Abstract
African-Americans compared to non-Hispanic-White-Americans have higher systolic, diastolic blood pressure and rates of prehypertension/hypertension. Reasons for these adverse findings remain obscure. Analyses here focused on relations of foods/nutrients/urinary metabolites to higher African-American blood pressure for 369 African-Americans compared to 1,190 non-Hispanic-White-Americans ages 40-59 from 8 population samples. Standardized data were from four 24-hour dietary recalls/person, two 24-h urine collections, 8 blood pressure measurements; multiple linear regression quantitating role of foods, nutrients, metabolites in higher African-American blood pressure. Compared to non-Hispanic-White-Americans, African-Americans average systolic/diastolic pressure was higher by 4.7/3.4 mm Hg (men) and 9.0/4.8 mm Hg (women). Control for higher body mass index of African-American women reduced excess African-American systolic/diastolic pressure to 6.8/3.8 mm Hg. African American intake of multiple foods, nutrients related to blood pressure was less favorable - - less vegetables, fruits, grains, vegetable protein, glutamic acid, starch, fiber, minerals, potassium; more processed meats, pork, eggs, sugar-sweetened beverages, cholesterol, higher sodium to potassium ratio. Control for 11 nutrient and 10 non-nutrient correlates reduced higher African-American systolic/diastolic pressure to 2.3/2.3 mm Hg (52% and 33% reduction) (men) and to 5.3/2.8 mm Hg (21% and 27% reduction) (women). Control also for foods/urinary metabolites had little further influence on higher African-American blood pressure. Multiple nutrients with less favorable intakes by African-Americans than non-Hispanic-White-Americans account at least in part for higher African-American blood pressure. Improved dietary patterns can contribute to prevention/control of more adverse African-American blood pressure levels.
Keywords: African-American, blood pressure, nutrient, food intake, urinary metabolites
INTRODUCTION
Adverse blood pressure (BP) is an established major risk factor for cardiovascular diseases (CVD). Repeated U.S. population surveys, including the International Collaborative Study on Macro-/Micronutrients and Blood Pressure (INTERMAP), document that BP is higher in African-Americans (AA) than non-Hispanic-White- Americans (NHWA).1-3 Etiopathogenesis of this BP difference remains unexplained, i.e., it continues to be - - theoretically and practically - - a major unsolved challenge for CVD research. Here we hypothesize that multiple AA-NHWA differences in food/nutrient intake and urinary metabolites account for higher AA BP; we use INTERMAP data to test this hypothesis.3-6
METHODS
Population Samples, Field Methods (1996-1999)
Participants are 369 AA and 1,190 NHWA women and men ages 40-59 years recruited as eight stratified random U.S. population samples (Online Table S1). Participants attended four times - - two visits on consecutive days, two further visits on consecutive days.3 Demographic data were obtained by interviewer-administered questionnaire. Height and weight were measured at two visits. Each participant provided two timed 24-hour urine collections, aliquots were air-freighted frozen to the Central Laboratory (Leuven, Belgium) for urinary biochemistry, and to Imperial College London for proton nuclear magnetic resonance (1H NMR) spectroscopy.6 Dietary data were collected and computerized by a certified interviewer using the multi-pass 24-hour recall method.3,7 Institutional ethics committee approval was obtained for each site; all participants gave written informed consent.
Blood Pressure Measurements
Systolic and diastolic BP (first and fifth Korotkoff sounds) were measured twice/visit by a certified technician using a random-zero sphygmomanometer - -participant seated for at least five minutes in a quiet room, bladder empty, arm at heart level.3
Statistical Methods
Measurements/person were averaged for BP and nutrients across the four visits; for 24-hour urinary excretions, across the two collections.3,7 Inter-ethnic differences were assessed for statistical significance by Student's t-test or χ2 test. Approximate reliability - - observed univariate regression coefficient as percent of theoretical ‘true’ coefficient - - was estimated for relevant variables.8,9
Based on AA-NHWA differences in dietary and urinary variables, multiple linear regression was used to examine relations of these traits to inter-ethnic differences in BP.5,6,10 Model A included age, gender, sample, and an indicator for African-American; Model B added other nondietary factors; model C added body mass index (BMI). Then each dietary/urinary factor was added to model C separately; percentage reduction from the model C AA-NHWA BP difference was calculated to assess influence of the added variable on higher BP of AA. Finally, dietary/urinary variables were included in combinations to assess joint impact on higher AA BP.
RESULTS
Descriptive Statistics
African-Americans had higher average BP than NHWA and higher prevalence rates of hypertension (Online Table S2). AA women had higher average BMI and rates of overweight/obesity than NHWA women. AA and NHWA of both genders differed in intake of multiple foods/nutrients (Online Tables S2 and S3).
African-American intake lower, foods/nutrients with possible favorable relation to BP
African-Americans had lower intakes of total and raw vegetables, fresh fruits, pasta/rice, total grains; vegetable protein, glutamic acid, starch, fiber, calcium (Ca), magnesium (Mg), phosphorus (P), potassium (K), iron (Fe), non-heme Fe.
African-American intake higher, foods/nutrients with possible adverse relation to BP
African-Americans had higher intakes of processed meats, pork, eggs, sugar-sweetened beverages; dietary cholesterol, total sugars, fructose/glucose/sucrose, glycine, sodium (Na)/K ratio.
African-American intakes were relatively favorable for only a few foods/nutrients
African-American intake higher, foods/nutrients with possible favorable relation to BP
Fish/fish roe/shellfish, poultry; polyunsaturated fatty acids (PFA), PFA/saturated fatty acid ratio, oleic acid.
African-American intake lower, foods/nutrients with possible adverse relation to BP
Cream, cheese, dairy product recipes, alcoholic beverages; no nutrients.
Urinary metabolites
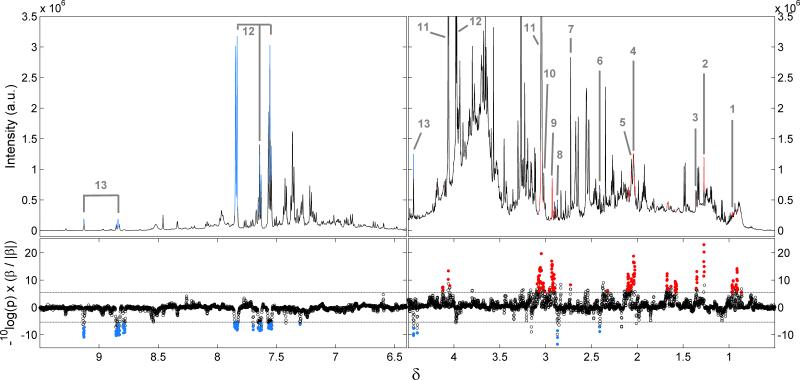
The median urinary 600 MHz 1H NMR spectra for AA and NHWA subsamples are shown in Figure 1. Urinary metabolites significantly12 higher in AA than NHWA included creatinine, 3-hydroxyisovalerate, N-acetyls of glycoprotein fragments, dimethylglycine, lysine, N-acetyl neuraminic acid, leucine, dimethylamine (DMA), taurine, and 2-hydroxyisobutyrate; metabolites significantly higher in NHWA, trimethylamine, N-methyl nicotinic acid (NMNA), hippurate, succinate (Table 1). Hippurate and NMNA quantified 24-h excretions are in Online Table S4.
Figure 1.
Top: median urinary 1H NMR spectrum of INTERMAP U.S. AA and NHWA participants, based on the first urine collection (N=1,455). Bottom: Manhattan plot indicating the significant spectral variables. Metabolites higher in AA individuals compared to NHWA are shown in red; in blue for metabolites higher in NHWA individuals compared to AA. Key: 1, Leucine; 2, 3-hydroxyisovalerate; 3, 2-hydroxyisobutyrate; 4, N-acetyls of glycoprotein fragments; 5, N-acetyl neuraminic acid; 6, Succinate; 7, Dimethylamine; 8, Trimethylamine; 9, Dimethylglycine; 10, Lysine; 11, Creatinine; 12, Hippurate; 13, N-methyl nicotinic acid.
Table 1.
1H NMR-derived urinary metabolites differing significantly* between African-Americans (AA) and non-Hispanic-White Americans (NHWA) participants, all and by gender
| Metabolites | Chemical Shifts, ppm (multiplicity) | Minimum P-value† |
|||||
|---|---|---|---|---|---|---|---|
| All | Men | Women | |||||
| 1st collection | 2nd collection | 1st collection | 2nd collection | 1st collection | 2nd collection | ||
| Higher in AA (154 men and 184 women) | |||||||
| Creatinine | 3.05 (s); 4.05 (s) | 3.8x10−25 | 1.8×10−20 | 4.4×10−13 | 1.1×10−11 | 5.8×10−14 | 9.3×10−10 |
| 3-hydroxyisovalerate | 1.27 (s); 2.37 (s) | 1.0×10−23 | 4.6×10−24 | 4.1×10−13 | 3.6×10−17 | 1.8×10−11 | 4.3×10−9 |
| N-acetyls of glycoprotein fragments | 2.02 -2.04 (s) | 1.6×10−22 | 1.5×10−19 | 3.9×10−12 | 1.5×10−9 | 1.4×10−12 | 3.1×10−12 |
| Dimethylglycine | 2.93 (s); 3.72 (s) | 5.2×10−18 | 9.7×10−18 | 4.1×10−15 | 2.2×10−11 | ||
| Lysine | 3.02 (t) | 1.3×10−9 | 4.2×10−15 | ||||
| N-acetyl neuraminic acid | 2.06 (s) | 2.9×10−14 | 3.0×10−11 | 4.3×10−10 | 2.7×10−9 | ||
| Leucine | 0.96 (d) | 1.7×10−10 | 3.9×10−11 | ||||
| Dimethylamine | 2.73 (s) | 3.3×10−10 | 4.7 ×10−9 | 4.1×10−10 | 1.7×10−11 | ||
| 2-hydroxyisobutyrate | 1.36 (s) | 5.3×10−10 | 8.6×10−8 | ||||
| Hiaher in NHWA (594 men and 523 women) | |||||||
| Trimethylamine | 2.87 (s) | 2.6×10−15 | 9.5×10-12 | 9.8×10−8 | 4.0×10−9 | ||
| A/-methyl nicotinic acid | 4.44 (s); 8.84 (t); | 3.1×10−13 | 1.2×10−10 | 4.1×10−8 | 2.2×10−7 | ||
| 9.13 (s) | |||||||
| Hippurate | 3.98 (d); 7.55 (t); | 5.9×10−10 | 3.1×10−9 | ||||
| 7.64 (t); 7.84 (d) | |||||||
| Succinate | 2.41 (s) | 1.5×10−9 | 4.0×10−10 | 3.9×10−9 | 3.4×10−8 | ||
Abbreviations: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet.
Mean population differences in peak intensity for spectral variables were assessed for statistical significance using Family Wise Error Rate <0.01 (P <4×10−6 for group mean population differences by Student's t) for the two urine collections considered separately.
Minimum P-values for mean population differences in peak intensity assigned to a particular metabolite, obtained separately for first and second urine collections, give a ranking of the discriminatory strength of the metabolites.
Partial correlations
Partial correlation coefficients >0.5 (adjusted for age, gender, sample) were recorded for many pairs of foods/nutrients (Online Table S5).
Reliability data
For foods, 29 of 68 reliability estimates were >50%, without apparent pattern across gender/ethnic strata (Online Table S6). Nutrient reliability estimates were generally higher than or foods; 75 of 108 were >50%, somewhat lower for AA than for NHWA. Reliability estimates were high for hippurate, NMNA, systolic BP (SBP), diastolic BP (DBP).
Relation of Foods, Nutrients, Urinary Metabolites to Higher AA BP
With control for possible non-dietary confounders, SBP/DBP was significantly higher for AA than NHWA by 4.7/3.4 mm Hg (men) and 9.0/4.8 mm Hg (women) (Online Table S6, row B). With BMI in the model (Online Table S7, row C), these differences remained about the same for men; for women, they were reduced to 6.8/3.8 mm Hg.
Foods considered singly
Of 17 foods with significantly different intakes by AA and NHWA, most had only a small influence on the higher BP of AA (Online Table S7).
Nutrients and urinary metabolites considered singly
Addition to the regression model of individual nutrients generally led to greater influences on SBP and DBP (Online Table S7) than addition of individual foods: 24-h urinary K excretion (inversely related to BP and lower in AA than NHWA) produced the largest effect in men, reduction of higher AA SBP by 1.2 mm Hg (26%), DBP by 0.8 mm Hg (24%), but without effect in women. Dietary glycine (% total protein) (directly related to BP and higher in AA) had a similar SBP effect in men and the largest effect in women. Inclusion of Ca, Mg, P singly (inversely related to BP and lower in AA than NHWA) produced 0.5 to 0.6 mm Hg (>10%) reductions in higher SBP of AA men, and qualitatively similar effects for AA women.
With inclusion of urinary hippurate, lower in AA men (Online Table S4) and inversely related to BP, higher SBP and DBP of AA men and women were reduced by only 0.1 mm Hg (Online Table S7). With addition to the model of NMNA, lower in AA than NHWA men and women (reportedly not related to BP), higher SBP and DBP of AA men and women were increased, not decreased.
Foods, nutrients, urinary metabolites in combination
With 10 foods considered together (all with significantly less favorable intakes for AA than NHWA), effects on higher AA SBP/DBP were modest (Table 2, row D). With 13 nutrients combined (most with significantly less favorable intakes for AA than NHWA), effects on higher AA BP were larger, particularly for men - - e.g., male SBP/DBP difference reduced to 2.3/2.3 mm Hg (Z-scores 1.72 and 2.41), a decrease of 2.2/1.1 mm Hg (−52% and −33%) (Table 2, row E1). Female AA SBP/DBP differences remained substantial, with nutrient related smaller effect of 1.5/1.0 mm Hg (−21% and −27%). No significant gender interaction was found (data not tabulated).
Table 2.
Relation of combinations of foods/nutrients/urinary metabolites, significantly different in AA and NHWA, to higher BP of AA than NHWA, by gender
| Model | Systolic BP, mm Hg | Diastolic BP, mm Hg | ||||
|---|---|---|---|---|---|---|
| BP difference | (Z-score) | % change from C* | BP difference | (Z-score) | % change from C* | |
| Men (N=165 AA, 620 NHWA) | ||||||
| A: age, sample | 5.41 | (4.40) | 3.46 | (3.94) | ||
| B: A+ medical history of CVD/diabetes, family history of hypertension, physical activity, special diet, supplement use | 4.68 | (3.80) | 3.38 | (3.84) | ||
| C: B+ BMI | 4.76 | (4.04) | 3.43 | (4.05) | ||
| Combination of foods | ||||||
| D: C+ raw vegetables, fresh fruit, pasta/rice, total grains, eggs, sugar-sweetened beverages, cream/cheese/ice cream/milk & cheese recipes, pork, processed meats, alcoholic beverages | 3.64 | (3.00) | −23.5 | 2.88 | (3.28) | −16.1 |
| Combinations of nutrients | ||||||
| E1: C+ vegetable protein, glutamic acid %kcal, starch, fiber, Ca, non-heme Fe, riboflavin, urinary K, Σ long chain PFA, dietary cholesterol, glycine %protein, 14-day alcohol, urinary Na | 2.28 | (1.72) | −52.1 | 2.31 | (2.41) | −32.7 |
| E2: as E1 except removing 14-day alcohol & urinary Na | 2.51 | (1.89) | −47.3 | 2.43 | (2.53) | −29.2 |
| E3: as E1 except glutamic acid as %protein (instead of %kcal) | 2.77 | (2.12) | −41.9 | 2.61 | (2.75) | −24.1 |
| E4: as E1 except Mg instead of Ca | 2.41 | (1.82) | −49.4 | 2.44 | (2.55) | −28.9 |
| E5: as E1 except P instead of Ca | 2.39 | (1.81) | −49.9 | 2.41 | (2.52) | −29.8 |
| E6: as E1 except urinary Na/K ratio instead of urinary K & Na | 2.47 | (1.88) | −48.1 | 2.51 | (2.63) | −27.0 |
| E7: E1+ Mg, P | 2.29 | (1.72) | −52.0 | 2.33 | (2.42) | −32.2 |
| Combinations of foods/nutrients | ||||||
| F1: including variables with largest SBP differences for men, D+E1 | 2.17 | (1.62) | −54.3 | 2.17 | (2.04) | −36.7 |
| F2: as F1, excluding variables correlated partial r >0.5† | 2.25 | (1.69) | −52.7 | 2.14 | (2.21) | −37.8 |
| Men with Quantitated Urinary Metabolites (N=146 AA, 578 NHWA) | ||||||
| Combinations of nutrients and urinary metabolite variables | ||||||
| G: as C except based on above N | 3.81 | (3.15) | 3.12 | (3.57) | ||
| H: G+ hippurate, A/-methylnicotinic acid | 4.10 | (3.29) | 7.6 | 3.09 | (3.44) | −0.8 |
| I: as E1 except based on above N | 2.05 | (1.51) | −46.2 | 2.46 | (2.50) | −21.1 |
| J: I+ hippurate, A/-methyl nicotinic acid | 2.44 | (1.78) | −36.0 | 2.55 | (2.56) | −18.2 |
| Women (N=204 AA, 570 NHWA) | ||||||
| A: age, sample | 9.66 | (8.02) | 5.03 | (6.52) | ||
| B: A+ medical history of CVD/diabetes, family history of hypertension, physical activity, special diet, supplement use | 9.00 | (7.42) | 4.83 | (6.20) | ||
| C: B+ BMI | 6.76 | (5.76) | 3.77 | (4.87) | ||
| Combination of foods | ||||||
| D: C+ raw vegetables, fresh fruit, pasta/rice, total grains, eggs, sugar-sweetened beverages, cream/cheese/ice cream/milk & cheese recipes, pork, processed meats, alcoholic beverages | 6.18 | (4.74) | −8.5 | 3.58 | (4.14) | −5.2 |
| Combinations of nutrients | ||||||
| E1: C+ vegetable protein, glutamic acid %kcal, starch, fiber, Ca, non-heme Fe, riboflavin, urinary K, I long chain PFA, dietary cholesterol, glycine %protein, 14-day alcohol, urinary Na | 5.31 | (3.82) | −21.4 | 2.76 | (3.01) | −26.8 |
| E2: as E1 except removing 14-day alcohol & urinary Na | 5.22 | (3.78) | −22.8 | 2.61 | (2.86) | −30.9 |
| E3: as E1 except glutamic acid as %protein (instead of %kcal) | 5.42 | (3.90) | −19.8 | 2.78 | (3.02) | −26.2 |
| E4: as E1 except Mg instead of Ca | 5.46 | (3.94) | −19.2 | 2.80 | (3.05) | −25.8 |
| E5: as E1 except P instead of Ca | 5.39 | (3.89) | −20.2 | 2.73 | (2.98) | −27.6 |
| E6: as E1 except urinary Na/K ratio instead of urinary K & Na | 5.13 | (3.69) | −24.1 | 2.65 | (2.88) | −29.7 |
| E7: E1+ Mg, P | 5.40 | (3.87) | −20.2 | 2.84 | (3.09) | −24.6 |
| Combinations of foods/nutrients | ||||||
| F1: including variables with largest SBP differences for women, D + E6 | 5.38 | (3.79) | −20.4 | 3.04 | (3.23) | −19.4 |
| F2: As F1, excluding variables correlated partial r >0.5‡ | 5.14 | (3.68) | −24.0 | 2.83 | (3.06) | −25.0 |
| Women with Quantitated Urinary Metabolites (N=188 AA, 514 NHWA) | ||||||
| Combinations of nutrients and urinary metabolite variables | ||||||
| G: as C except based on above N | 6.80 | (5.48) | 3.78 | (4.60) | ||
| H: G+ hippurate, A/-methyl nicotinic acid | 6.92 | (5.52) | 1.6 | 4.01 | (4.83) | 6.0 |
| I: as E1 except based on above N | 5.37 | (3.63) | −21.1 | 2.77 | (2.82) | −26.8 |
| J: I+ hippurate, A/-methyl nicotinic acid | 5.33 | (3.60) | −21.7 | 2.80 | (2.86) | −26.0 |
Abbreviations: Σ, sum of; Ca, calcium; Fe, iron; K, potassium; Mg, magnesium; Na, sodium; SFA, saturated fatty acids; P, phosphorus; PFA, polyunsaturated fatty acids.
Units for nutrients are %kcal, g/1,000kcal, or mg/1,000kcal; for glutamic acid and glycine, also % total protein; for urinary hippurate and N-methyl nicotinic acid, mmol/24-hours.
Z-score = regression coefficient/standard error. |Z| ≥ 1.96, uncorrected P ≤ 0.05; |Z| ≥ 2.58, uncorrected P≤0.01; |Z| ≥ 3.29, uncorrected P≤0.001.
For rows H, I and J, % change from row G.
Model F2 (men) includes: raw vegetables, fresh fruit, pasta/rice, eggs, sugar-sweetened beverages, cream/cheese/ice cream/milk & cheese recipes, pork, processed meats, vegetable protein, glutamic acid %kcal, riboflavin, urinary K, Σ long chain PFA, glycine %protein, 14-day alcohol, urinary Na.
Model F2 (women) includes: raw vegetables, fresh fruit, pasta/rice, total grains, sugar- sweetened beverages, cream/cheese/ice cream/milk & cheese recipes, pork, processed meats, alcoholic beverages, glutamic acid %kcal, cholesterol, riboflavin, glycine %protein, urinary Na/K ratio.
Combinations of nutrients and foods yielded little or no additional reduction in higher AA BP (Table 2, row F2). With the two quantified urinary metabolites in the model together (without and with nutrients), higher AA SBP/DBP was not reduced (Table 2, rows H, J).
DISCUSSION
Main findings here on higher SBP/DBP of AA than NHWA are: multiple nutrients, possibly related to BP, with less favorable intakes by AA than NHWA, account in part for higher AA SBP/DBP. These include vegetable protein, its main amino acid glutamic acid, starch, fiber, K, Ca and/or Mg and/or P, non-heme Fe; also, dietary cholesterol and glycine.11-25
To the best of our knowledge, these INTERMAP findings are unique. The ARIC population study, involving over 8,000 nonhypertensive women and men ages 45-64, reported that Whites consuming three or more daily servings of low-fat milk, compared to those consuming less than one, had a 2.7 mm Hg smaller SBP increase with 9 year follow-up; an association not prevailing for AA.26 No data were given on relations of dietary variables to BP differences between AAs and Whites. The CARDIA young adult population study reported that with 10 year follow-up, intake of low-fat dairy products was associated with lower incidence of high BP in AA and Whites.27 The study did not assess whether these or other foods/nutrients related to higher AA BP. The Third U.S. National Health and Nutrition Survey (NHANES III) (1988-1994) reported lower serum 25-hydroxyvitamin D (25(OH)D) of AA than NHWA, and an inverse relation of 25(OH)D to BP.28 These differences were estimated to “explain” about half the greater AA high BP prevalence. No dietary data were given.
The INTERMAP findings here are reproducible, supporting the inference that they are etiologically significant, they have important implications, especially need for greater efforts to improve AA nutrition. Adoption of dietary recommendations for prevention/control of adverse BP, e.g., per Dietary Approaches to Stop Hypertension (DASH)-low Na or Optimal Macronutrient Intake Trial for Heart Health (OMNIHEART) plus low Na,15,16 results in such improved nutrition. To extend their use among AA, specific factors influencing AA diet need to be considered, e.g., ethnic traditions; lower average income; reduced accessibility to modern supermarkets.29 For AA women there is a particular need to reduce their higher BMI, known to relate importantly to higher BP.
Metabolome-wide association analysis revealed 12 urinary metabolites that differed significantly between AA and NHWA. Hippurate (higher in NHWA) is a gut-microbial co-metabolite produced by bacterial metabolism of plant phenols;30,31 hippurate related inversely to BP of INTERMAP participants.6 Observed differences in DMA and dimethylglycine (both higher in AA) also likely relate to inter-ethnic microbiotal differences.33,33 Creatinine and guanidinoacetate (involved in creatinine metabolism) were higher in AA than NHWA. Creatinine excretion is related to muscle turnover;34 these metabolite differences could reflect greater AA physical activity and muscle mass. Lower AA excretion of N-methyl nicotinic acid, a product of nicotinic acid/nicotinamide metabolism, could reflect lower AA dietary intakes of niacin and tryptophan (observed; data not tabulated). Trimethylamine (lower in AA) is linked to dietary choline-induced atherosclerosis;35 this difference could reflect lower AA dietary intakes of the B-complex vitamins.
Multiple nutrients accounted only in part for higher AA BP. This may reflect regression dilution bias and other limitations in the nutrient data, despite their quality.5,9 In this regard, two prior INTERMAP investigations - - on higher BP of less educated Americans, and higher BP of northern than southern Chinese - - showed that multiple nutrients accounted completely for the higher BP.13,25 Thus, the fact that here multiple foods/nutrients/metabolites apparently account only in part for higher AA BP suggests that other traits may operate, e.g., in utero influences, early life dietary patterns, psychosocial factors, genetic factors.36-40
Study strengths
Findings here are solidly based, with participants from 8 diverse U.S. random samples, and dietary/metabolite/BP data collected by standardized, quality-controlled repetitive methods.
Study limitations
Data are cross-sectional, subject to random regression dilution bias despite multiple measurements, and - - in regard to the dietary data - - non-random biases inherent in the methodology.9
PERSPECTIVES
Delineation of factors responsible for the more adverse BP patterns of AA compared to other Americans is a long-term major unresolved research challenge. Its importance relates not only to the need to overcome this inequality in health of African-Americans. It also stems from the likelihood that resolving this problem will clarify understanding on the etiopathogenesis of epidemic prehypertension/hypertension in all strata of the population. The INTERMAP data reported here show that about a quarter (SBP, women) to a half (SBP, men) of the higher BP of AA is attributable to less favorable AA intake of multiple nutrients, and that greater obesity among AA women also is a significant factor. Improved AA eating patterns can help prevent/control adverse AA BP patterns.
NOVELTY AND SIGNIFICANCE.
Novelty: To the best of our knowledge, the data here are the first reported on multiple food/nutrient intakes/urinary metabolites that apparently account - - at least in part - - for the more adverse BP patterns of AA compared to NHWA.
Relevance: These data point the way to specific dietary enhancements for the prevention/control of adverse BP patterns in AA.
Summary: Data here from the population-based US samples of the INTERMAP Study support the conclusion that the inordinately high rates of prehypertension/hypertension -- common among AA - - can be ameliorated by improved nutrition.
ACKNOWLEDGMENTS
It is our privilege to dedicate this paper to our colleague of many years duration, Professor Hugo Kesteloot, who died 5 October 2010. NMR signal processing and in-house software were developed by Drs. Cloarec, Ebbels, Veselkov, Keun, and Rantalainen (Department of Surgery and Cancer, Imperial College London). This observational study is registered at www.clinicaltrials.gov as NCT00005271.
FUNDING SOURCES
Supported by grants (R01-HL50490, and R01-HL84228) from the National Heart, Lung, and Blood Institute, National Institutes of Health, and by the National Institutes of Health Office on Dietary Supplements (Bethesda, Maryland, USA); also by national agencies in Japan, PRC, and the UK.
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks: US population data. Arch Intern Med. 1993;153:598–615. doi: 10.1001/archinte.153.5.598. [DOI] [PubMed] [Google Scholar]
- 2.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population: results from the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 3.Stamler J, editor. Special Issue—INTERMAP: International study of macro- and micro-nutrients and blood pressure. J Hum Hypertens. 2003;17:589–775. doi: 10.1038/sj.jhh.1001609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elliott P, Stamler J. Primary prevention of high blood pressure. In: Marmot M, Elliot P, editors. Coronary Heart Disease Epidemiology: From Aetiology to Public Health. Second Edition. Oxford University Press; London: 2005. pp. 751–768. [Google Scholar]
- 5.Stamler J, Brown IJ, Elliott P, Daviglus ML, Dyer AR, Garside D, Van Horn L, Appel LJ, Chan Q, Tzoulaki I, Kesteloot H, Miura K, Okuda N, Ueshima H, Zhao L. Improved nutrition: key to solving the populationwide blood pressure problem. In: Mancini M, Ordovas J, Riccardi G, Rubba P, Strazzullo P, editors. Nutritional and Metabolic Bases of Cardiovascular Disease. Blackwell Publishing; London: pp. 303–324. [Google Scholar]
- 6.Holmes E, Loo RL, Stamler J, Bictash M, Yap IK, Chan Q, Ebbels T, De Iorio M, Brown IJ, Veselkov K, Daviglus ML, Kesteloot H, Ueshima H, Zhao L, Nicholson JK, Elliott P. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature. 2008;453:396–400. doi: 10.1038/nature06882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schakel SF, Dennis BH, Wold AC, Conway R, Zhao L, Okuda N, Okayama A, Moag-Stahlberg A, Robertson C, Van Heel N, Buzzard IM, Stamler J. Enhancing data on nutrient composition of foods eaten by participants in the INTERMAP Study in China, Japan, the United Kingdom and the United States. J Food Comp Anal. 2003;16:395–408. doi: 10.1016/S0889-1575(03)00043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grandits GA, Bartsch GE, Stamler J. Methods issues in dietary data analyses in the Multiple Risk Factor Intervention Trial. Am J Clin Nutr. 1997;65(1 Suppl):211S–227S. doi: 10.1093/ajcn/65.1.211S. Chapter 4. [DOI] [PubMed] [Google Scholar]
- 9.Dyer AR, Liu K, Sempos CT. Nutrient data analysis techniques and strategy. In: Berdanier CD, Dwyer J, Feldman EB, editors. Handbook of Nutrition and Food. Second Edition CRC Press; New York: 2008. pp. 93–103. [Google Scholar]
- 10.Chadeau-Hyam M, Ebbels TMD, Brown IJ, Chan Q, Stamler J, Huang CC, Daviglus ML, Ueshima H, Zhao L, Holmes E, Nicholson JK, Elliott P, De Iorio M. Metabolic profiling and the metabolome-wide association study: significance level for biomarker identification. J Proteome Res. 2010;9:4620–4627. doi: 10.1021/pr1003449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou BF, Stamler J, Dennis B, Moag-Stahlberg A, Okuda N, Robertson C, Zhao L, Chan Q, Elliott P. Nutrient intakes of middle-aged men and women in China, Japan, United Kingdom, and United States in the late 1990s: the INTERMAP Study. J Hum Hypertens. 2003;17:623–630. doi: 10.1038/sj.jhh.1001605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueshima H, Okayama A, Saitoh S, Nakagawa H, Rodriguez B, Sakata K, Okuda N, Choudhury S, Curb JD. Differences in cardiovascular disease risk factors between Japanese in Japan and Japanese-Americans in Hawaii: the INTERLIPID Study. J Hum Hypertens. 2003;17:631–639. doi: 10.1038/sj.jhh.1001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stamler J, Elliott P, Appel L, Chan Q, Buzzard M, Dennis B, Dyer AR, Elmer P, Greenland P, Jones D, Kesteloot H, Kuller L, Labarthe D, Liu K, Moag-Stahlberg A, Nichaman M, Okayama A, Okuda N, Robertson C, Rodriguez B, Stevens M, Ueshima H, Horn LV, Zhou B. Higher blood pressure in middle-aged American adults with less education—role of multiple dietary factors: the INTERMAP Study. J Hum Hypertens. 2003;17:655–664. doi: 10.1038/sj.jhh.1001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miura K, Greenland P, Stamler J, Liu K, Daviglus ML, Nakagawa H. Relation of vegetable, fruit, and meat intake to 7-year blood pressure change in middle-aged men. The Chicago Western Electric Study. Am J Epidemiol. 2004;159:572–580. doi: 10.1093/aje/kwh085. [DOI] [PubMed] [Google Scholar]
- 15.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, 3rd, Simons-Morton DG, Karanja N, Lin PH. Effects on blood pressure of reduced sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. New Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 16.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER, 3rd, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM, Charleston J, McCarron P, Bishop LM. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. J Am Med Assoc. 2005;294:2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 17.Elliott P, Stamler J, Dyer AR, Appel L, Dennis B, Kesteloot H, Ueshima H, Okayama A, Chan Q, Garside DB, Zhou B. Association between protein intake and blood pressure. Arch Intern Med. 2006;166:79–87. doi: 10.1001/archinte.166.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ueshima H, Stamler J, Elliott P, Chan Q, Brown IJ, Carnethon MR, Daviglus ML, He K, Moag-Stahlberg A, Rodriguez BL, Steffen LM, Van Horn L, Yarnell J, Zhou B. Food omega-3 fatty acid intake of individuals (total, linolenic acid, long-chain) and their blood pressure: INTERMAP Study. Hypertension. 2007;50:313–319. doi: 10.1161/HYPERTENSIONAHA.107.090720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elliott P, Kesteloot H, Appel LJ, Dyer AR, Ueshima H, Chan Q, Brown IJ, Zhao L, Stamler J. Dietary phosphorus and blood pressure. International Population Study on Macronutrients and Blood Pressure. Hypertension. 2008;51:669–675. doi: 10.1161/HYPERTENSIONAHA.107.103747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miura K, Stamler J, Nakagawa H, Elliott P, Ueshima H, Chan Q, Brown IJ, Tzoulaki I, Saitoh S, Dyer AR, Daviglus ML, Kesteloot H, Okayama A, Curb JD, Rodriguez BL, Elmer PJ, Steffen LM, Robertson C, Zhao L. Relationship of dietary linoleic acid to blood pressure. The International Study of Macro-Micronutrients and Blood Pressure. Hypertension. 2008;52:408–414. doi: 10.1161/HYPERTENSIONAHA.108.112383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tzoulaki I, Brown IJ, Chan Q, Van Horn L, Ueshima H, Zhao L, Stamler J, Elliott P. Relation of iron and red meat intake to blood pressure: cross sectional epidemiological study. Brit Med J. 2008;337:a258. doi: 10.1136/bmj.a258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown IJ, Elliott P, Robertson CE, Chan Q, Daviglus ML, Dyer AR, Huang CC, Rodriguez BL, Sakata K, Ueshima H, Van Horn L, Zhao L, Stamler J. Dietary starch intake of individuals and their blood pressure: the International Study of Macronutrients and Micronutrients and Blood Pressure. J Hypertens. 2009;27:231–236. doi: 10.1097/HJH.0b013e32831a7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stamler J, Brown IJ, Daviglus ML, Chan Q, Kesteloot H, Ueshima H, Zhao L, Elliott P. Glutamic acid, the main dietary amino acid, and blood pressure. The INTERMAP Study (International Collaborative Study of Macronutrients, Micronutrients and Blood Pressure). Circulation. 2009;120:221–228. doi: 10.1161/CIRCULATIONAHA.108.839241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yap IKS, Brown IJ, Chan Q, Wijeyesekera A, Garcia-Perez I, Bictash M, Loo RL, Chadeau-Hyam M, Ebbels T, De Iorio M, Maibaum E, Zhao L, Kesteloot H, Daviglus ML, Stamler J, Nicholson JK, Elliott P, Holmes E. Metabolome-wide association study identifies multiple biomarkers that discriminate north and south Chinese populations at differing risks of cardiovascular disease: INTERMAP Study. J Proteome Res. 2010;9:6647–6654. doi: 10.1021/pr100798r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao L, Stamler J, Yan LL, Zhou B, Wu Y, Liu K, Daviglus ML, Dennis BH, Elliott P, Ueshima H, Yang J, Zhu L, Guo D. Blood pressure differences between northern and southern Chinese: role of dietary factors—the INTERMAP Study. Hypertension. 2004;43:1–6. doi: 10.1161/01.HYP.0000128243.06502.bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alonso A, Steffen LM, Folsom AR. Dairy intake and changes in blood pressure over 9 years: the ARIC study. Eur J Clin Nutr. 2009;63:1272–1275. doi: 10.1038/ejcn.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pereira MA, Jacobs DR, Jr, Van Horn L, Slattery ML, Kartashov AI, Ludwig DS. Dairy consumption, obesity, and the insulin resistance syndrome in young adults: the CARDIA Study. J Am Med Assoc. 2002;287:2081–2089. doi: 10.1001/jama.287.16.2081. [DOI] [PubMed] [Google Scholar]
- 28.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens. 2007;20:713–719. doi: 10.1016/j.amjhyper.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 29.Zenk SN, Schulz AJ, Israel BA, James SA, Bao S, Wilson ML. Neighborhood racial composition, neighborhood poverty, and the spatial accessibility of supermarkets in metropolitan Detroit. Am J Public Health. 2005;95:660–667. doi: 10.2105/AJPH.2004.042150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwab AJ, Tao L, Yoshimura T, Simard A, Barker F, Pang KS. Hepatic uptake and metabolism of benzoate: a multiple indicator dilution, perfused rat liver study. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1124–G1136. doi: 10.1152/ajpgi.2001.280.6.G1124. [DOI] [PubMed] [Google Scholar]
- 31.Asatoor AM. Aromatisation of quinic acid and shikimic acid by bacteria and the production of urinary hippurate. Biochim Biophys Acta. 1965;100:290–292. doi: 10.1016/0304-4165(65)90455-1. [DOI] [PubMed] [Google Scholar]
- 32.Zeisel SH, DaCosta KA, Fox JG. Endogenous formation of dimethylamine. Biochem J. 1985;232:403–408. doi: 10.1042/bj2320403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith JL, Wishnok JS, Deen WM. Metabolism and excretion of methylamines in rats. Toxicol Appl Pharmacol. 1994;125:296–308. doi: 10.1006/taap.1994.1076. [DOI] [PubMed] [Google Scholar]
- 34.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, DuGar B, Feldstein AE, Britt EB, Fu X, Chung Y, Wu Y, Schauer P, Smtih JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gleiberman L. Sodium, blood pressure, and ethnicity: what have we learned? Am J Hum Biol. 2009;21:679–686. doi: 10.1002/ajhb.20921. [DOI] [PubMed] [Google Scholar]
- 37.Turban S, Miller ER, 3rd, Ange B, Appel LJ. Racial differences in urinary potassium excretion. J Am Soc Nephrol. 2008;19:1396–1402. doi: 10.1681/ASN.2007101142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Järvelin M-R, Sovio U, King V, Lauren L, Xu B, McCarthy MI, Hartikainen A-L, Laitinen J, Zitting P, Rantakallio P, Elliott P. Early life factors and blood pressure at age 31 years in the 1966 Northern Finland Birth Cohort. Hypertension. 2004;44:838–846. doi: 10.1161/01.HYP.0000148304.33869.ee. [DOI] [PubMed] [Google Scholar]
- 39.Thorpe RJ, Jr, Brandon DT, LaVeist LA. Social context as an explanation for race disparities in hypertension: findings from the Exploring Health Disparities in Integrated Communities (EHDIC) Study. Soc Sci Med. 2008;67:1604–1611. doi: 10.1016/j.socscimed.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adeyemo A, Gerry N, Chen G, Herbert A, Doumatey A, Huang H, Zhou J, Lashley K, Chen Y, Christman M, Rotimi C. A genome-wide association study of hypertension and blood pressure in African Americans. PLoS Genet. 2009;5:e1000564. doi: 10.1371/journal.pgen.1000564. [DOI] [PMC free article] [PubMed] [Google Scholar]