Abstract
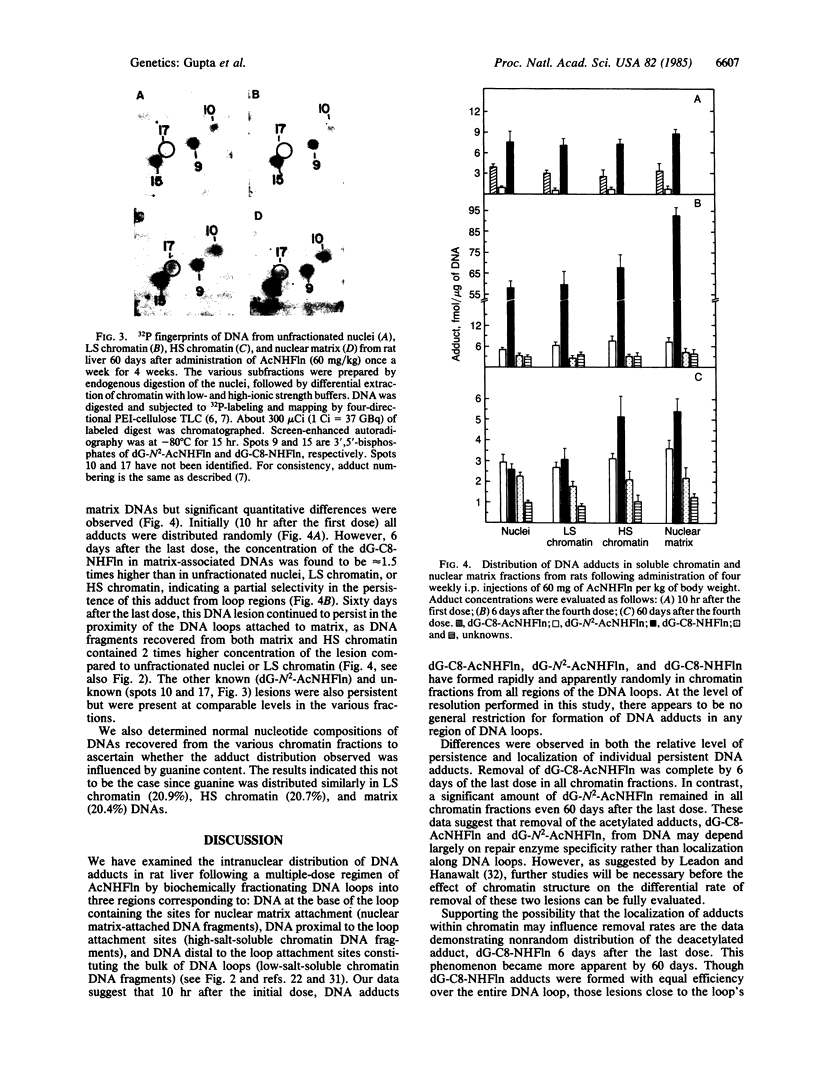
The intranuclear distribution of initial and persistent DNA adducts induced in vivo after four weekly injections of the hepatocarcinogen 2-acetylaminofluorene was examined in rat liver by using a protocol that fractionates chromatin from various regions of each of the multiple nuclear DNA loops. Ten hours after the initial dose, two acetylated [(N-acetyl-N-(deoxyguanosin-8-yl)-2-aminofluorene and 3-(deoxyguanosin-N2-yl)-2-acetylaminofluorene] and one deacetylated [N-(deoxyguanosin-8-yl)-2-aminofluorene] adduct were detected by a 32P-labeling assay and were found to have a random genomic distribution, as evident by their relative concentrations in various chromatin fractions. These data suggest that all regions of the DNA loops are equally susceptible to adduct formation. A nonrandom persistence of the deacetylated adduct in the regions where the DNA loops are constrained by the nuclear matrix was evident by 6 days after the last dose and was markedly apparent by 60 days. In contrast, all chromatin fractions had equally inefficient removal of the N2-acetylated adduct by 6 days as well as 60 days but had complete removal of the C8-acetylated adduct. These findings suggest that pronounced regional differences in adduct repair along the DNA loops may play a role in chemically induced hepatocarcinogenesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basler J., Hastie N. D., Pietras D., Matsui S. I., Sandberg A. A., Berezney R. Hybridization of nuclear matrix attached deoxyribonucleic acid fragments. Biochemistry. 1981 Nov 24;20(24):6921–6929. doi: 10.1021/bi00527a027. [DOI] [PubMed] [Google Scholar]
- Beland F. A., Dooley K. L., Jackson C. D. Persistence of DNA adducts in rat liver and kidney after multiple doses of the carcinogen N-hydroxy-2-acetylaminofluorene. Cancer Res. 1982 Apr;42(4):1348–1354. [PubMed] [Google Scholar]
- Beranek D. T., White G. L., Heflich R. H., Beland F. A. Aminofluorene-DNA adduct formation in Salmonella typhimurium exposed to the carcinogen N-hydroxy-2-acetylaminofluorene. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5175–5178. doi: 10.1073/pnas.79.17.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezney R., Buchholtz L. A. Dynamic association of replicating DNA fragments with the nuclear matrix of regenerating liver. Exp Cell Res. 1981 Mar;132(1):1–13. doi: 10.1016/0014-4827(81)90076-8. [DOI] [PubMed] [Google Scholar]
- Bodnar J. W., Jones C. J., Coombs D. H., Pearson G. D., Ward D. C. Proteins tightly bound to HeLa cell DNA at nuclear matrix attachment sites. Mol Cell Biol. 1983 Sep;3(9):1567–1579. doi: 10.1128/mcb.3.9.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr V. A., Smith C. A., Okumoto D. S., Hanawalt P. C. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985 Feb;40(2):359–369. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- Capon D. J., Seeburg P. H., McGrath J. P., Hayflick J. S., Edman U., Levinson A. D., Goeddel D. V. Activation of Ki-ras2 gene in human colon and lung carcinomas by two different point mutations. Nature. 1983 Aug 11;304(5926):507–513. doi: 10.1038/304507a0. [DOI] [PubMed] [Google Scholar]
- Ciejek E. M., Tsai M. J., O'Malley B. W. Actively transcribed genes are associated with the nuclear matrix. Nature. 1983 Dec 8;306(5943):607–609. doi: 10.1038/306607a0. [DOI] [PubMed] [Google Scholar]
- Cook P. R., Lang J., Hayday A., Lania L., Fried M., Chiswell D. J., Wyke J. A. Active viral genes in transformed cells lie close to the nuclear cage. EMBO J. 1982;1(4):447–452. doi: 10.1002/j.1460-2075.1982.tb01189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch J. T., Klug A. Solenoidal model for superstructure in chromatin. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1897–1901. doi: 10.1073/pnas.73.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. C., Dighe N. R. Formation and removal of DNA adducts in rat liver treated with N-hydroxy derivatives of 2-acetylaminofluorene, 4-acetylaminobiphenyl, and 2-acetylaminophenanthrene. Carcinogenesis. 1984 Mar;5(3):343–349. doi: 10.1093/carcin/5.3.343. [DOI] [PubMed] [Google Scholar]
- Gupta R. C. Nonrandom binding of the carcinogen N-hydroxy-2-acetylaminofluorene to repetitive sequences of rat liver DNA in vivo. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6943–6947. doi: 10.1073/pnas.81.22.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. C., Reddy M. V., Randerath K. 32P-postlabeling analysis of non-radioactive aromatic carcinogen--DNA adducts. Carcinogenesis. 1982;3(9):1081–1092. doi: 10.1093/carcin/3.9.1081. [DOI] [PubMed] [Google Scholar]
- Hemminki K., Vainio H. Preferential binding of benzo[a]pyrene into nuclear matrix fraction. Cancer Lett. 1979 Mar;6(3):167–173. doi: 10.1016/s0304-3835(79)80028-2. [DOI] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Leadon S. A., Hanawalt P. C. Ultraviolet irradiation of monkey cells enhances the repair of DNA adducts in alpha DNA. Carcinogenesis. 1984 Nov;5(11):1505–1510. doi: 10.1093/carcin/5.11.1505. [DOI] [PubMed] [Google Scholar]
- Metzger G., Wilhelm F. X., Wilhelm M. L. Non-random binding of a chemical carcinogen to the DNA in chromatin. Biochem Biophys Res Commun. 1977 Apr 11;75(3):703–710. doi: 10.1016/0006-291x(77)91529-7. [DOI] [PubMed] [Google Scholar]
- Miller E. C. Some current perspectives on chemical carcinogenesis in humans and experimental animals: Presidential Address. Cancer Res. 1978 Jun;38(6):1479–1496. [PubMed] [Google Scholar]
- Miller J. A. Carcinogenesis by chemicals: an overview--G. H. A. Clowes memorial lecture. Cancer Res. 1970 Mar;30(3):559–576. [PubMed] [Google Scholar]
- Mirkovitch J., Mirault M. E., Laemmli U. K. Organization of the higher-order chromatin loop: specific DNA attachment sites on nuclear scaffold. Cell. 1984 Nov;39(1):223–232. doi: 10.1016/0092-8674(84)90208-3. [DOI] [PubMed] [Google Scholar]
- Mironov N. M., Grover P. L., Sims P. Preferential binding of polycyclic hydrocarbons to matrix-bound DNA in rat-liver nuclei. Carcinogenesis. 1983;4(2):189–193. doi: 10.1093/carcin/4.2.189. [DOI] [PubMed] [Google Scholar]
- Moyer G. H., Gumbiner B., Austin G. E. Binding of N-hydroxy acetylaminofluorene to eu- and heterochromatin fractions of rat liver in vivo. Cancer Lett. 1977 Mar;2(4-5):259–265. doi: 10.1016/s0304-3835(77)80030-x. [DOI] [PubMed] [Google Scholar]
- Poirier M. C., True B., Laishes B. A. Formation and removal of (guan-8-yl)-DNA-2-acetylaminofluorene adducts in liver and kidney of male rats given dietary 2-acetylaminofluorene. Cancer Res. 1982 Apr;42(4):1317–1321. [PubMed] [Google Scholar]
- Rabkin S. D., Strauss B. S. A role for DNA polymerase in the specificity of nucleotide incorporation opposite N-acetyl-2-aminofluorene adducts. J Mol Biol. 1984 Sep 25;178(3):569–594. doi: 10.1016/0022-2836(84)90239-0. [DOI] [PubMed] [Google Scholar]
- Ramanathan R., Rajalakshmi S., Sarma D. S., Farber E. Nonrandom nature of in vivo methylation of dimethylnitrosamine and the subsequent removal of methylated products from rat liver chromatin DNA. Cancer Res. 1976 Jun;36(6):2073–2079. [PubMed] [Google Scholar]
- Reddy E. P., Reynolds R. K., Santos E., Barbacid M. A point mutation is responsible for the acquisition of transforming properties by the T24 human bladder carcinoma oncogene. Nature. 1982 Nov 11;300(5888):149–152. doi: 10.1038/300149a0. [DOI] [PubMed] [Google Scholar]
- Robinson S. I., Nelkin B. D., Vogelstein B. The ovalbumin gene is associated with the nuclear matrix of chicken oviduct cells. Cell. 1982 Jan;28(1):99–106. doi: 10.1016/0092-8674(82)90379-8. [DOI] [PubMed] [Google Scholar]
- Schwartz E. L., Braselton W. E., Jr, Goodman J. I. Factors influencing the binding of N-2-fluorenylacetamide to specific regions of the hepatic genome in vivo in rats. J Natl Cancer Inst. 1981 Apr;66(4):667–672. [PubMed] [Google Scholar]
- Smith H. C., Berezney R. DNA polymerase alpha is tightly bound to the nuclear matrix of actively replicating liver. Biochem Biophys Res Commun. 1980 Dec 31;97(4):1541–1547. doi: 10.1016/s0006-291x(80)80041-6. [DOI] [PubMed] [Google Scholar]
- Smith H. C., Berezney R. Nuclear matrix-bound deoxyribonucleic acid synthesis: an in vitro system. Biochemistry. 1982 Dec 21;21(26):6751–6761. doi: 10.1021/bi00269a021. [DOI] [PubMed] [Google Scholar]
- Tabin C. J., Bradley S. M., Bargmann C. I., Weinberg R. A., Papageorge A. G., Scolnick E. M., Dhar R., Lowy D. R., Chang E. H. Mechanism of activation of a human oncogene. Nature. 1982 Nov 11;300(5888):143–149. doi: 10.1038/300143a0. [DOI] [PubMed] [Google Scholar]
- Ueyama H., Matsuura T., Nomi S., Nakayasu H., Ueda K. Binding of benzo(a)pyrene to rat liver nuclear matrix. Life Sci. 1981 Aug 17;29(7):655–661. doi: 10.1016/0024-3205(81)90017-5. [DOI] [PubMed] [Google Scholar]
- Walker M. S., Becker F. F., Rodriguez L. V. In vivo binding of N-2-acetylaminofluorene and its N-hydroxy derivative to the DNA of fractionated rat liver chromatin. Chem Biol Interact. 1979 Oct;27(2-3):177–190. doi: 10.1016/0009-2797(79)90124-8. [DOI] [PubMed] [Google Scholar]
- Werner D., Chanpu S., Müller M., Spiess E., Plagens U. Antibodies to the most tightly bound proteins in eukaryotic DNA. Formation of immuno-complexes with 'nuclear matrix' components. Exp Cell Res. 1984 Apr;151(2):384–395. doi: 10.1016/0014-4827(84)90389-6. [DOI] [PubMed] [Google Scholar]