Abstract
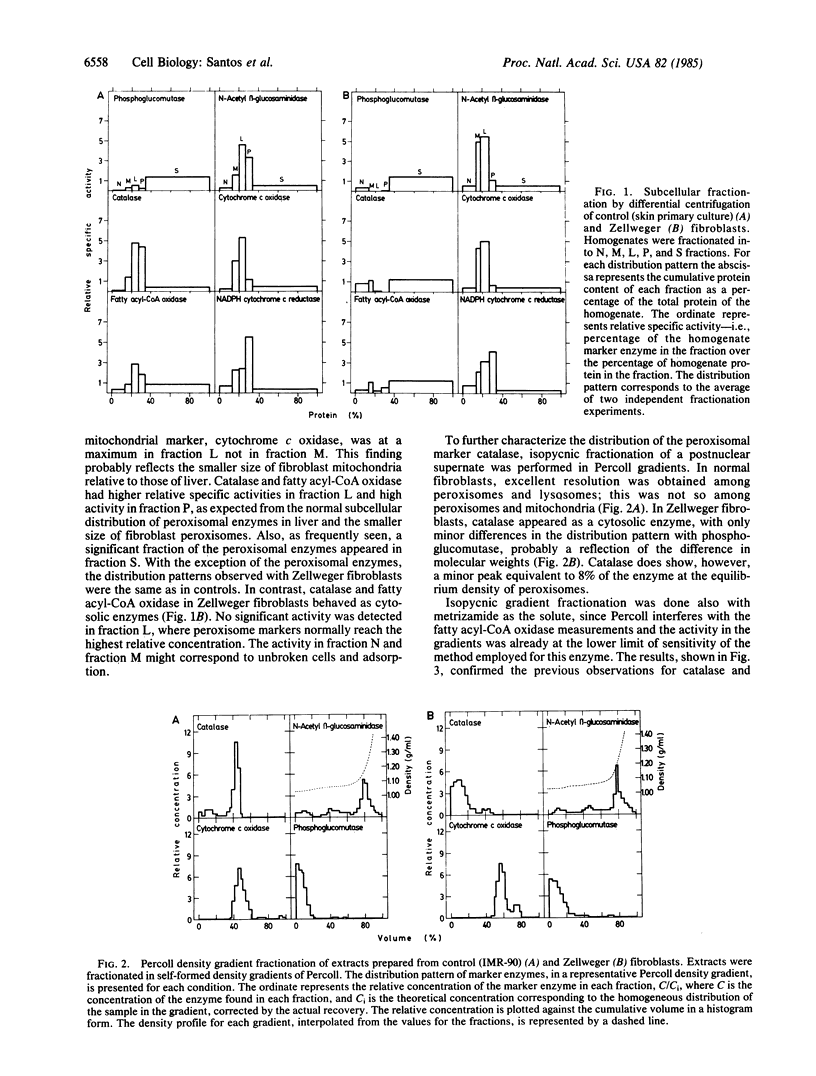
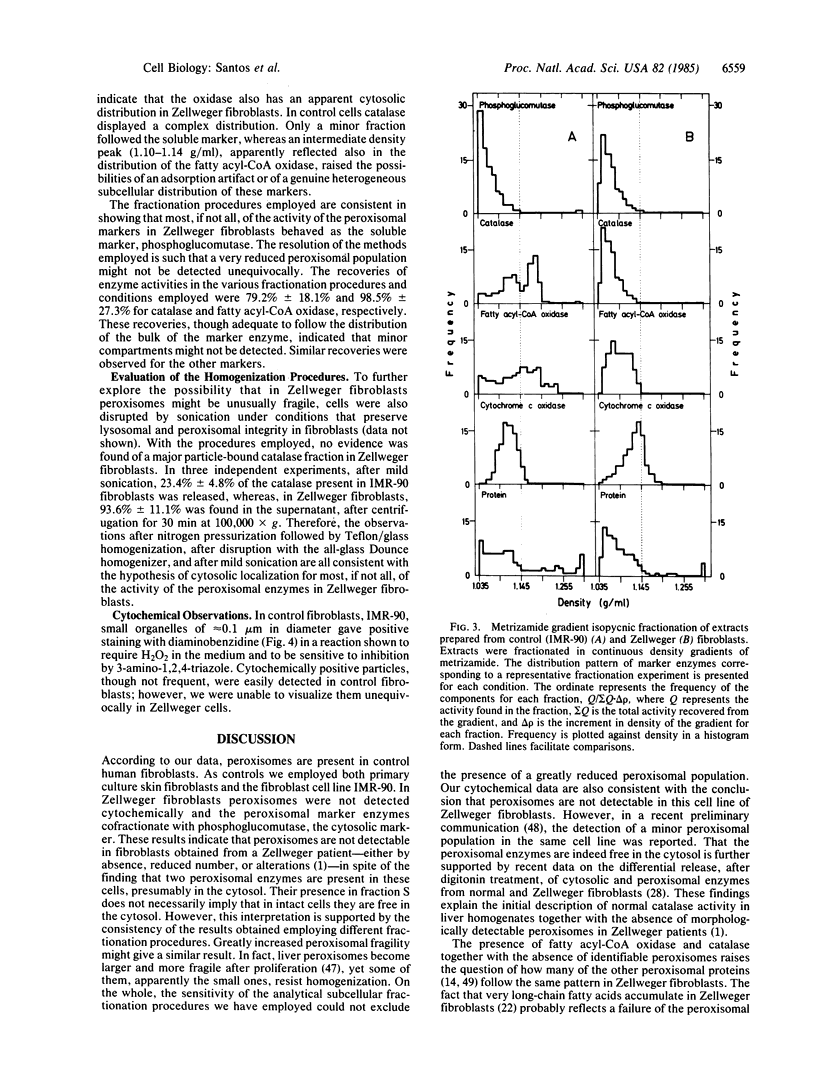
The reported absence of morphologically detectable peroxisomes in liver and kidney tissue cells from patients affected by the autosomic recessive, inherited metabolic disease known as cerebrohepatorenal, or Zellweger, syndrome was studied in fibroblasts, assuming it to be a generalized defect. Normal cultured fibroblasts were shown to contain peroxisomes according to morphological, biochemical, and subcellular fractionation criteria: particle-bound catalase and fatty acyl-CoA oxidase copurify in subcellular fractionation by differential centrifugation or isopycnic equilibrium in continuous density gradients and peroxidase-positive organelles of approximately equal to 0.1 micron in diameter are detected in the cytoplasm. In Zellweger cultured fibroblasts, these peroxisomal enzymes are present; however, they behave as cytosolic enzymes in the different subcellular fractionation procedures employed and peroxisomes are not detected cytochemically. These findings support the hypothesis that the lack of peroxisomes in this genetic disease is the consequence of a defect in the assembly of the peroxisomal constituents. Furthermore, the value of fibroblasts for subcellular analysis of peroxisomal defects is illustrated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaufay H., Amar-Costesec A., Feytmans E., Thinès-Sempoux D., Wibo M., Robbi M., Berthet J. Analytical study of microsomes and isolated subcellular membranes from rat liver. I. Biochemical methods. J Cell Biol. 1974 Apr;61(1):188–200. doi: 10.1083/jcb.61.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaufay H., Jacques P., Baudhuin P., Sellinger O. Z., Berthet J., De Duve C. Tissue fractionation studies. 18. Resolution of mitochondrial fractions from rat liver into three distinct populations of cytoplasmic particles by means of density equilibration in various gradients. Biochem J. 1964 Jul;92(1):184–205. doi: 10.1042/bj0920184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers W. E., de Duve C. Lysosomes in lymphoid tissue. II. Intracellular distribution of acid hydrolases. J Cell Biol. 1967 Feb;32(2):339–348. doi: 10.1083/jcb.32.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfman M., Inestrosa N. C., Nervi F. O., Leighton F. Acyl-CoA synthetase and the peroxisomal enzymes of beta-oxidation in human liver. Quantitative analysis of their subcellular localization. Biochem J. 1984 Dec 15;224(3):709–720. doi: 10.1042/bj2240709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown F. R., 3rd, McAdams A. J., Cummins J. W., Konkol R., Singh I., Moser A. B., Moser H. W. Cerebro-hepato-renal (Zellweger) syndrome and neonatal adrenoleukodystrophy: similarities in phenotype and accumulation of very long chain fatty acids. Johns Hopkins Med J. 1982 Dec;151(6):344–351. [PubMed] [Google Scholar]
- COOPERSTEIN S. J., LAZAROW A. A microspectrophotometric method for the determination of cytochrome oxidase. J Biol Chem. 1951 Apr;189(2):665–670. [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danks D. M., Tippett P., Adams C., Campbell P. Cerebro-hepato-renal syndrome of Zellweger. A report of eight cases with comments upon the incidence, the liver lesion, and a fault in pipecolic acid metabolism. J Pediatr. 1975 Mar;86(3):382–387. doi: 10.1016/s0022-3476(75)80967-x. [DOI] [PubMed] [Google Scholar]
- De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966 Apr;46(2):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- Goldfischer S., Moore C. L., Johnson A. B., Spiro A. J., Valsamis M. P., Wisniewski H. K., Ritch R. H., Norton W. T., Rapin I., Gartner L. M. Peroxisomal and mitochondrial defects in the cerebro-hepato-renal syndrome. Science. 1973 Oct 5;182(4107):62–64. doi: 10.1126/science.182.4107.62. [DOI] [PubMed] [Google Scholar]
- Goldfischer S., Reddy J. K. Peroxisomes (microbodies) in cell pathology. Int Rev Exp Pathol. 1984;26:45–84. [PubMed] [Google Scholar]
- Hajra A. K., Bishop J. E. Glycerolipid biosynthesis in peroxisomes via the acyl dihydroxyacetone phosphate pathway. Ann N Y Acad Sci. 1982;386:170–182. doi: 10.1111/j.1749-6632.1982.tb21415.x. [DOI] [PubMed] [Google Scholar]
- Hanson R. F., Szczepanik-VanLeeuwen P., Williams G. C., Grabowski G., Sharp H. L. Defects of bile acid synthesis in Zellweger's syndrome. Science. 1979 Mar 16;203(4385):1107–1108. doi: 10.1126/science.424737. [DOI] [PubMed] [Google Scholar]
- Heymans H. S., Schutgens R. B., Tan R., van den Bosch H., Borst P. Severe plasmalogen deficiency in tissues of infants without peroxisomes (Zellweger syndrome). Nature. 1983 Nov 3;306(5938):69–70. doi: 10.1038/306069a0. [DOI] [PubMed] [Google Scholar]
- Kawamura N., Moser H. W., Kishimoto Y. Very long chain fatty acid oxidation in rat liver. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1216–1225. doi: 10.1016/0006-291x(81)90749-x. [DOI] [PubMed] [Google Scholar]
- Kelley R. I. Review: the cerebrohepatorenal syndrome of Zellweger, morphologic and metabolic aspects. Am J Med Genet. 1983 Dec;16(4):503–517. doi: 10.1002/ajmg.1320160409. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton F., Coloma L., Koenig C. Structure, composition, physical properties, and turnover of proliferated peroxisomes. A study of the trophic effects of Su-13437 on rat liver. J Cell Biol. 1975 Nov;67(2PT1):281–309. doi: 10.1083/jcb.67.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R. I., Sondik E. J. Large-scale clinical trials: are they worth the cost? Ann N Y Acad Sci. 1982;382:411–422. doi: 10.1111/j.1749-6632.1982.tb55234.x. [DOI] [PubMed] [Google Scholar]
- Merion M., Poretz R. D. The resolution of two populations of lysosomal organelles containing endocytosed Wistaria floribunda agglutinin from murine fibroblasts. J Supramol Struct Cell Biochem. 1981;17(4):337–346. doi: 10.1002/jsscb.380170405. [DOI] [PubMed] [Google Scholar]
- Miura S., Mori M., Takiguchi M., Tatibana M., Furuta S., Miyazawa S., Hashimoto T. Biosynthesis and intracellular transport of enzymes of peroxisomal beta-oxidation. J Biol Chem. 1984 May 25;259(10):6397–6402. [PubMed] [Google Scholar]
- Mortensen P. B., Kølvraa S., Gregersen N., Rasmussen K. Cyanide-insensitive and clofibrate enhanced beta-oxidation of dodecanedioic acid in rat liver. An indication of peroxisomal beta-oxidation of N-dicarboxylic acids. Biochim Biophys Acta. 1982 Nov 12;713(2):393–397. doi: 10.1016/0005-2760(82)90258-2. [DOI] [PubMed] [Google Scholar]
- Moser A. E., Singh I., Brown F. R., 3rd, Solish G. I., Kelley R. I., Benke P. J., Moser H. W. The cerebrohepatorenal (Zellweger) syndrome. Increased levels and impaired degradation of very-long-chain fatty acids and their use in prenatal diagnosis. N Engl J Med. 1984 May 3;310(18):1141–1146. doi: 10.1056/NEJM198405033101802. [DOI] [PubMed] [Google Scholar]
- Nichols W. W., Murphy D. G., Cristofalo V. J., Toji L. H., Greene A. E., Dwight S. A. Characterization of a new human diploid cell strain, IMR-90. Science. 1977 Apr 1;196(4285):60–63. doi: 10.1126/science.841339. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M. Microperoxisomes and peroxisomes in relation to lipid metabolism. Ann N Y Acad Sci. 1982;386:138–152. doi: 10.1111/j.1749-6632.1982.tb21412.x. [DOI] [PubMed] [Google Scholar]
- Pfeifer U., Sandhage K. Licht- und Elektronenmikroskopische Leberbefunde beim Cerebro-Hepato-Renalen Syndrom nach Zellweger (Peroxisomen-Defizienz). Virchows Arch A Pathol Anat Histol. 1979 Oct;384(3):269–284. doi: 10.1007/BF00428229. [DOI] [PubMed] [Google Scholar]
- Rachubinski R. A., Fujiki Y., Mortensen R. M., Lazarow P. B. Acyl-Coa oxidase and hydratase-dehydrogenase, two enzymes of the peroxisomal beta-oxidation system, are synthesized on free polysomes of clofibrate-treated rat liver. J Cell Biol. 1984 Dec;99(6):2241–2246. doi: 10.1083/jcb.99.6.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy J. K., Ohno S. Testicular feminization of the mouse: paucity of peroxisomes in Leydig cell of the testis. Am J Pathol. 1981 Apr;103(1):96–104. [PMC free article] [PubMed] [Google Scholar]
- Robbins A. R. Isolation of lysosomal alpha-mannosidase mutants of Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1911–1915. doi: 10.1073/pnas.76.4.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roels F., Goldfischer S. Cytochemistry of human catalase. The demonstration of hepatic and renal peroxisomes by a high temperature procedure. J Histochem Cytochem. 1979 Nov;27(11):1471–1477. doi: 10.1177/27.11.92501. [DOI] [PubMed] [Google Scholar]
- Rome L. H., Garvin A. J., Allietta M. M., Neufeld E. F. Two species of lysosomal organelles in cultured human fibroblasts. Cell. 1979 May;17(1):143–153. doi: 10.1016/0092-8674(79)90302-7. [DOI] [PubMed] [Google Scholar]
- SELLINGER O. Z., BEAUFAY H., JACQUES P., DOYEN A., DE DUVE C. Tissue fractionation studies. 15. Intracellular distribution and properties of beta-N-acetylglucosaminidase and beta-galactosidase in rat liver. Biochem J. 1960 Mar;74:450–456. doi: 10.1042/bj0740450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaumburg H. H., Powers J. M., Raine C. S., Suzuki K., Richardson E. P., Jr Adrenoleukodystrophy. A clinical and pathological study of 17 cases. Arch Neurol. 1975 Sep;32(9):577–591. doi: 10.1001/archneur.1975.00490510033001. [DOI] [PubMed] [Google Scholar]
- Schutgens R. B., Romeyn G. J., Wanders R. J., van den Bosch H., Schrakamp G., Heymans H. S. Deficiency of acyl-CoA: dihydroxyacetone phosphate acyltransferase in patients with Zellweger (cerebro-hepato-renal) syndrome. Biochem Biophys Res Commun. 1984 Apr 16;120(1):179–184. doi: 10.1016/0006-291x(84)91430-x. [DOI] [PubMed] [Google Scholar]
- Singh I., Moser H. W., Moser A. B., Kishimoto Y. Adrenoleukodystrophy: impaired oxidation of long chain fatty acids in cultured skin fibroblasts an adrenal cortex. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1223–1229. doi: 10.1016/s0006-291x(81)80142-8. [DOI] [PubMed] [Google Scholar]
- Small G. M., Brolly D., Connock M. J. Palmityl-CoA oxidase: detection in several guinea pig tissues and peroxisomal localisation in mucosa, of small intestine. Life Sci. 1980 Nov 10;27(19):1743–1751. doi: 10.1016/0024-3205(80)90441-5. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E., Essner E. Microbodies: peroxisomes and glyoxysomes. J Cell Biol. 1981 Dec;91(3 Pt 2):271s–283s. doi: 10.1083/jcb.91.3.271s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E. Metabolic pathways in peroxisomes and glyoxysomes. Annu Rev Biochem. 1981;50:133–157. doi: 10.1146/annurev.bi.50.070181.001025. [DOI] [PubMed] [Google Scholar]
- Trijbels J. M., Berden J. A., Monnens L. A., Willems J. L., Janssen A. J., Schutgens R. B., van den Broek-Van Essen M. Biochemical studies in the liver and muscle of patients with Zellweger syndrome. Pediatr Res. 1983 Jun;17(6):514–517. doi: 10.1203/00006450-198306000-00018. [DOI] [PubMed] [Google Scholar]
- Tulkens P., Beaufay H., Trouet A. Analytical fractionation of homogenates from cultured rat embryo fibroblasts. J Cell Biol. 1974 Nov;63(2 Pt 1):383–401. doi: 10.1083/jcb.63.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vamecq J., Van Hoof F. Implication of a peroxisomal enzyme in the catabolism of glutaryl-CoA. Biochem J. 1984 Jul 1;221(1):203–211. doi: 10.1042/bj2210203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders R. J., Kos M., Roest B., Meijer A. J., Schrakamp G., Heymans H. S., Tegelaers W. H., van den Bosch H., Schutgens R. B., Tager J. M. Activity of peroxisomal enzymes and intracellular distribution of catalase in Zellweger syndrome. Biochem Biophys Res Commun. 1984 Sep 28;123(3):1054–1061. doi: 10.1016/s0006-291x(84)80240-5. [DOI] [PubMed] [Google Scholar]



