Abstract
Decreased arterial compliance is an early manifestation of adverse structural and functional changes within the vessel wall. Its correlation with left ventricular (LV) area on computed tomography (CT), a marker of LV remodeling, has not been well demonstrated. We tested the hypothesis that decreasing aortic compliance and increasing arterial stiffness is independently associated with increased LV area. The study population consisted of 3,540 (61±10 years, 46% men) from the MESA study who underwent aortic distensibility (AD) assessment on magnetic resonance imaging (MRI) and LV area measurement on CT (adjusted to body surface area). Multivariable logistic regression was performed to assess the association between body surface area (BSA) normalized LV area >75th percentile and AD after adjusting for baseline clinical, historical and imaging covariates. The mean LV area /BSA was 2,153 cm2 and mean AD was 1.84 mm Hg−1 x103. Subjects in the lowest AD quartile were older with higher prevalence of hypertension, diabetes, and hypercholesterolemia (p<0.05 for all comparisons). Using multivariate linear regression adjusting for demographics, traditional risk factors, coronary artery calcium and C-reactive protein, each standard deviation decrease was associated with 18 cm2 increase in the LV area. In addition, decreasing AD quartiles were independently associated with increased BSA LV area defined as >75th percentile. In this multi-ethnic cohort, reduced AD was associated with increased LV area. Longitudinal studies are needed to determine if decreased distensibility precedes and directly influences increased LV area.
Keywords: Arterial compliance, Left ventricular area, Computed tomography, Aortic Distensibility
Introduction
Decreased arterial compliance is an early manifestation of adverse structural and functional changes within the vessel wall.1 The use of different imaging techniques optimized for assessment of vascular elasticity and quantification of luminal and vessel wall parameters allows for a comprehensive and detailed view of the vascular system. 2 The distensibility coefficient (a measure of compliance) has been validated in large study populations and has been used to predict adverse cardiovascular outcomes. 2 Several studies have also documented the prognostic importance of arterial stiffness in various populations as an independent predictor of cardiovascular morbidity and all-cause mortality.3–6 Increased left ventricular (LV) mass is also well established as an independent predictor of cardiovascular morbidity and mortality.7–11 Recently, LV area has been shown to be an accurate and highly reproducible surrogate of LV mass and volumes.12, 13 It is easily obtained from gated chest computed tomography (CT). While the correlation between LV mass and aortic distensibility (AD) has been well demonstrated, the correlation between LV area on CT and AD has not been studied. 14–16 Thus, we tested the hypothesis that decreasing aortic compliance and increasing arterial stiffness are independently associated with increased LV area.
Methods
The Multi-Ethnic Study of Atherosclerosis (MESA) investigated the prevalence, correlates, and progression of sub-clinical cardiovascular disease (CVD) in a population-based sample of 6,814 men and women aged 45–84 years free of known cardiovascular disease at baseline. The study objectives and design have been published before.17 In brief, this prospective cohort study includes recruited subjects from six U.S. communities (Baltimore, MD; Chicago, Ill; Forsyth County, North Carolina; Los Angeles County, California; northern Manhattan, New York; and St. Paul, MN). In this analysis, we included all individuals who had baseline aortic distensibility (AD) assessment on MRI as well as LV area on non contrast computed tomography. We used baseline data from MESA (2000–2002). A total of 3,540 subjects (61±10 years, 46% males) had both LV area and AD on MRI measured and formed the study cohort. Institutional Review Board approval was obtained at all MESA sites, and written informed consent obtained from all participants.
Medical history, anthropometric measurements, and laboratory data for the present study were taken from the first examination of the MESA cohort (July 2000 to August 2002). Information about age, gender, ethnicity, a family history of coronary heart disease and medical history were obtained by questionnaires. Current smoker was defined as having smoked a cigarette in the last 30 days. Diabetes mellitus (DM) was defined as a fasting glucose ≥126 mg/dL or use of hypoglycemic medications. Resting blood pressure was measured 3 times in the seated position, and the average of the second and third readings was recorded. Hypertension was defined as a systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or use of medication prescribed for hypertension. Body mass index (BMI) was calculated from the equation weight (kg)/ height (m2). Total and high-density lipoprotein (HDL) cholesterol were measured from blood samples obtained after a 12-hour fast. Low-density lipoprotein cholesterol (LDL) was estimated by the Friedewald equation.18 Estimated glomerular filtration rate (GFR) was calculated using the creatinine-based four-variable Modification of Diet in Renal Disease (MDRD) equation.19
All participants underwent CT scans at the same time for evaluation of coronary calcification in exam 1. The image acquisition protocol has been described before.20–23 In summary, computed tomography scanning of the chest in supine position was performed either with an ECG-triggered (at 80% of the RR interval) electron-beam CT scanner or with prospectively ECG-triggered scan acquisition at 50% of the RR interval with a multidetector CT system that acquired 4 simultaneous 2.5mm slices for each cardiac cycle in a sequential or axial scan mode. A minimum of 35 contiguous images with a 2.5 or 3mm slice thickness was obtained, starting above the left main coronary artery to the bottom of both ventricles. Each scan was obtained in a single breath hold, field of view of 35 cm, and matrix of 512 × 512 were used to reconstruct raw image data. The nominal section thickness was 3.0 mm for electron beam CT and 2.5 mm for 4–detector row CT. Spatial resolution is described by the smallest volume element, or voxel, for the protocol for each system: 1.15 mm3 for 4–detector row CT (0.68 × 0.68 × 2.50 mm) and 1.38 mm3 for electron beam CT (0.68 × 0.68 × 3.00 mm).
LV area was determined using a single mid-slice area at the level containing the coronary sinus slice or the first level below the left atrium during mid-diastole. In the core lab, the LV traces were done around region bounded by the outer border, the anterior interventricular groove (where the left anterior descending coronary artery resides) and the left posterior interventricular groove. This area included both the LV mass and LV intracavitary volume, as well as part of the interventricular septum. The LV area calculated was adjusted to body-surface area (BSA) in all participants. Ascending and descending thoracic aortic calcium (TAC) was measured in the aorta from the lower edge of the pulmonary artery bifurcation to the cardiac apex (imaged on every study of coronary calcium). Coronary Calcium was traced in all three coronary arteries. The coronary calcium score as well as the TAC score were calculated by the method of Agatston 24.
The MESA Gradient-echo phase-contrast cine MRI with electrocardiographic gating was performed to evaluate the distensibility of the descending aorta. Images of the descending aorta were obtained in the transverse plane at the level of the right pulmonary artery perpendicular to the vessel lumen. To determine AD, the minimum and maximum cross-sectional areas of the ascending aorta vessel were determined using an automated contour routine using the software FLOW (MEDIS Medical Imaging Systems). AD was calculated using this described by Gamble23:
Where ΔPD was the difference between systolic and diastolic measurements of blood pressure, and ΔD was the difference between maximum and minimal cross-sectional aortic diameters. Blood pressure was measured immediately before and after the MRI aortic measurements, while the patient was in the supine position on the MRI scanner gantry; the average systolic and diastolic values were then used to calculate pulse pressure. The MRI reader was blinded to all variables of the study subjects.
Categorical data are presented as percent frequencies and compared between groups by chi-square test. Continuous variables are presented as mean ± standard deviation and compared using Student’s t-test. Non-normally distributed variables are presented as median and Interquartile range and were compared using non-parametric Kruskal-Wallis test. Multivariable linear regression analysis was used to determine the association between BSA normalized left ventricular and AD after adjusting for baseline clinical, historical and imaging covariates. The primary analysis was to assess the association between BSA normalized left ventricular area >75th percentile and AD after adjusting for baseline clinical, historical and imaging covariates by multivariable logistic regression. The 4th quartile (most distensible) is used as the reference group for subsequent analysis. For all multivariate modeling, the threshold for variable entry into models was p<0.05 using forward selection conditional logistic regression. Care was given to avoid model over-fitting by maintaining an outcome: covariate ratio of at least 20:1. In addition to LV area, model 1 included age, gender and ethnicity. Model 2 was adjusted for age, gender, ethnicity, hypertension, DM, cigarette smoking, family history of coronary artery disease, low density lipoprotein cholesterol levels, cholesterol lowering medications Model 3 includes all the variables in model 2 plus the Log transformed CAC+1 and Log transformed C-reactive protein (CRP). Statistical analyses were performed using SPSS (version 17.0; SPSS Inc, Chicago, IL) and SAS (version 9.1 Cary, North Carolina).
Results
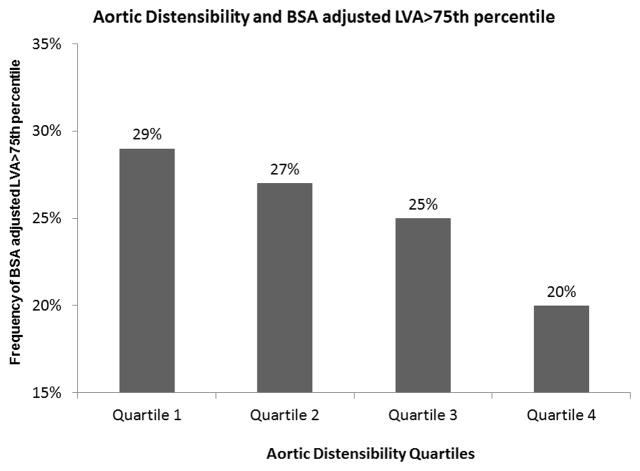
A total of 3,540 subjects are included in this analysis. The mean difference between the CT and MRI was 26 days. Participants were divided into 4 AD quartiles as shown in table 1. With the decrease in AD quartile (increasing aortic stiffness), participants were older, more likely to be males and black. The decrease in aortic distensibility was associated with an increase in the prevalence of traditional atherosclerotic risk factors including hypertension, diabetes, hypercholesterolemia, decreasing renal function and 10 year Framingham risk score (p<0.0001, Table 1) and a higher prevalence of patients with thoracic and coronary aortic calcium. The LV area increased significantly across decreasing quartiles of AD (p<0.0001). The subjects with least distensible aorta had the highest prevalence of BSA normalized LV area in the highest quartile. (Figure 1)
Table 1.
Baseline Characteristics of the study cohort according to the Four Quartiles of Aortic Distensibility
| Variable | Aortic Distensibility (mm Hg−1 x103) | P Value | |||
|---|---|---|---|---|---|
| ≥2.33 (N=885) | 1.57–2.32 (N=885) | 1.05–1..56 (N=885) | ≤1.04 (N=885) | ||
| Age (Years) | 54±7 | 58±9 | 63±9 | 69±10 | <0.0001 |
| Women | 412(47%) | 445(50%) | 387(44%) | 373(42%) | 0.003 |
| White | 364(41%) | 386(44%) | 370(42%) | 364(41%) | <0.001 |
| Chinese | 129(15%) | 98(11%) | 93(11%) | 78(9%) | |
| Black | 203(23%) | 244(28%) | 295(33%) | 313(35%) | |
| Hispanic | 189(21%) | 157(18%) | 127(14%) | 130(15%) | |
| Anti-Hypertension | 183(20%) | 309(35%) | 424(48%) | 553(62%) | <0.0001 |
| Hypertension medications | 181(20%) | 263(30%) | 356(40%) | 444(50%) | <0.0001 |
| Diabetes Mellitus | 46(5%) | 48(5%) | 81(9%) | 107(12%) | <0.0001 |
| Smoker (Former) | 288(32%) | 295(33%) | 330(37%) | 337(38%) | 0.06 |
| Smoker (Current) | 111(13%) | 130(15%) | 114(13%) | 106(12%) | 0.60 |
| Cholesterol lowering Medication | 109(12%) | 108(12%) | 147(17%) | 174(20%) | <0.0001 |
| High Sensitivity C-reactive protein (mg/l) * | 2.1 (0.9 –4.4) | 1.8 (0.8 –4.2) | 1.8 (0.8 –4.1) | 1.7 (0.7 –4.0) | 0.013 |
| Presence of TAC | 64(7%) | 148(17%) | 273(31%) | 376(42%) | <0.0001 |
| CAC = 0 | 512 (58%) | 471 (53%) | 373 (42%) | 276 (31%) | <0.0001 |
| BMI (kg/m2) | 27.6±4.8 | 27.6±4.9 | 28.1±5.2 | 27.9±4.9 | 0.51 |
| Systolic blood pressure (mmHg) | 113±16 | 123±19 | 129±20 | 136±23 | <0.0001 |
| Diastolic blood pressure (mmHg) | 69±11 | 72±10 | 72±11 | 74±11 | <0.0001 |
| LDL (mg/dl) | 118±31 | 117±30 | 117±30 | 116±30 | 0.38 |
| HDL (mg/dl) | 51±15 | 51±15 | 53±16 | 53±15 | 0.024 |
| Triglycerides (mg/dl) * | 110 (77–160) | 73 (51 –105) | 75 (56 –107) | 107 (75 –157) | 0.21 |
| Estimated Glomerular Filtration Rate, (mL/min/1.73 m2) | 84±15 | 83±17 | 81±17 | 78±11 | <0.0001 |
median and interquartile range
Figure 1.
Prevalence of increased body surface area adjusted left ventricular area in the highest quartile across the different aortic distensibility quartiles. There is an increase in the prevalence of increased LV area across aortic distensibility quartiles. Quartile 4 AD≥2.33 mm Hg−1 x103; Quartile 3 AD1.57–2.32 mm Hg−1 x103; Quartile 2 AD 1.05–1.56 mm Hg−1 x103; Quartile 1 AD ≤1.04 mm Hg−1 x103
Using multivariable analysis, the adjusted mean BSA normalized LV area is shown in table 2. After adjustment for age, gender and ethnicity, lower AD was associated with a significant increase in the BSA normalized LV area. (P<0.0001) To determine whether AD was independently associated with having BSA normalized LV area highest quartiles, we constructed several logistic regression models adjusting for multiple baseline characteristics (Table 2). After adjusting for age, gender and ethnicity, decreasing AD was independently associated with having BSA normalized LV area in the highest quartile (p<0.0001). This relation persisted after adjusting for body mass index, hypertension, diabetes mellitus, cigarette smoking, family history of heart attack, LDL cholesterol levels, cholesterol lowering medications (p<0.0001) as well as adjusting for coronary artery calcium and high sensitivity C-reactive protein (p<0.0001).
Table 2.
Adjusted odds ratios (95% confidence interval) for presence of BSA normalized left ventricular area >75th percentile with reduced aortic distensibility
| Model | Aortic Distensibility (mm Hg−1 x103) | ≤1.04 (N=885) | ||
|---|---|---|---|---|
| ≥2.33 (N=885) | 1.57–2.32 (N=885) | 1.05–1..56 (N=885) | ||
| Mean LVA/BSA (cm2) | 2119±277 | 2142±312 | 2168±312 | 2183±306 |
| Model 1 | 1.00 (Ref) | 1.35(1.07 – 1.71) | 1.67 (1.31 – 2.13) | 1.82 (1.41 – 2.35) |
| Model 2 | 1.00 (Ref) | 1.21 (0.95 –1.54) | 1.40 (1.09 – 1.80) | 1.34 (1.03 –1.76) |
| Model 3 | 1.00 (Ref) | 1.20 (0.94 –1.53) | 1.39 (1.08 – 1.80) | 1.32 (1.01 –1.74) |
Abbreviations: Ref: Reference
Model 1: adjusted for age, gender, ethnicity
Model 2: Further adjusted for hypertension, diabetes mellitus, cigarette smoking, family history of heart attack, low density lipoprotein cholesterol levels, cholesterol lowering medications
Model 3: Further adjusted for coronary artery calcium and C-reactive protein
Discussion
Our analysis demonstrates that decreased AD was independently associated with increased LV area after adjusting for age, ethnicity, gender, atherosclerotic risk factors, and coronary artery calcium. Participants in the lowest quartile of AD (least compliant aorta) had a 30% increased risk of having LV area in the highest quartile.
Previous analysis from the MESA study has shown that decreased AD (a stiffer aorta) is associated with older age, hypertension, African American ethnicity, and increased aortic calcifications25. Additionally, decreased AD was present in current smokers and in subjects with higher HDL cholesterol levels 5. In this analysis, we extended this observation and showed that decreased AD is associated with higher LV area, an easy and quick measurement that could be easily obtained from non contrast chest CT. With the increase in atherosclerotic risk factors, especially hypertension, the left ventricular mass and size increases. In addition, the aorta stiffens with aging, and the age-related structural and functional degenerative changes are accelerated by arterial hypertension. Our analysis suggests that both pathologic processes, the decreasing aortic compliance and increased left ventricular area, correlate well and are concomitantly present in many subjects.
MRI evaluation of different regions of arterial stiffness provides additional information about vascular changes. However, the use of MRI derived AD has been restricted by the limited availability and cost. Previous work has demonstrated that AD is altered by smoking, exercise, valvular disease as well as medical therapy26–28. Decreased aortic stiffness has been previously associated with a significant morbidity and increased all-cause and cardiovascular mortality in hypertensive participants 4, 29, 30. Similar relations had been shown between left ventricular hypertrophy and different outcomes. In this analysis we used left ventricular area as a surrogate of left ventricular mass and showed that it correlates well with increased AD. Whether there is a significant correlation between LV area and other atherosclerotic changes (outward remodeling, increased non calcified plaque) is not known and needs further evaluation.
This analysis should be interpreted in the context of its limitations. The cross-sectional design of our analysis would not allow us to address whether decreased AD precedes the development or is caused by increased LV area. We did not evaluate subsequent cardiovascular events and thus, it is unclear whether AD adds incremental prognostic value over increased LV area in predicting cardiovascular endpoints.
Acknowledgments
We thank the other investigators, the staff, and the participants of the MESA for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org
Sources of Funding: This research was supported by R01 HL071739 and contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute.
Footnotes
Disclosures: M.J. Budoff is a member of the Speakers’ Bureau for General Electric
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nelson AJ, Worthley SG, Cameron JD, Willoughby SR, Piantadosi C, Carbone A, Dundon BK, Leung MC, Hope SA, Meredith IT, Worthley MI. Cardiovascular magnetic resonance-derived aortic distensibility: Validation and observed regional differences in the elderly. J Hypertens. 2009;27:535–542. doi: 10.1097/hjh.0b013e32831e4599. [DOI] [PubMed] [Google Scholar]
- 2.Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness current understanding and future directions. J Am Coll Cardiol. 2011;57:1511–1522. doi: 10.1016/j.jacc.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Kupari M, Hekali P, Keto P, Poutanen VP, Tikkanen MJ, Standerstkjold-Nordenstam CG. Relation of aortic stiffness to factors modifying the risk of atherosclerosis in healthy people. Arterioscler Thromb. 1994;14:386–394. doi: 10.1161/01.atv.14.3.386. [DOI] [PubMed] [Google Scholar]
- 4.Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, Boutouyrie P. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34:1203–1206. doi: 10.1161/01.STR.0000065428.03209.64. [DOI] [PubMed] [Google Scholar]
- 5.Malayeri AA, Natori S, Bahrami H, Bertoni AG, Kronmal R, Lima JA, Bluemke DA. Relation of aortic wall thickness and distensibility to cardiovascular risk factors (from the multi-ethnic study of atherosclerosis [MESA]) Am J Cardiol. 2008;102:491–496. doi: 10.1016/j.amjcard.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sen S, Hinderliter A, Sen PK, Simmons J, Beck J, Offenbacher S, Ohman EM, Oppenheimer SM. Aortic arch atheroma progression and recurrent vascular events in patients with stroke or transient ischemic attack. Circulation. 2007;116:928–935. doi: 10.1161/CIRCULATIONAHA.106.671727. [DOI] [PubMed] [Google Scholar]
- 7.Carluccio E, Tommasi S, Bentivoglio M, Buccolieri M, Filippucci L, Prosciutti L, Corea L. Prognostic value of left ventricular hypertrophy and geometry in patients with a first, uncomplicated myocardial infarction. Int J Cardiol. 2000;74:177–183. doi: 10.1016/s0167-5273(00)00264-3. [DOI] [PubMed] [Google Scholar]
- 8.Larstorp AC, Okin PM, Devereux RB, Olsen MH, Ibsen H, Dahlof B, Kjeldsen SE, Wachtell K. Changes in electrocardiographic left ventricular hypertrophy and risk of major cardiovascular events in isolated systolic hypertension: The life study. J Hum Hypertens. 2011;25:178–185. doi: 10.1038/jhh.2010.52. [DOI] [PubMed] [Google Scholar]
- 9.Okin PM, Kjeldsen SE, Julius S, Hille DA, Dahlof B, Edelman JM, Devereux RB. All-cause and cardiovascular mortality in relation to changing heart rate during treatment of hypertensive patients with electrocardiographic left ventricular hypertrophy. Eur Heart J. 2010;31:2271–2279. doi: 10.1093/eurheartj/ehq225. [DOI] [PubMed] [Google Scholar]
- 10.Okin PM, Oikarinen L, Viitasalo M, Toivonen L, Kjeldsen SE, Nieminen MS, Edelman JM, Dahlof B, Devereux RB. Prognostic value of changes in the electrocardiographic strain pattern during antihypertensive treatment: The losartan intervention for end-point reduction in hypertension study (life) Circulation. 2009;119:1883–1891. doi: 10.1161/CIRCULATIONAHA.108.812313. [DOI] [PubMed] [Google Scholar]
- 11.Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Gattobigio R, Zampi I, Porcellati C. Prognostic value of a new electrocardiographic method for diagnosis of left ventricular hypertrophy in essential hypertension. J Am Coll Cardiol. 1998;31:383–390. doi: 10.1016/s0735-1097(97)00493-2. [DOI] [PubMed] [Google Scholar]
- 12.Nasir K, Katz R, Mao S, Takasu J, Bomma C, Lima JA, Bluemke DA, Kronmal R, Carr JJ, Budoff MJ. Comparison of left ventricular size by computed tomography with magnetic resonance imaging measures of left ventricle mass and volumes: The multi-ethnic study of atherosclerosis. J Cardiovasc Comput Tomogr. 2008;2:141–148. doi: 10.1016/j.jcct.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Schlett CL, Kwait DC, Mahabadi AA, Bamberg F, O’Donnell CJ, Fox CS, Hoffmann U. Simple area-based measurement for multidetector computed tomography to predict left ventricular size. Eur Radiol. 2010;20:1590–1596. doi: 10.1007/s00330-010-1720-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nitta K, Akiba T, Uchida K, Otsubo S, Otsubo Y, Takei T, Ogawa T, Yumura W, Kabaya T, Nihei H. Left ventricular hypertrophy is associated with arterial stiffness and vascular calcification in hemodialysis patients. Hypertension research. 2004;27:47–52. doi: 10.1291/hypres.27.47. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto Y, Hamada M, Hiwada K. Aortic distensibility is closely related to the progression of left ventricular hypertrophy in patients receiving hemodialysis. Angiology. 2000;51:933–941. doi: 10.1177/000331970005101106. [DOI] [PubMed] [Google Scholar]
- 16.Robinson RF, Nahata MC, Sparks E, Daniels C, Batisky DL, Hayes JR, Mahan JD. Abnormal left ventricular mass and aortic distensibility in pediatric dialysis patients. Pediatr Nephrol. 2005;20:64–68. doi: 10.1007/s00467-004-1667-x. [DOI] [PubMed] [Google Scholar]
- 17.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: Objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical chemistry. 1972;18:499–502. [PubMed] [Google Scholar]
- 19.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G. National kidney foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Annal Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 20.Budoff MJ, Katz R, Wong ND, Nasir K, Mao SS, Takasu J, Kronmal R, Detrano RC, Shavelle DM, Blumenthal RS, O’Brien KD, Carr JJ. Effect of scanner type on the reproducibility of extracoronary measures of calcification: The multi-ethnic study of atherosclerosis. Academic Radiology. 2007;14:1043–1049. doi: 10.1016/j.acra.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 21.Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, Flores FR, Callister TQ, Raggi P, Berman DS. Long-term prognosis associated with coronary calcification: Observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 22.Detrano RC, Anderson M, Nelson J, Wong ND, Carr JJ, McNitt-Gray M, Bild DE. Coronary calcium measurements: Effect of ct scanner type and calcium measure on rescan reproducibility--MESA study. Radiology. 2005;236:477–484. doi: 10.1148/radiol.2362040513. [DOI] [PubMed] [Google Scholar]
- 23.Katz R, Wong ND, Kronmal R, Takasu J, Shavelle DM, Probstfield JL, Bertoni AG, Budoff MJ, O’Brien KD. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the multi-ethnic study of atherosclerosis. Circulation. 2006;113:2113–2119. doi: 10.1161/CIRCULATIONAHA.105.598086. [DOI] [PubMed] [Google Scholar]
- 24.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 25.Al-Mallah MH, Nasir K, Katz R, Takasu J, Lima JA, Bluemke DA, Hundley G, Blumenthal RS, Budoff MJ. Thoracic aortic distensibility and thoracic aortic calcium (from the multi-ethnic study of atherosclerosis [mesa]) Am J Cardiol. 2010;106:575–580. doi: 10.1016/j.amjcard.2010.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hundley WG, Kitzman DW, Morgan TM, Hamilton CA, Darty SN, Stewart KP, Herrington DM, Link KM, Little WC. Cardiac cycle-dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001;38:796–802. doi: 10.1016/s0735-1097(01)01447-4. [DOI] [PubMed] [Google Scholar]
- 27.Wiesmann F, Petersen SE, Leeson PM, Francis JM, Robson MD, Wang Q, Choudhury R, Channon KM, Neubauer S. Global impairment of brachial, carotid, and aortic vascular function in young smokers: Direct quantification by high-resolution magnetic resonance imaging. J Am Coll Cardiol. 2004;44:2056–2064. doi: 10.1016/j.jacc.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 28.Murai S, Hamada S, Ueguchi T, Khankan AA, Sumikawa H, Inoue A, Tsubamoto M, Honda O, Tomiyama N, Johkoh T, Nakamura H. Aortic compliance in patients with aortic regurgitation: Evaluation with magnetic resonance imaging. Radiat Med. 2005;23:236–241. [PubMed] [Google Scholar]
- 29.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 30.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: The rotterdam study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]