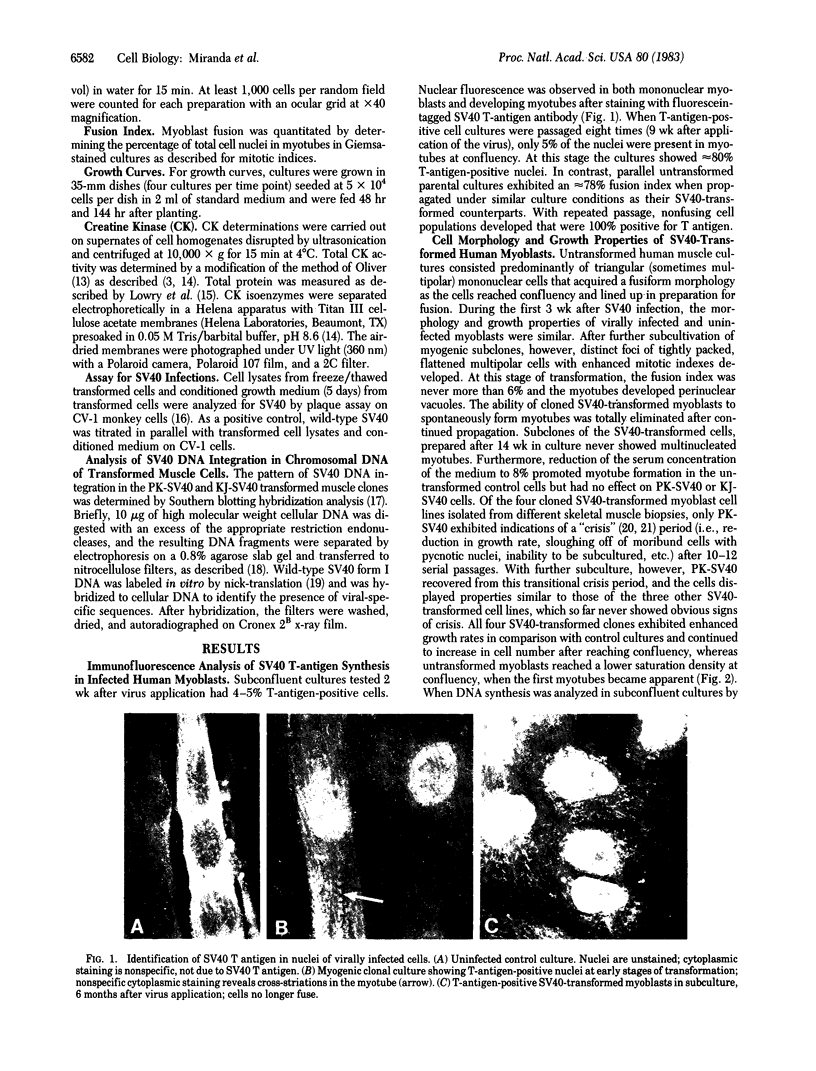
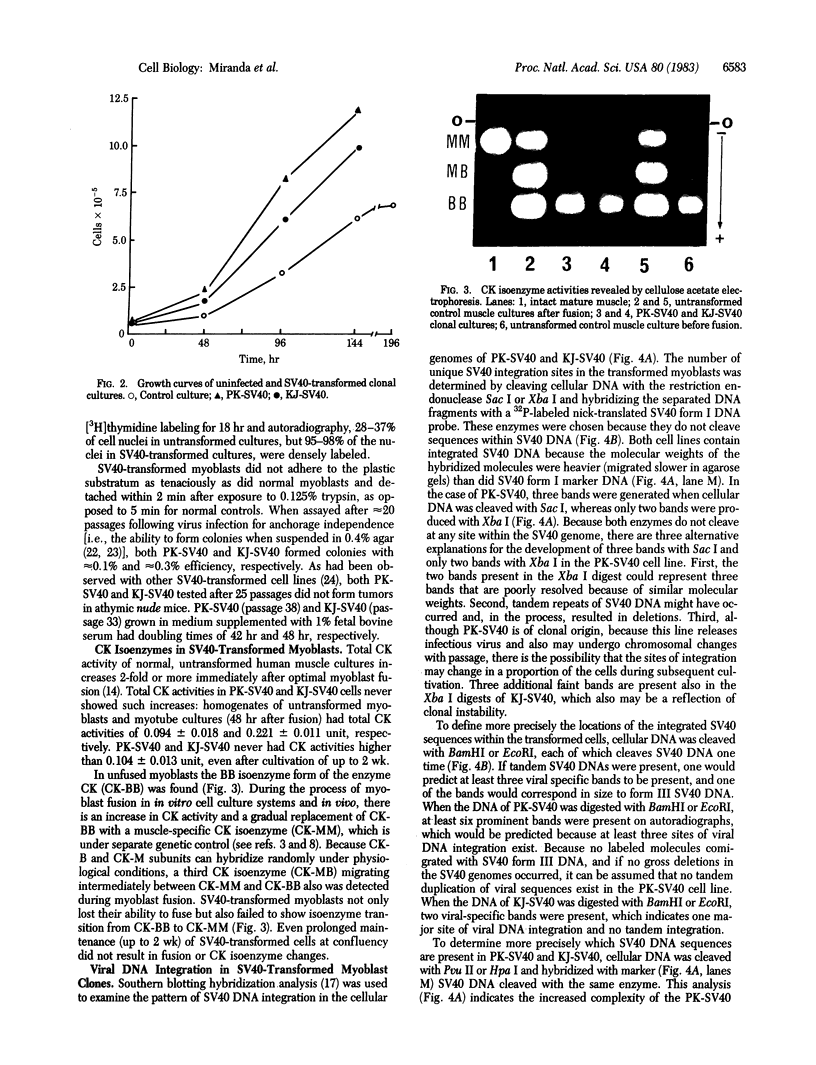
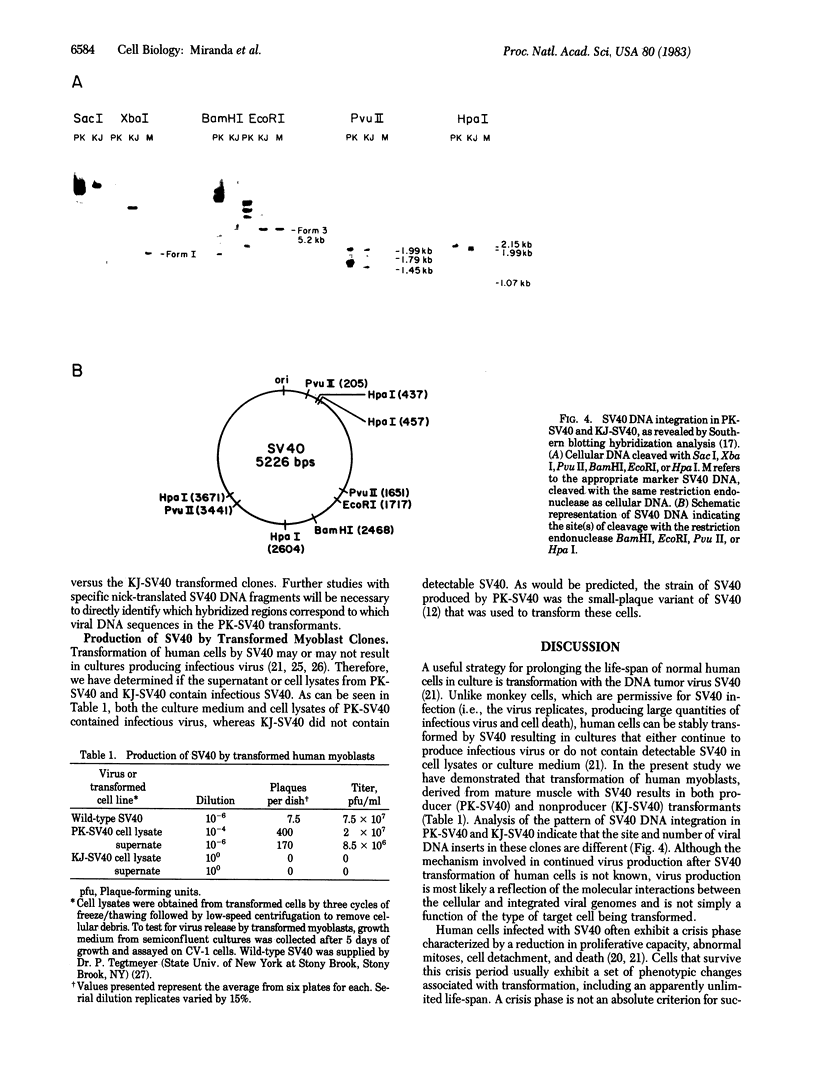
Abstract
Molecular studies of the biochemical alterations involved in human myopathies have been restricted because of the finite life-span and slow growth rate of cultures derived from primary tissue. Because the tumor virus simian virus 40 (SV40) can alter both the growth properties and longevity of human cells, we have infected skeletal muscle cultures derived from four biopsies with a small-plaque variant of SV40 and analyzed the biological and biochemical properties of cloned myoblast derivatives. At early times after infection, myoblasts fused normally into multinucleated myotubes, and both unfused and fused cells contained SV40 tumor antigen (T antigen). After six to eight subcultures after infection, the ability of myoblasts to fuse diminished, and clonal cell lines were generated with increased growth rates and saturation densities. Transformed cultures also lost contact inhibition of growth and became anchorage independent. Unlike untransformed myoblasts, SV40-transformed clones did not undergo an increase in creatine kinase activity or a transition of creatine kinase isoenzymes from the BB form to the muscle-specific MM form. Analysis of the pattern of SV40 DNA integration by Southern blotting hybridization analysis in two cloned SV40-transformed myoblast cell lines (KJ-SV40 and PK-SV40) indicated that KJ-SV40 contained at least one site of SV40 DNA integration into chromosomal DNA and PK-SV40 contained at least three sites of SV40 DNA covalently linked to cellular DNA. Cell lysates and growth medium from PK-SV40 transformants contained infectious small-plaque variant SV40, whereas KJ-SV40 did not contain or produce detectable virus. These studies demonstrate that human myoblasts can be immortalized by SV40. This procedure may prove useful for generating large quantities of genetically deficient human cells for biochemical and molecular analysis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askanas V., Engel W. K. A new program for investigating adult human skeletal muscle grown aneurally in tissue culture. Neurology. 1975 Jan;25(1):58–67. doi: 10.1212/wnl.25.1.58. [DOI] [PubMed] [Google Scholar]
- Banks-Schlegel S. P., Howley P. M. Differentiation of human epidermal cells transformed by SV40. J Cell Biol. 1983 Feb;96(2):330–337. doi: 10.1083/jcb.96.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau H. M., Webster C. Isolation and characterization of human muscle cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5623–5627. doi: 10.1073/pnas.78.9.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman W. W., Lee T. N., Nathans D. The evolution of new species of viral DNA during serial passage of simian virus 40 at high multiplicity. Virology. 1973 Aug;54(2):384–397. doi: 10.1016/0042-6822(73)90151-7. [DOI] [PubMed] [Google Scholar]
- Carlson B. M., Gutmann E. Regneration in free grafts of normal and denervated muscles in the rat: morphology and histochemistry. Anat Rec. 1975 Sep;183(1):47–62. doi: 10.1002/ar.1091830106. [DOI] [PubMed] [Google Scholar]
- Chou J. Y. Human placental cells transformed by tsA mutants of simian virus 40: a model system for the study of placental functions. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1409–1413. doi: 10.1073/pnas.75.3.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna K., Nathans D. Specific cleavage of simian virus 40 DNA by restriction endonuclease of Hemophilus influenzae. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2913–2917. doi: 10.1073/pnas.68.12.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deftos L. J., Rabson A. S., Buckle R. M., Aurbach G. D., Potts J. T., Jr Parathyroid hormone production in vitro by human parathyroid cells transformed by Simian virus 40. Science. 1968 Jan 26;159(3813):435–436. doi: 10.1126/science.159.3813.435. [DOI] [PubMed] [Google Scholar]
- Dorsch-Häsler K., Fisher P. B., Weinstein I. B., Ginsberg H. S. Patterns of viral DNA integration in cells transformed by wild type or DNA-binding protein mutants of adenovirus type 5 and effect of chemical carcinogens on integration. J Virol. 1980 May;34(2):305–314. doi: 10.1128/jvi.34.2.305-314.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P. B., Bryson V., Schaffner C. P. Polyene macrolide antibiotic cytotoxicity and membrane permeability alterations. II. Phenotypic expression in intraspecific and interspecific somatic cell hybrids. J Cell Physiol. 1979 Aug;100(2):335–342. doi: 10.1002/jcp.1041000214. [DOI] [PubMed] [Google Scholar]
- Fisher P. B., Goldstein N. I., Weinstein I. B. Phenotypic properties and tumor promotor-induced alterations in rat embryo cells transformed by adenovirus. Cancer Res. 1979 Aug;39(8):3051–3057. [PubMed] [Google Scholar]
- Fisher P. B., Miranda A. F., Mufson R. A., Weinstein L. S., Fujiki H., Sugimura T., Weinstein I. B. Effects of teleocidin and the phorbol ester tumor promoters on cell transformation, differentiation, and phospholipid metabolism. Cancer Res. 1982 Jul;42(7):2829–2835. [PubMed] [Google Scholar]
- Fogel M., Defendi V. Infection of muscle cultures from various species with oncogenic DNA viruses (SV40 and polyoma). Proc Natl Acad Sci U S A. 1967 Sep;58(3):967–973. doi: 10.1073/pnas.58.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIRARDI A. J., JENSEN F. C., KOPROWSKI H. SV40-INDUCED TRANFORMATION OF HUMAN DIPLOID CELLS: CRISIS AND RECOVERY. J Cell Physiol. 1965 Feb;65:69–83. doi: 10.1002/jcp.1030650110. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Fareed G. C. Transformation of cultured human vascular endothelium by SV40 DNA. Cell. 1976 Dec;9(4 Pt 2):685–693. doi: 10.1016/0092-8674(76)90132-x. [DOI] [PubMed] [Google Scholar]
- Girardi A. J., Weinstein D., Moorhead P. S. SV40 transformation of human diploid cells. A parallel study of viral and karyologic parameters. Ann Med Exp Biol Fenn. 1966;44(2):242–254. [PubMed] [Google Scholar]
- HABEL K., JENSEN F., PAGANO J. S., KOPROWSKI H. SPECIFIC COMPLEMENT-FIXING TUMOR ANTIGEN IN SV40-TRANSFORMED HUMAN CELLS. Proc Soc Exp Biol Med. 1965 Jan;118:4–9. doi: 10.3181/00379727-118-29741. [DOI] [PubMed] [Google Scholar]
- Jones R. E., Sanford E. J., Rohner T. J., Jr, Rapp F. In vitro viral transformation of human prostatic carcinoma. J Urol. 1976 Jan;115(1):82–85. doi: 10.1016/s0022-5347(17)59076-x. [DOI] [PubMed] [Google Scholar]
- KONIGSBERG I. R., MCELVAIN N., TOOTLE M., HERRMANN H. The dissociability of deoxyribonucleic acid synthesis from the development of multinuclearity of muscle cells in culture. J Biophys Biochem Cytol. 1960 Oct;8:333–343. doi: 10.1083/jcb.8.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mong F. S., Hays A. P., Miranda A. F. Creatine kinase isoenzyme transitions in muscle grafts of mice. Cell Differ. 1982 May;11(3):141–145. doi: 10.1016/0045-6039(82)90004-5. [DOI] [PubMed] [Google Scholar]
- OLIVER I. T. A spectrophotometric method for the determination of creatine phosphokinase and myokinase. Biochem J. 1955 Sep;61(1):116–122. doi: 10.1042/bj0610116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima R. G., Pellett O. L., Robb J. A., Schneider J. A. Transformation of human cystinotic fibroblasts by SV40: characteristics of transformed cells with limited and unlimited growth potential. J Cell Physiol. 1977 Oct;93(1):129–136. doi: 10.1002/jcp.1040930116. [DOI] [PubMed] [Google Scholar]
- RABSON A. S., MALMGREN R. A., O'CONOR G. T., KIRSCHSTEIN R. L. Simian vacuolating virus (SV40) infection in cell cultures derived from adult human thyroid tissue. J Natl Cancer Inst. 1962 Dec;29:1123–1145. [PubMed] [Google Scholar]
- Richler C., Yaffe D. The in vitro cultivation and differentiation capacities of myogenic cell lines. Dev Biol. 1970 Sep;23(1):1–22. doi: 10.1016/s0012-1606(70)80004-5. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sack G. H., Jr Human cell transformation by simian virus 40--a review. In Vitro. 1981 Jan;17(1):1–19. doi: 10.1007/BF02618025. [DOI] [PubMed] [Google Scholar]
- Santoli D., Wroblewska Z., Gilden D. H., Girardi A., Koprowski H. Human brain in tissue culture. III. PML-SV40-induced transformation of brain cells and establishment of permanent lines. J Comp Neurol. 1975 Jun 1;161(3):317–328. doi: 10.1002/cne.901610304. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinberg M. L., Defendi V. Altered pattern of growth and differentiation in human keratinocytes infected by simian virus 40. Proc Natl Acad Sci U S A. 1979 Feb;76(2):801–805. doi: 10.1073/pnas.76.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles C. D., Desmond W., Jr, Sato G., Saier M. H., Jr Failure of human cells transformed by simian virus 40 to form tumors in athymic nude mice. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4971–4975. doi: 10.1073/pnas.72.12.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Purkis P., Lane E. B., McKay I. A., Chang S. E. Effects of SV40 transformation on the cytoskeleton and behavioural properties of human keratinocytes. Cell Differ. 1982 May;11(3):169–180. doi: 10.1016/0045-6039(82)90008-2. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Function of simian virus 40 gene A in transforming infection. J Virol. 1975 Mar;15(3):613–618. doi: 10.1128/jvi.15.3.613-618.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski J. A. Diseased muscle cells in culture. Biol Rev Camb Philos Soc. 1977 Nov;52(4):431–476. doi: 10.1111/j.1469-185x.1977.tb00856.x. [DOI] [PubMed] [Google Scholar]
- Yasin R., Kundu D., Thompson E. J. Growth of adult human cells in culture at clonal densities. Cell Differ. 1981 May;10(3):131–137. doi: 10.1016/0045-6039(81)90033-6. [DOI] [PubMed] [Google Scholar]