar]
- 4.Rowe RG, Weiss SJ. Navigating ECM barriers at the invasive front: the cancer cell-stroma interface. Annu Rev Cell Dev Biol. 2009;25:567–95. doi: 10.1146/annurev.cellbio.24.110707.175315. [DOI] [PubMed] [Google Scholar]
- 5.Holmbeck K, Bianco P, Birkedal-Hansen H. MT1-mmp: a collagenase essential for tumor cell invasive growth. Cancer Cell. 2003;4:83–4. doi: 10.1016/S1535-6108(03)00196-X. [DOI] [PubMed] [Google Scholar]
- 6.Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer. 2006;6:764–75. doi: 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- 7.Madsen DH, Leonard D, Masedunskas A, Moyer A, Jürgensen HJ, Peters DE, Amornphimoltham P, Selvaraj A, Yamada SS, Brenner DA, et al. M2-like macrophages are responsible for collagen degradation through a mannose receptor-mediated pathway. J Cell Biol. 2013;202:951–66. doi: 10.1083/jcb.201301081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leitinger B, Hohenester E. Mammalian collagen receptors. Matrix Biol. 2007;26:146–55. doi: 10.1016/j.matbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 9.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gocheva V, Wang HW, Gadea BB, Shree T, Hunter KE, Garfall AL, Berman T, Joyce JA. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010;24:241–55. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]