Abstract
With the high prevalence of gastrointestinal disorders, there is great interest in establishing in vitro models of human intestinal disease and in developing drug-screening platforms that more accurately represent the complex physiology of the intestine. We will review how recent advances in developmental and stem cell biology have made it possible to generate complex, three-dimensional, human intestinal tissues in vitro through directed differentiation of human pluripotent stem cells. These are currently being used to study human development, genetic forms of disease, intestinal pathogens, metabolic disease and cancer.
Keywords: Directed differentiation, Embryonic stem cells, Gastrointestinal disease, Gut tube, Intestinal morphogenesis, Pluripotent stem cells
Introduction
The intestinal tract is one of the most architecturally and functionally complex organs of the body. The human intestine is 8 m in length (Hounnou et al., 2002) and is subdivided into five functional domains along the proximal-to-distal axis: the duodenum, jejunum and ileum are segments of the small intestine; and the cecum and colon make up the large intestine. The intestine comprises specialized cell and tissue types from all three germ layers. Intestinal tissues include endoderm-derived epithelium, which houses specialized intestinal stem cells (ISCs) (Sato and Clevers, 2013), mesoderm-derived smooth muscle, vasculature, lymphatics and immune cells, and the ectoderm-derived enteric nervous system (ENS). The contributions from all of the germ layers are required to help coordinate a myriad of complex intestinal functions.
The intestine is best known for its digestive function to orchestrate the breakdown of macronutrients, regulate absorption and secrete waste. Less appreciated is the central role of the intestinal endocrine system in coordinating systemic nutrient levels and feeding behavior with other organ systems via endocrine hormones. In addition, the epithelium of the intestine acts as a selective barrier by restricting microbes to the gut lumen (Turner, 2009). These epithelial functions are largely carried out by four cell types: absorptive enterocytes and the three secretory lineages. Secretory cells involved in barrier function include goblet and Paneth cells, which secrete mucin and antimicrobial factors. The enteroendocrine cells are a rare but diverse population of cell types that secrete hormones that regulate satiety, motility, absorption, β-cell proliferation, secretion of other hormones and digestive enzymes, among other things (Engelstoft et al., 2013). The epithelium of the intestine turns over every 5 days in mice, and this regenerative capacity is driven by a resident population of ISCs (Creamer et al., 1961; Sato et al., 2009). The mechanical functions of the intestine are controlled by complex interactions between the epithelium, smooth muscle and ENS, which regulate peristalsis to ensure unidirectional movement of luminal contents.
Given the cellular and functional complexity of the intestine, it is no wonder that there are a staggering number of people impacted by intestinal dysfunction. Common intestinal disorders include infections, irritable bowel syndrome (IBS), malabsorption and fecal incontinence. Other debilitating diseases include inflammatory bowel disease (IBD), colon cancer and diseases that, in some cases, have a genetic basis, such as Hirschsprung’s Disease. Additionally, since most oral drugs are absorbed in the intestine, the most common drug side effects are intestinal. Given the plethora of functions carried out by the intestine, there is significant interest in preventing or reversing intestinal disease by manipulation of intestinal cell biology; for example, by stimulating ISC growth as a means to protect or re-establish epithelial integrity and barrier function following injury (Zhou et al., 2013). However, gastrointestinal (GI) disease research has largely relied on in vivo animal studies, which are intrinsically low throughput and sometimes do not adequately mimic human physiology. Therefore, in vitro-derived human intestinal tissues represent a powerful tool for functional modeling of congenital defects in human intestinal development, preclinical screening for drug efficacy and toxicity testing, and for establishing models to study the mechanistic basis of diseases including IBD and cancer.
In this Primer, we discuss the current understanding of intestine development and how this information has been used to direct the differentiation of human pluripotent stem cells (PSCs) into intestinal tissue in vitro. This approach requires the temporal manipulation of signaling pathways in a step-wise manner that recapitulates early intestinal development (Fig. 1). These main developmental steps include the formation of definitive endoderm, posterior endoderm patterning, gut tube formation, and intestinal growth and morphogenesis (Fig. 2). Success in this area is largely due to a shift from two- to three-dimensional growth conditions and the presence of mesenchymal cell types that result in a level of tissue complexity that more closely resembles the developing intestine in vivo. We will also evaluate how these tissues can be used to model human intestinal development and disease.
Fig. 1.
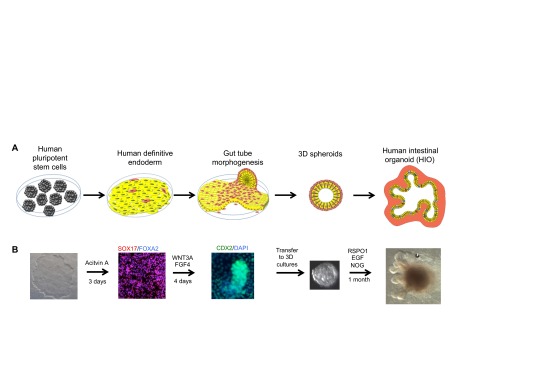
Intestinal differentiation and morphogenesis in a dish. (A) Directed differentiation of human PSCs into human intestinal organoids (HIOs). Pluripotent stem cells (PSCs) are first differentiated into definitive endoderm (DE) (yellow) and, during differentiation, a small population of cells differentiate into mesoderm (red). Upon the activation of WNT and FGF signaling, endoderm begins to express gut-specific transcription factors (CDX2, red nucleus), which persists in the epithelium throughout intestinal development. In addition, the mesenchymal cells proliferate and coalesce with the endoderm to form three-dimensional (3D) ‘spheroids’, consisting of a mesenchymal layer and a polarized epithelial layer with a lumen. Spheroids are then grown under 3D conditions in vitro and form HIOs. HIOs contain most epithelial cell types of the developing intestine, including goblet cells, Paneth cells, enteroendocrine cells and enterocytes. Other cell types have not been explicitly identified. The mesenchyme (light red) also differentiates into smooth muscle and fibroblastic cell types. (B) Directed differentiation of human PSCs (far left image shows one colony) is induced by the nodal mimetic activin A, resulting in the formation of SOX17+/FOXA2+ DE. Human DE is then differentiated into CDX2+ gut/intestinal tissue by inducing high levels of FGF and WNT signaling (for example, by using recombinant FGF4+WNT3A). During this differentiation process, endoderm and mesoderm undergo morphogenesis to form CDX2+ tube-like structures (middle panel) and 3D spheres that delaminate from the tissue culture dish (fourth image from the left shows a delaminated spheroid). Three-dimensional spheroids contain CDX2+ endoderm and mesoderm, and are grown in a 3D matrix in the presence of EGF, noggin (NOG) and R-spondin (RSPO1). Over the course of 1 month, spheroids expand and differentiate into HIOs, which contain multiple differentiated cell types. HIOs can be repeatedly passaged every 10-14 days for more than 1 year.
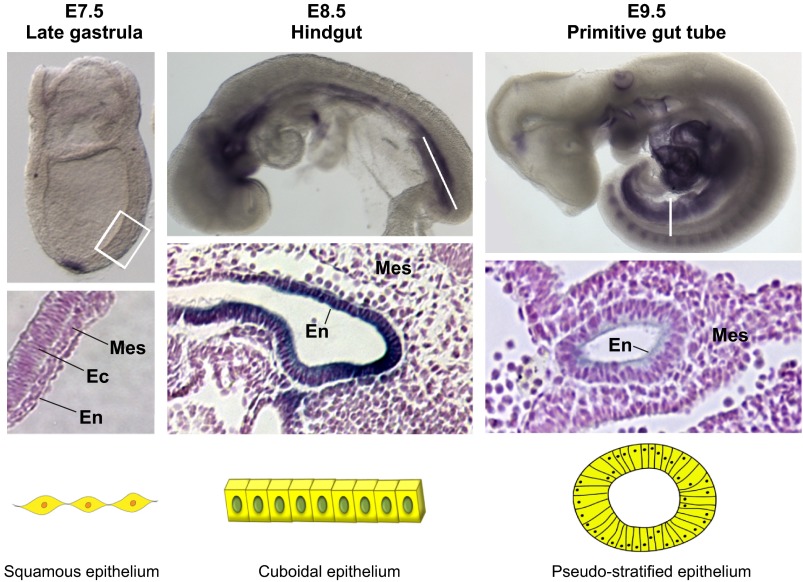
Fig. 2.
Gut tube formation in the developing mouse embryo. Three primary germ layers, mesoderm (Mes), ectoderm (Ec) and endoderm (En), are specified during gastrulation. The gut tube is specified from the endoderm, which has a flat and squamous-like morphology (left, E7.5). The developing gut tube epithelium forms along the anterior-posterior axis (middle, E8.5), stained here by whole-mount in situ hybridization for NM_029639 (Moore-Scott et al., 2007). At this point in time, the anterior endoderm has formed the foregut tube, the posterior endoderm has formed hindgut tube and the middle region is forming the midgut. Stained sections of the mid/hindgut region show that the epithelium has acquired a cuboidal-like morphology. Gut tube formation is completed by E9.5 in the mouse embryo (right), with the pseudo-stratified epithelium fully encased in mesoderm-derived mesenchyme. Staining for desmoplakin highlights the pseudo-stratified morphology of the gut tube. The boxed area and lines indicate the regions or sections enlarged in the panels underneath.
The first step: endoderm formation
Generating intestinal cells from PSCs requires a step to mimic the process of gastrulation and formation of definitive endoderm (DE) (Fig. 1). Studies of gastrulation using frog, fish, chick and mouse embryos have been essential in identifying a conserved molecular pathway that directs gastrulating cells into the endoderm and mesoderm lineages (reviewed in Zorn and Wells, 2009; Zorn and Wells, 2007). Central to these processes is the Nodal family of TGFβ signaling proteins, which in frog embryos act both to initiate gastrulation and to guide the specification of the mesoderm and endoderm lineages in a dose-dependent manner (Green and Smith, 1990). Nodal acts through its cognate Type I (Alk4/7) and type II (ActRIIA or B) receptors, and also requires the Crypto co-receptor for robust activation of signaling in vivo (Gritsman et al., 1999). The serine-threonine kinase activity of the Nodal-receptor complex mediates phosphorylation and nuclear localization of a Smad2/3/4 complex. Once in the nucleus, Smads interact with transcriptional co-factors such as FoxH1/FAST1 or Mix-like homeodomain proteins to activate a highly conserved endoderm gene regulatory network (Hoodless et al., 2001; Tremblay et al., 2000). The core of this transcriptional network in vertebrates contains the transcription factors Sox17, Foxa2, Mix, Gata4/6 and Eomes. Although the exact roles of these factors might vary slightly between vertebrates, they act to coordinate the induction the endoderm lineage (Sinner et al., 2006).
Establishing posterior identity from the DE
At the end of gastrulation there are four major signaling pathways that promote the posterior patterning of the vertebrate embryo: Wnt, Fgf, retinoic acid (RA) and Bmp. In Xenopus embryos, high levels of β-catenin dependent Wnt (Wnt/β-catenin) signaling promotes posterior gene expression in endoderm while at the same time inhibiting anterior gene expression in the posterior (McLin et al., 2007; Zorn and Wells, 2009). Conversely, repression of Wnt by the Sfrp family of secreted Wnt antagonists is required to help establish the stomach-duodenum boundary (Kim et al., 2005). Bmp ligands are expressed in the posterior mesoderm and act in a paracrine manner to promote posterior fate of endoderm in frog, fish and chick embryos (Kumar et al., 2003; Rankin et al., 2011; Roberts et al., 1995; Tiso et al., 2002). Similarly, Fgf ligands that are expressed in posterior mesoderm act on adjacent ectoderm and endoderm to promote a posterior fate (Dessimoz et al., 2006; Wells and Melton, 2000). Finally, RA is also known to promote posterior patterning of endoderm (Bayha et al., 2009; Huang et al., 1998; Niederreither et al., 2003; Stafford and Prince, 2002; Wang et al., 2006b).
One of the primary means by which these pathways promote a posterior fate is through direct regulation of Caudal homeobox (Cdx) genes, first identified as master regulators of posterior fate in Drosophila (Macdonald and Struhl, 1986; Mlodzik et al., 1985). One family member, Cdx2, is expressed in a temporally and spatially dynamic manner, first being expressed in all three germ layers in early gestation, then becoming restricted to the intestinal epithelium by midgestation. Initial insights into the critical role for Cdx2 during gut/intestine development came through tetraploid complementation assays (Chawengsaksophak et al., 2004). These experiments showed that Cdx2-null mesoderm and neurectoderm were properly specified, whereas the hindgut failed to express endodermal Shh and had delayed formation of the hindgut. Subsequent studies showed that conditional deletion of Cdx2 in endoderm using Foxa3-Cre resulted in mutant mice that formed a gut tube that was truncated at the cecum, completely lacked the colon and terminated in a blind-ended sac. Mutant intestines failed to undergo villus morphogenesis and expressed foregut-specific genes and an esophageal program, with the epithelium morphologically resembling the squamous epithelium of the esophagus. Collectively, these data suggested that in the absence of Cdx2, a foregut program is ectopically activated in the region of the intestine (Gao et al., 2009).
Studies in vitro and in vivo suggest that Wnt signaling mediates posterior fate by regulating Cdx2 expression. For example, electroporation of a constitutively active form of β-catenin in the mouse foregut endoderm, genetic activation of β-catenin or stabilization of β-catenin using a Gsk3b inhibitor at E8.25 resulted in ectopic induction of Cdx2 and repression of Sox2 in the foregut (Sherwood et al., 2011). Fgf, RA and BMP signaling pathways similarly regulate posterior endoderm specification by regulating expression of the Cdx genes (Bayha et al., 2009; Dale et al., 1992; Dessimoz et al., 2006; Keenan et al., 2006; Kinkel et al., 2008; Kumar et al., 2003; Lickert and Kemler, 2002; Northrop and Kimelman, 1994). In many cases, these pathways directly regulate Cdx expression through Wnt, RA and Fgf responsive elements (Haremaki et al., 2003; Rankin et al., 2011; Tiso et al., 2002). Finally, Cdx factors feed back on these posteriorizing pathways by regulating expression of key signaling components such as Wnt3, Fgf8 and the RA synthesizing enzyme Cyp26a1. These data suggest that there is a posterior regulatory network involving the synergistic activities of Fgf, Wnt and RA, which act to regulate expression of the Cdx family of transcription factors.
Using embryonic patterning and inductive cues to generate intestinal tissue in vitro
Activation of Nodal signaling has been the primary approach to generate DE cells from mouse and human embryonic stem cells (ESCs) (D’Amour et al., 2005; Kubo et al., 2004). This requires culturing ESCs in high levels of activin A (100 ng/ml) that, over the course of 3 or 4 days, result in monolayer cultures of DE cells expressing endoderm markers such as Sox17 and Foxa2. The reason most protocols use the nodal mimetic activin is because it does not require the crypto co-receptor and is many-fold more active than nodal in promoting endoderm formation from human PSCs or in Xenopus animal caps (Tada et al., 2005). Although the DE derived from Nodal verses activin treatment is remarkably similar, recent studies indicate that there are some differences in gene expression and the in vivo differentiation potential of activin versus nodal-generated DE (Chen et al., 2013). In part, this could be due to the significant differences in the activity of activin versus nodal as the levels of nodal signaling can impact the anterior-posterior (A-P) nature of the endoderm. For example, high levels of nodal/activin signaling in the anterior primitive streak of mice promote anterior endoderm fate (Chen et al., 2013; Spence et al., 2011b). Consistent with this, longer activin treatment directs ESCs into an anterior definitive endoderm fate. Further differences between nodal and activin-generated DE could be due their differential ability to synergize with Wnt signaling to promote anterior endoderm fate, as has been shown in fish and frogs (Ho et al., 1999; Zorn et al., 1999), and in ESCs (Sumi et al., 2008).
Manipulation of the posterior regulatory network has been the focus of efforts to direct the intestinal differentiation of mouse and human PSC-derived DE (Ameri et al., 2009; Cao et al., 2010; McCracken et al., 2011; Ogaki et al., 2013; Sherwood et al., 2011; Spence et al., 2011b; Ueda et al., 2010). Activation of the Wnt pathway in DE cultures derived from mouse ESCs directs a posterior fate, as demonstrated by Cdx2 expression. However, Cao and colleagues reported that Cdx2 expression required the presence of fibroblast-conditioned medium (Cao et al., 2010), suggesting that other signaling factors secreted by fibroblasts were acting with Wnt to promote posterior specification. Indeed, WNT and FGF pathways were shown to act synergistically to induce a posterior fate in human PSC cultures, as marked by broad CDX2 expression (Fig. 1B) (McCracken et al., 2011; Spence et al., 2011b). Moreover, these studies demonstrated there was a temporal signaling requirement such that prolonged activation of the FGF and WNT signaling pathways together was required to irreversibly specify a posterior fate. For example, extended treatment of human DE cultures with FGF4 and WNT3A proteins for 4 days was required for stable and irreversible CDX2 expression, and was crucial for intestinal specification. By contrast, shorter treatment for 2 days resulted in transient, reversible induction of CDX2 and was not sufficient for intestinal specification (Spence et al., 2011b). As discussed above, the WNT and FGF pathways may have multiple nodes of intersection with RA and BMP signaling during establishment of the posterior axis in embryos. Thus, it seems reasonable to expect a combinatorial role for these pathways in activating the posterior regulatory network in PSC cultures; however, this has not yet been demonstrated.
Contribution of other germ layers to the developing intestine
The intestines are assembled from progenitors arising from the three germ layers. Although most studies focus on the endoderm-derived epithelium, the mesoderm and ectoderm-derived tissues play central roles in intestinal development and function. The mesoderm that gives rise to the intestinal mesenchyme is generated during gastrulation and is distributed laterally along the trunk of the vertebrate embryo. This lateral plate mesoderm separates into somatic and splanchnic mesoderm, the latter of which is a distinct population of cells adjacent to the developing intestinal epithelium prior to gut tube closure in the avian embryo (Thomason et al., 2012). As the gut tube closes and the epithelium matures, the splanchnic mesoderm forms a mesenchyme that encases the primitive gut tube. This mesenchyme forms multiple layers with distinct cell types and functions, including subepithelial myofibroblasts and fibrobasts, as well as circular and longitudinal smooth muscle. In addition, the mesoderm-derived endothelial cells and pericytes form a complex vascular plexus that requires a close proximity to epithelial cells for nutrient and hormone transport (Powell et al., 2011; Zorn and Wells, 2009; Zorn and Wells, 2007).
As well as giving rise to multiple cell types, the mesenchyme is also a crucial player in villus formation (Mathan et al., 1976; Karlsson et al., 2000) (see Fig. 3). Aggregates of mesenchyme adjacent to the intestinal epithelium are first visible at E14.5, and express platelet-derived growth factor A (Pdgfra) (Karlsson et al., 2000). At later stages, as the epithelium remodels to give rise to the villi projecting into the lumen, the mesenchymal clusters remain associated with the tip of the villus epithelium and express several signaling ligands, including Bmp2 and Bmp4. In addition, both the epithelium adjacent to the mesenchymal clusters and the cluster itself appear to be non-proliferative, suggesting that clusters may act as a signaling center to coordinate villus emergence and withdraw from the cell cycle (Karlsson et al., 2000). Consistent with this, inhibition of Bmp signaling leads to excessive proliferation and ectopic formation of crypt-like structures on the villus epithelium, similar to what is seen in human juvenile polyposis (Haramis, 2004; Batts et al., 2006).
Fig. 3.
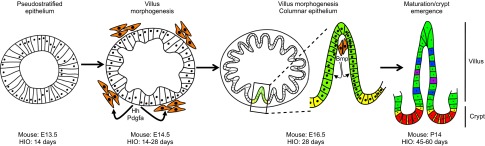
Milestones in murine and HIO intestinal development. The intestinal epithelium is present as a pseudostratified epithelium at ∼E12.5 through E13.5 in the mouse, and within 14 days of HIO specification. After this point, the appearance of mesenchymal clusters (orange), which respond to hedgehog (Hh) and platelet-derived growth factor A (Pdgfa) secreted from the epithelium, is coincident with the onset of villus morphogenesis. In the mouse, villus morphogenesis proceeds between E14.5 and E16.5, whereas in HIOs this stage takes considerably longer. As the epithelium remodels, the columnar epithelium forms with stereotypical villus (green) and proliferative intervillus (yellow) domains. Mesenchymal clusters remain associated with the villus tip and act as a postmitotic signaling center, signaling to the epithelium via secreted growth factors (including Bmp ligands, among others), which prevent epithelial proliferation. The fetal intervillus domain gives rise to the adult crypt by postnatal day 14 in the mouse, and after approximately 8 weeks in HIOs. The mature adult crypt houses the proliferative intestinal stem cells (yellow) and secretory Paneth cells (red), as well as several additional cell types, the most prevalent of which are enterocytes (green), goblet cells (blue) and enteroendocrine cells (purple).
More recent work has demonstrated that mesenchymal clusters respond to, and are dependent on, epithelial hedgehog (Hh) signaling (Walton et al., 2012). Hh signaling has been previously shown to be crucial for villus morphogenesis (Madison et al., 2005; Mao et al., 2010). The study by Walton and colleagues described a mechanism by which Hh signaling controls the size and formation of Pdgfra+ mesenchymal clusters, thus allowing villus morphogenesis to proceed. In the absence of Hh signaling, both the mesenchymal clusters and the villi fail to form (Walton et al., 2012). In addition to cluster formation, new work has also revealed the importance of the mesenchyme-derived intestinal smooth muscle layers, which generate compressive forces necessary for intestinal folding seen in a variety of vertebrates, and which leads to an increased intestinal surface area (Shyer et al., 2013).
Ectoderm-derived tissue is also crucial for intestine development and function. Within the intestinal mesoderm are two neural plexuses that control intestinal motility. These neurons, collectively called the enteric nervous system (ENS), coordinate the muscular contractions of peristalsis, which control the mixing of food with digestive enzymes and the movement of luminal contents through the GI tract. The peripheral nervous system is derived from a subset of trunk neural crest cells (NCCs) referred to as vagal neural crest cells, which originate just caudal to the hindbrain in the region between somites 1 and 7 (Burns et al., 2002; Durbec et al., 1996). Vagal NCCs migrate ventrally and invade the mesenchyme surrounding the primitive foregut tube in early somite stage embryos (reviewed by Kuo and Erickson, 2010). NCCs migrate caudally to colonize the developing small and large intestine, and migration is regulated by several pathways including netrin, Bmp and endothelin signaling (Goldstein et al., 2005; Jiang et al., 2003; Nataf et al., 1996). Last, NCCs differentiate into neuronal and glial lineages, and this involves the repression of several signaling pathways, including Bmp and Notch (Chalazonitis et al., 2004; Goldstein et al., 2005).
Harnessing embryonic morphogenesis and tissue-tissue interactions to generate more functional intestinal tissues from PSCs
Multilineage contributions and tissue-tissue interactions are critical for the development a functional intestine. There is an emerging concept that one reason for the lack of functionality of many PSC-derived cells and tissues is due the absence of organ morphogenesis in PSC monolayer cultures. For example, monolayer culture-derived pancreatic endocrine cells lack the ability to secrete insulin in response to glucose (D’Amour et al., 2006) and liver hepatocytes have an enzyme expression profile that is fetal in nature (DeLaForest et al., 2011). These non-physiological, two-dimensional (2D) monolayer cultures do not recapitulate the many morphogenetic processes that occur during endoderm organ development. Efforts to use more three-dimensional (3D) approaches for differentiation of PSCs have largely used spontaneous aggregation into embryoid bodies. However the stochastic nature of this approach makes it hard to efficiently direct differentiation into specific cell and tissue types. Moreover, there is little evidence that normal morphogenesis occurs in embryoid bodies. Here, we will discuss morphogenetic processes that are important for intestinal development and how they have been use to direct the morphogenesis of PSCs into complex, 3D human intestinal ‘organoids’ (HIOs; see Box 1).
Box 1. PSC-derived versus primary intestinal epithelial cultures: what is the difference?
One of the major advances in gastrointestinal biology includes long-term growth and expansion of individual adult ISCs in three-dimensional ‘enteroid’ cultures (Ootani et al., 2009; Sato et al., 2009; Sato and Clevers, 2013; Sato et al., 2011). Enteroids are also commonly referred to as ‘mini-guts’, as well as ‘organoids’ (Sato and Clevers, 2013). Human intestinal organoids (HIOs), however, are derived from pluripotent stem cells and are significantly different from enteroids, as recently discussed at length (Finkbeiner and Spence, 2013). Briefly, enteroids are derived from fully committed adult intestinal crypts (or single ISCs) that are isolated from human or mouse intestine (Gracz et al., 2013; Wang et al., 2013). Enteroids consist of epithelium only and are biased towards an undifferentiated stem-cell fate due to the culture conditions (Miyoshi et al., 2012). Enteroids have proven to be an unparalleled model in which to study molecular mechanisms by which adult intestinal stem cells are regulated and maintained, and are well suited to adult disease studies (de Lau et al., 2011; Dekkers et al., 2013; Zhou et al., 2013). HIOs, however, are generated by recapitulation of a developmental program and are therefore an unparalleled model in which to study human gastrointestinal development (Du et al., 2012). Both HIOs and adult enteroids have great potential as new in vitro models for drug screening, infectious disease and cancer.
Transition from 2D to 3D: formation of the primitive gut tube in the embryo
Shortly after gastrulation, all three germ layers undergo morphogenesis that will result in the formation of complex 3D structures such as the neural tube, somites and the gut tube. Gut tube morphogenesis in birds and mammals initiates at the anterior and posterior ends of the embryo with the formation of the foregut and hindgut, and is largely completed within 36 hours. During this process, the 2D DE undergoes dramatic cell shape changes, starting as flat squamous cells, but then transitioning into a cuboidal epithelium during the morphogenesis of the foregut and hindgut. The entire process of gut tube formation is completed within 2 days after the end of gastrulation in mice, at which time the gut tube epithelium has a pseudostratified morphology (Fig. 2). In addition, cell lineage tracing experiments and embryo imaging experiments demonstrate that endoderm and mesoderm cells are actively migrating laterally and along the anterior-posterior axis between the gastrula and gut tube stages of development (Kimura et al., 2006; Lawson et al., 1991; Lawson et al., 1986; Lawson and Pedersen, 1987).
The signaling pathways that control posterior fate, such as FGF and WNT, also regulate endoderm and mesoderm cell behaviors that are involved in morphogenesis of the gut tube. Non-canonical Wnt signaling is involved in hindgut morphogenesis and elongation. Mutations in genes encoding either Wnt5a or the downstream mediators of non-canonical Wnt signaling, such as the disheveled-interacting protein Dapper1 (Dact1), perturb both hindgut formation and subsequent intestinal elongation (Cervantes et al., 2009; Marlow et al., 2004; Rauch et al., 1997; Tai et al., 2009; Yamaguchi et al., 1999). Fgf signaling may also play a role in morphogenesis by regulating migration of presumptive hindgut mesoderm. In chick, Fgf8 and Fgf4 act as a chemoattractant and repellant, respectively, to regulate mesoderm migration laterally and posteriorly as the embryo and hindgut extend caudally (Yang et al., 2002).
Triggering gut tube morphogenesis in PSC cultures
Although the Wnt and Fgf pathways play distinct roles during embryonic hindgut morphogenesis, studies using human PSC cultures suggest that FGF and WNT synergistically act both to promote expression of CDX2 and to initiate a morphogenetic process that is similar to gut tube morphogenesis in vivo (Figs 1, 2) (Spence et al., 2011b). For example, in vivo, embryonic DE forms as a flat sheet of cells with a squamous-like morphology. However, as gut tube morphogenesis commences, DE cells transition to the cuboidal type of epithelium that lines the developing mid- and hindgut (Fig. 2). Similar transitions occur with PSC-derived DE, which starts as a flat sheet of cells, much like a squamous epithelium. However, following 4 days of treatment with both FGF4 and WNT3a, DE cells condense into the cuboidal epithelium and form gut tube-like structures.
In addition to the epithelial morphogenesis observed in PSC cultures, the mesoderm undergoes transitions that are similar to those observed during gut tube morphogenesis in vivo: FGF ligands mediate mesoderm specification, proliferation and migration (Yang et al., 2002). In PSC cultures, FGF4 mediated an expansion of mesoderm that formed the mesenchyme surrounding the epithelium of these gut tube-like structures. It is likely that these gut tube structures were fully committed to the intestinal lineage, as growth in 3D pro-intestinal culture conditions (Sato et al., 2009) resulted in the formation intestinal tissue, and not other gut tube derivatives.
In addition to intestinal epithelium, PSC-derived HIOs have a significant level of mesenchymal complexity. The mesenchyme in HIOs comes from mesoderm, which populates the early DE cultures and expands in response to FGF4 to form a layer of primitive mesenchyme surrounding the developing organoid (Spence et al., 2011b) (see Fig. 1). This primitive mesenchyme differentiates in parallel with the epithelium and forms several differentiated cell types, such as smooth muscle, subepithelial myofibroblasts and fibroblasts, consistent with in vivo development (Spence et al., 2011b).
Milestones during midgestational intestine development are mirrored in PSC-derived intestine
In the mouse embryo, immediately after gut tube formation (around E9.0), the hindgut is a simple cuboidal epithelium that undergoes a series of changes over the next 6-7 days that culminates in the emergence of the stereotypical villus structures of the postnatal intestine (Fig. 3) (Spence et al., 2011a). Between E9.5 and E12.5, the intestinal epithelium proliferates and expands in cell number, girth and luminal size, with the intestinal epithelium and lumen shaped like an oval (Sbarbati, 1982). Recent studies using genetic lineage tracing and 3D confocal microscopy demonstrate that the epithelium becomes pseudostratified around E12.5 (Grosse et al., 2011). Villus morphogenesis begins at E14.5, and gives rise to a columnar epithelium and the stereotypical villus structures that project into the lumen. The intestinal mesenchyme is also involved and acts as a signaling center to drive villus morphogenesis (Karlsson et al., 2000; Madison et al., 2005; Walton et al., 2012).
Shortly after the villi have formed, around E16.5 in mouse, proliferative intervillus progenitor cells give rise to newly differentiated cells on the villi, which include enterocytes, goblet and enteroendocrine cells. Of note, Paneth cells do not differentiate until around postnatal day 14 (P14) in mice and their differentiation occurs simultaneously with tissue remodeling events that are responsible for crypt emergence (Calvert and Pothier, 1990; Kim et al., 2012). In humans, crypts and Paneth cells are present by 22 weeks of gestation (Trier and Moxey, 1979).
Many of the epithelial milestones that occur at these stages of intestinal development also take place during PSC-derived intestine ‘development’ in vitro, i.e. during the formation of HIOs (Fig. 3) (McCracken et al., 2011; Spence et al., 2011b). The 3D cuboidal epithelium of the early intestinal organoids gives rise to a pseudostratified epithelium, which undergoes a process mimicking villus morphogenesis, with a few exceptions. As organoids continue to grow in culture, the pseudostratified epithelium transitions to columnar epithelium; however, true villi do not form. Instead, villus-like structures form, which are made up of epithelial protrusions extending into the lumen. These structures are not true villi due their lack of the lamina propria, which is the underlying mesenchymal tissue made up of nerve, vascular, immune and support cells normally found in the core of the villus. Although true villi do not seem to develop in HIOs, the villus-like structures still behave similarly to their in vivo counterpart and proliferation is restricted to the base of the villus-like structure, equivalent to the intervillus progenitor domain, and preferentially expresses molecular markers of the intervillus domain, including Sox9. Furthermore, HIOs generate cells that express markers for the major intestinal cell types: enterocytes, goblet, enterendocrine and Paneth cells. The co-expression of Gata4/Gata6 and presence of Paneth cells in the HIOs suggest that they are most similar to the small intestine. In addition to the major cell types mentioned above, the small intestine in vivo possesses other cell types, including tuft, cup and M-cells; however, it is unclear at this point whether HIOs possess these cell types. HIOs also appear to undergo a process similar to crypt emergence. In HIOs, the intestinal stem cell marker Lgr5 is not expressed robustly during early stages of organoid ‘development’, but is expressed in discreet epithelial domains after prolonged culture. It is important to point out that to date, functional validation of all cell types in organoids has not been carried out, and many of the conclusions we have drawn are based on marker analysis. Therefore, as this system becomes more widely used, it will be interesting to see additional levels of functional validation for individual cell types.
Future directions
Building a better intestine in vitro
PSC-derived HIOs exhibit several basic functional properties; for example, they secrete mucus into the lumen and they are competent to absorb dipeptides, indicating a functional dipeptide transport system. Despite these observations, it is still unclear whether HIOs represent a ‘mature’ or ‘immature’ tissue. Recent studies have suggested that human PSC-derived intestinal tissues are more similar to fetal human intestine than to adult human intestine (Fordham et al., 2013). Importantly, however, different methods used to generate HIOs and maintain their growth in vitro confound comparisons from one study to another. In general, the generation of functional, mature cells or tissues from human PSC cultures has proven challenging across many tissue types (Dolnikov et al., 2005; Si-Tayeb et al., 2010; D’Amour et al., 2006; Kroon et al., 2008). Recently, the transcription factor Blimp1 (also know an Prdm1) was identified as a key regulator of the transition from fetal to mature intestinal epithelium, including the formation of crypts (Harper et al., 2011; Muncan et al., 2011). Therefore, identification of signaling pathways that repress Blimp1 may be one approach to promote maturation of HIOs.
One potential reason for lack of maturation and/or functionality is because in vitro-derived tissues lack important cell types that are crucial for organ function. For example, even though HIOs contain important mesoderm-derived cell types that are crucial for intestinal function, such as smooth muscle myocytes, fibroblasts and subepithelial myofibroblasts (Powell et al., 2011), they are missing a vascular plexus and the enteric nervous system (ENS) that coordinates the muscular contractions of peristalsis. The lack of the vascular and neural plexus limits the physiological relevance of drug absorption studies and makes it impossible to study intestinal motility disorders. Given that protocols exist for generating vascular endothelial cells and NCCs from human PSCs (Bajpai et al., 2010; Lee et al., 2007; Levenberg et al., 2002), it should be possible to incorporate vascular and ENS progenitors into developing HIOs in an attempt to generate vascular and neural networks. An example of this approach was recently demonstrated whereby vascular progenitors were added during the differentiation of PSCs into hepatocyte progenitors (Takebe et al., 2013). The addition of vascular cells resulted in the formation of 3D liver buds with a well-formed vascular plexus and greatly improved functionality in vivo.
Development and disease research
Although generating transplantable segments of intestine in vitro is still years (or decades) away, in vitro-derived human intestinal tissue allows for unprecedented studies of human development, homeostasis and disease. Agonists and antagonists can be used to dissect the role of signaling pathways during intestinal morphogenesis, patterning and cell fate specification. Using standard genetic gain- and loss-of-function approaches, it is straightforward to study gene function and identify gene regulatory networks involved in lineage development and morphogenesis. Alternatively, iPSC technology can be used to identify the molecular basis of human congenital disorders affecting the intestines such as cystic fibrosis and genetic forms of malabsorption (Wang et al., 2006a). Because of the ease of manipulation and imaging, human organoid-based approaches are a powerful tool to study wide variety of other disease processes. Introduction of viral and bacterial pathogens into the lumen of organoids can allow for new studies of infectious disease and facilitate identification of pathways mediating host-pathogen interactions (Finkbeiner et al., 2012). Organoids derived from PSCs or adult tissues may also be useful to study the environmental and genetic causes of cancer initiation and progression (Ootani et al., 2009). Last, many commonly prescribed drugs have intestinal side effects, making it a possibility that HIOs could be used as a rapid screening tool for drugs with good absorption and fewer intestinal complications.
As for using in vitro-derived intestinal tissues in transplantation-based therapies, current efforts have focused on seeding damaged intestinal epithelia with adult ISCs (Yui et al., 2012). This approach has resulted in engraftment and expansion of transplanted cells; however, it is technically challenging and might be a difficult approach to use broadly, particularly in more proximal regions of the intestinal tract. Moreover, this approach is not helpful for individuals suffering from short gut syndrome. Although in vitro derivation of transplantable intestinal segments is not likely to occur in the immediate future, recent advances in tissue engineering have made it possible to generate an artificial trachea for transplantation (Jungebluth et al., 2011). Similar approaches using endogenous or biosynthetic scaffolds should be possible for intestine.
Human organoids are fueling a renaissance of in vitro studies of human intestinal development and disease. Organoid-based research is especially powerful when combined with new technologies such as gene targeting, real-time high content imaging, and fluidics-based platforms to grow multiple tissue-type organoids on chips to mimic systemic organ interactions.
Acknowledgments
We thank our colleagues for many helpful discussions and apologize for not including additional references due to space limitations.
Footnotes
Competing interests
The authors declare no competing financial interests.
Funding
J.R.S. is supported by the University of Michigan Center for Organogenesis, the University of Michigan Biological Sciences Scholar Program, The National Institute of Diabetes and Digestive and Kidney Diseases and a March of Dimes Basil O’Connor new investigator award. J.M.W. is supported by the National Institutes of Health. Deposited in PMC for release after 12 months.
References
- Ameri J., Ståhlberg A., Pedersen J., Johansson J. K., Johannesson M. M., Artner I., Semb H. (2009). FGF2 specifies hESC-derived definitive endoderm into foregut/midgut cell lineages in a concentration-dependent manner. Stem Cells 28, 45–56 [DOI] [PubMed] [Google Scholar]
- Bajpai R., Chen D. A., Rada-Iglesias A., Zhang J., Xiong Y., Helms J., Chang C. P., Zhao Y., Swigut T., Wysocka J. (2010). CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature 463, 958–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batts L. E., Polk D. B., Dubois R. N., Kulessa H. (2006). Bmp signaling is required for intestinal growth and morphogenesis. Dev. Dyn. 235, 1563–1570 [DOI] [PubMed] [Google Scholar]
- Bayha E., Jørgensen M. C., Serup P., Grapin-Botton A. (2009). Retinoic acid signaling organizes endodermal organ specification along the entire antero-posterior axis. PLoS ONE 4, e5845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns A. J., Delalande J. M., Le Douarin N. M. (2002). In ovo transplantation of enteric nervous system precursors from vagal to sacral neural crest results in extensive hindgut colonisation. Development 129, 2785–2796 [DOI] [PubMed] [Google Scholar]
- Calvert R., Pothier P. (1990). Migration of fetal intestinal intervillous cells in neonatal mice. Anat. Rec. 227, 199–206 [DOI] [PubMed] [Google Scholar]
- Cao L., Gibson J. D., Miyamoto S., Sail V., Verma R., Rosenberg D. W., Nelson C. E., Giardina C. (2010). Intestinal lineage commitment of embryonic stem cells. Differentiation 81, 1–10 [DOI] [PubMed] [Google Scholar]
- Cervantes S., Yamaguchi T. P., Hebrok M. (2009). Wnt5a is essential for intestinal elongation in mice. Dev. Biol. 326, 285–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalazonitis A., D’Autréaux F., Guha U., Pham T. D., Faure C., Chen J. J., Roman D., Kan L., Rothman T. P., Kessler J. A., et al. (2004). Bone morphogenetic protein-2 and -4 limit the number of enteric neurons but promote development of a TrkC-expressing neurotrophin-3-dependent subset. J. Neurosci. 24, 4266–4282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawengsaksophak K., de Graaff W., Rossant J., Deschamps J., Beck F. (2004). Cdx2 is essential for axial elongation in mouse development. Proc. Natl. Acad. Sci. USA 101, 7641–7645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A. E., Borowiak M., Sherwood R. I., Kweudjeu A., Melton D. A. (2013). Functional evaluation of ES cell-derived endodermal populations reveals differences between Nodal and Activin A-guided differentiation. Development 140, 675–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creamer B., Shorter R. G., Bamforth J. (1961). The turnover and shedding of epithelial cells. I. The turnover in the gastro-intestinal tract. Gut 2, 110–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amour K. A., Agulnick A. D., Eliazer S., Kelly O. G., Kroon E., Baetge E. E. (2005). Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 23, 1534–1541 [DOI] [PubMed] [Google Scholar]
- D’Amour K. A., Bang A. G., Eliazer S., Kelly O. G., Agulnick A. D., Smart N. G., Moorman M. A., Kroon E., Carpenter M. K., Baetge E. E. (2006). Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 24, 1392–1401 [DOI] [PubMed] [Google Scholar]
- Dale L., Howes G., Price B. M., Smith J. C. (1992). Bone morphogenetic protein 4: a ventralizing factor in early Xenopus development. Development 115, 573–585 [DOI] [PubMed] [Google Scholar]
- de Lau W., Barker N., Low T. Y., Koo B.-K., Li V. S. W., Teunissen H., Kujala P., Haegebarth A., Peters P. J., van de Wetering M., et al. (2011). Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476, 293–297 [DOI] [PubMed] [Google Scholar]
- Dekkers J. F., Wiegerinck C. L., de Jonge H. R., Bronsveld I., Janssens H. M., de Winter-de Groot K. M., Brandsma A. M., de Jong N. W. M., Bijvelds M. J. C., Scholte B. J., et al. (2013). A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat. Med. 19, 939–945 [DOI] [PubMed] [Google Scholar]
- DeLaForest A., Nagaoka M., Si-Tayeb K., Noto F. K., Konopka G., Battle M. A., Duncan S. A. (2011). HNF4A is essential for specification of hepatic progenitors from human pluripotent stem cells. Development 138, 4143–4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessimoz J., Opoka R., Kordich J. J., Grapin-Botton A., Wells J. M. (2006). FGF signaling is necessary for establishing gut tube domains along the anterior-posterior axis in vivo. Mech. Dev. 123, 42–55 [DOI] [PubMed] [Google Scholar]
- Dolnikov K., Shilkrut M., Zeevi-Levin N., Danon A., Gerecht-Nir S., Itskovitz-Eldor J., Binah O. (2005). Functional properties of human embryonic stem cell-derived cardiomyocytes. Ann. N. Y. Acad. Sci. 1047, 66–75 [DOI] [PubMed] [Google Scholar]
- Du A., McCracken K. W., Walp E. R., Terry N. A., Klein T. J., Han A., Wells J. M., May C. L. (2012). Arx is required for normal enteroendocrine cell development in mice and humans. Dev. Biol. 365, 175–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbec P. L., Larsson-Blomberg L. B., Schuchardt A., Costantini F., Pachnis V. (1996). Common origin and developmental dependence on c-ret of subsets of enteric and sympathetic neuroblasts. Development 122, 349–358 [DOI] [PubMed] [Google Scholar]
- Engelstoft M. S., Egerod K. L., Lund M. L., Schwartz T. W. (2013). Enteroendocrine cell types revisited. Curr. Opin. Pharmacol. 13, 912–921 [DOI] [PubMed] [Google Scholar]
- Finkbeiner S. R., Spence J. R. (2013). A gutsy task: generating intestinal tissue from human pluripotent stem cells. Dig. Dis. Sci. 58, 1176–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner S. R., Zeng X.-L., Utama B., Atmar R. L., Shroyer N. F., Estes M. K. (2012). Stem cell-derived human intestinal organoids as an infection model for rotaviruses. MBio 3, e00159–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordham R. P., Yui S., Hannan N. R. F., Soendergaard C., Madgwick A., Schweiger P. J., Nielsen O. H., Vallier L., Pedersen R. A., Nakamura T., et al. (2013). Transplantation of expanded fetal intestinal progenitors contributes to colon regeneration after injury. Cell Stem Cell 13, 734–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N., White P., Kaestner K. H. (2009). Establishment of intestinal identity and epithelial-mesenchymal signaling by Cdx2. Dev. Cell 16, 588–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. M., Brewer K. C., Doyle A. M., Nagy N., Roberts D. J. (2005). BMP signaling is necessary for neural crest cell migration and ganglion formation in the enteric nervous system. Mech. Dev. 122, 821–833 [DOI] [PubMed] [Google Scholar]
- Gracz A.D., Fuller M.K., Wang F., Li L., Stelzner M., Dunn J.C.Y., Martín M.G., Magness S.T. (2013). CD24 and CD44 mark human intestinal epithelial cell populations with characteristics of active and facultative stem cells. Stem Cells 31, 2024–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J. B., Smith J. C. (1990). Graded changes in dose of a Xenopus activin A homologue elicit stepwise transitions in embryonic cell fate. Nature 347, 391–394 [DOI] [PubMed] [Google Scholar]
- Gritsman K., Zhang J., Cheng S., Heckscher E., Talbot W. S., Schier A. F. (1999). The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell 97, 121–132 [DOI] [PubMed] [Google Scholar]
- Grosse A. S., Pressprich M. F., Curley L. B., Hamilton K. L., Margolis B., Hildebrand J. D., Gumucio D. L. (2011). Cell dynamics in fetal intestinal epithelium: implications for intestinal growth and morphogenesis. Development 138, 4423–4432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramis A. P. G. (2004). De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science 303, 1684–1686 [DOI] [PubMed] [Google Scholar]
- Haremaki T., Tanaka Y., Hongo I., Yuge M., Okamoto H. (2003). Integration of multiple signal transducing pathways on Fgf response elements of the Xenopus caudal homologue Xcad3. Development 130, 4907–4917 [DOI] [PubMed] [Google Scholar]
- Harper J., Mould A., Andrews R. M., Bikoff E. K., Robertson E. J. (2011). The transcriptional repressor Blimp1/Prdm1 regulates postnatal reprogramming of intestinal enterocytes. Proc. Natl. Acad. Sci. USA 108, 10585–10590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C. Y., Houart C., Wilson S. W., Stainier D. Y. (1999). A role for the extraembryonic yolk syncytial layer in patterning the zebrafish embryo suggested by properties of the hex gene. Curr. Biol. 9, 1131–S4 [DOI] [PubMed] [Google Scholar]
- Hoodless P. A., Pye M., Chazaud C., Labbé E., Attisano L., Rossant J., Wrana J. L. (2001). FoxH1 (Fast) functions to specify the anterior primitive streak in the mouse. Genes Dev. 15, 1257–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounnou G., Destrieux C., Desmé J., Bertrand P., Velut S. (2002). Anatomical study of the length of the human intestine. Surg. Radiol. Anat. 24, 290–294 [DOI] [PubMed] [Google Scholar]
- Huang D., Chen S. W., Langston A. W., Gudas L. J. (1998). A conserved retinoic acid responsive element in the murine Hoxb-1 gene is required for expression in the developing gut. Development 125, 3235–3246 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Liu M. T., Gershon M. D. (2003). Netrins and DCC in the guidance of migrating neural crest-derived cells in the developing bowel and pancreas. Dev. Biol. 258, 364–384 [DOI] [PubMed] [Google Scholar]
- Jungebluth P., Alici E., Baiguera S., Le Blanc K., Blomberg P., Bozóky B., Crowley C., Einarsson O., Grinnemo K.-H., Gudbjartsson T., et al. (2011). Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite: a proof-of-concept study. Lancet 378, 1997–2004 [DOI] [PubMed] [Google Scholar]
- Karlsson L., Lindahl P., Heath J. K., Betsholtz C. (2000). Abnormal gastrointestinal development in PDGF-A and PDGFR-(alpha) deficient mice implicates a novel mesenchymal structure with putative instructive properties in villus morphogenesis. Development 127, 3457–3466 [DOI] [PubMed] [Google Scholar]
- Keenan I. D., Sharrard R. M., Isaacs H. V. (2006). FGF signal transduction and the regulation of Cdx gene expression. Dev. Biol. 299, 478–488 [DOI] [PubMed] [Google Scholar]
- Kim B. M., Buchner G., Miletich I., Sharpe P. T., Shivdasani R. A. (2005). The stomach mesenchymal transcription factor Barx1 specifies gastric epithelial identity through inhibition of transient Wnt signaling. Dev. Cell 8, 611–622 [DOI] [PubMed] [Google Scholar]
- Kim T.-H., Escudero S., Shivdasani R. A. (2012). Intact function of Lgr5 receptor-expressing intestinal stem cells in the absence of Paneth cells. Proc. Natl. Acad. Sci. USA [Epub ahead of print] doi:10.1073/pnas.1113890109 [DOI] [PMC free article] [PubMed]
- Kimura W., Yasugi S., Stern C. D., Fukuda K. (2006). Fate and plasticity of the endoderm in the early chick embryo. Dev. Biol. 289, 283–295 [DOI] [PubMed] [Google Scholar]
- Kinkel M. D., Eames S. C., Alonzo M. R., Prince V. E. (2008). Cdx4 is required in the endoderm to localize the pancreas and limit beta-cell number. Development 135, 919–929 [DOI] [PubMed] [Google Scholar]
- Kroon E., Martinson L. A., Kadoya K., Bang A. G., Kelly O. G., Eliazer S., Young H., Richardson M., Smart N. G., Cunningham J., et al. (2008). Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 26, 443–452 [DOI] [PubMed] [Google Scholar]
- Kubo A., Shinozaki K., Shannon J. M., Kouskoff V., Kennedy M., Woo S., Fehling H. J., Keller G. (2004). Development of definitive endoderm from embryonic stem cells in culture. Development 131, 1651–1662 [DOI] [PubMed] [Google Scholar]
- Kumar M., Jordan N., Melton D., Grapin-Botton A. (2003). Signals from lateral plate mesoderm instruct endoderm toward a pancreatic fate. Dev. Biol. 259, 109–122 [DOI] [PubMed] [Google Scholar]
- Kuo B. R., Erickson C. A. (2010). Regional differences in neural crest morphogenesis. Cell Adh. Migr. 4, 567–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson K. A., Pedersen R. A. (1987). Cell fate, morphogenetic movement and population kinetics of embryonic endoderm at the time of germ layer formation in the mouse. Development 101, 627–652 [DOI] [PubMed] [Google Scholar]
- Lawson K. A., Meneses J. J., Pedersen R. A. (1986). Cell fate and cell lineage in the endoderm of the presomite mouse embryo, studied with an intracellular tracer. Dev. Biol. 115, 325–339 [DOI] [PubMed] [Google Scholar]
- Lawson K. A., Meneses J. J., Pedersen R. A. (1991). Clonal analysis of epiblast fate during germ layer formation in the mouse embryo. Development 113, 891–911 [DOI] [PubMed] [Google Scholar]
- Lee G., Kim H., Elkabetz Y., Al Shamy G., Panagiotakos G., Barberi T., Tabar V., Studer L. (2007). Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat. Biotechnol. 25, 1468–1475 [DOI] [PubMed] [Google Scholar]
- Levenberg S., Golub J. S., Amit M., Itskovitz-Eldor J., Langer R. (2002). Endothelial cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 99, 4391–4396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickert H., Kemler R. (2002). Functional analysis of cis-regulatory elements controlling initiation and maintenance of early Cdx1 gene expression in the mouse. Dev. Dyn. 225, 216–220 [DOI] [PubMed] [Google Scholar]
- Macdonald P. M., Struhl G. (1986). A molecular gradient in early Drosophila embryos and its role in specifying the body pattern. Nature 324, 537–545 [DOI] [PubMed] [Google Scholar]
- Madison B. B., Braunstein K., Kuizon E., Portman K., Qiao X. T., Gumucio D. L. (2005). Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development 132, 279–289 [DOI] [PubMed] [Google Scholar]
- Marlow F., Gonzalez E. M., Yin C., Rojo C., Solnica-Krezel L. (2004). No tail co-operates with non-canonical Wnt signaling to regulate posterior body morphogenesis in zebrafish. Development 131, 203–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J., Kim B. M., Rajurkar M., Shivdasani R. A., McMahon A. P. (2010). Hedgehog signaling controls mesenchymal growth in the developing mammalian digestive tract. Development 137, 1721–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathan M., Moxey P. C., Trier J. S. (1976). Morphogenesis of fetal rat duodenal villi. Am. J. Anat. 146, 73–92 [DOI] [PubMed] [Google Scholar]
- McCracken K. W., Howell J. C., Wells J. M., Spence J. R. (2011). Generating human intestinal tissue from pluripotent stem cells in vitro. Nat. Protoc. 6, 1920–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLin V. A., Rankin S. A., Zorn A. M. (2007). Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development 134, 2207–2217 [DOI] [PubMed] [Google Scholar]
- Miyoshi H., Ajima R., Luo C. T., Yamaguchi T. P., Stappenbeck T. S. (2012). Wnt5a potentiates TGF-β signaling to promote colonic crypt regeneration after tissue injury. Science 338, 108–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlodzik M., Fjose A., Gehring W. J. (1985). Isolation of caudal, a Drosophila homeo box-containing gene with maternal expression, whose transcripts form a concentration gradient at the pre-blastoderm stage. EMBO J. 4, 2961–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore-Scott B. A., Opoka R., Lin S.-C. J., Kordich J. J., Wells J. M. (2007). Identification of molecular markers that are expressed in discrete anterior-posterior domains of the endoderm from the gastrula stage to mid-gestation. Dev. Dyn. 236, 1997–2003 [DOI] [PubMed] [Google Scholar]
- Muncan V., Heijmans J., Krasinski S. D., Büller N. V., Wildenberg M. E., Meisner S., Radonjic M., Stapleton K. A., Lamers W. H., Biemond I., et al. (2011). Blimp1 regulates the transition of neonatal to adult intestinal epithelium. Nat. Commun. 2, 452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataf V., Lecoin L., Eichmann A., Le Douarin N. M. (1996). Endothelin-B receptor is expressed by neural crest cells in the avian embryo. Proc. Natl. Acad. Sci. USA 93, 9645–9650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederreither K., Vermot J., Le Roux I., Schuhbaur B., Chambon P., Dollé P. (2003). The regional pattern of retinoic acid synthesis by RALDH2 is essential for the development of posterior pharyngeal arches and the enteric nervous system. Development 130, 2525–2534 [DOI] [PubMed] [Google Scholar]
- Northrop J. L., Kimelman D. (1994). Dorsal-ventral differences in Xcad-3 expression in response to FGF-mediated induction in Xenopus. Dev. Biol. 161, 490–503 [DOI] [PubMed] [Google Scholar]
- Ogaki S., Shiraki N., Kume K., Kume S. (2013). Wnt and Notch signals guide embryonic stem cell differentiation into the intestinal lineages. Stem Cells 31, 1086–1096 [DOI] [PubMed] [Google Scholar]
- Ootani A., Li X., Sangiorgi E., Ho Q. T., Ueno H., Toda S., Sugihara H., Fujimoto K., Weissman I. L., Capecchi M. R., et al. (2009). Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 15, 701–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D. W., Pinchuk I. V., Saada J. I., Chen X., Mifflin R. C. (2011). Mesenchymal cells of the intestinal lamina propria. Annu. Rev. Physiol. 73, 213–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin S. A., Kormish J., Kofron M., Jegga A., Zorn A. M. (2011). A gene regulatory network controlling hhex transcription in the anterior endoderm of the organizer. Dev. Biol. 351, 297–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch G. J., Hammerschmidt M., Blader P., Schauerte H. E., Strähle U., Ingham P. W., McMahon A. P., Haffter P. (1997). Wnt5 is required for tail formation in the zebrafish embryo. Cold Spring Harb. Symp. Quant. Biol. 62, 227–234 [PubMed] [Google Scholar]
- Roberts D. J., Johnson R. L., Burke A. C., Nelson C. E., Morgan B. A., Tabin C. (1995). Sonic hedgehog is an endodermal signal inducing Bmp-4 and Hox genes during induction and regionalization of the chick hindgut. Development 121, 3163–3174 [DOI] [PubMed] [Google Scholar]
- Sato T., Clevers H. (2013). Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science 340, 1190–1194 [DOI] [PubMed] [Google Scholar]
- Sato T., Vries R. G., Snippert H. J., van de Wetering M., Barker N., Stange D. E., van Es J. H., Abo A., Kujala P., Peters P. J., et al. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 [DOI] [PubMed] [Google Scholar]
- Sato T., Stange D. E., Ferrante M., Vries R. G. J., Van Es J. H., Van den Brink S., Van Houdt W. J., Pronk A., Van Gorp J., Siersema P. D., et al. (2011). Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141, 1762–1772 [DOI] [PubMed] [Google Scholar]
- Sbarbati R. (1982). Morphogenesis of the intestinal villi of the mouse embryo: chance and spatial necessity. J. Anat. 135, 477–499 [PMC free article] [PubMed] [Google Scholar]
- Sherwood R. I., Maehr R., Mazzoni E. O., Melton D. A. (2011). Wnt signaling specifies and patterns intestinal endoderm. Mech. Dev. 128, 387–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyer A. E., Tallinen T., Nerurkar N. L., Wei Z., Gil E. S., Kaplan D. L., Tabin C. J., Mahadevan L. (2013). Villification: how the gut gets its villi. Science 342, 212–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si-Tayeb K., Noto F. K., Nagaoka M., Li J., Battle M. A., Duris C., North P. E., Dalton S., Duncan S. A. (2010). Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology 51, 297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinner D., Kirilenko P., Rankin S., Wei E., Howard L., Kofron M., Heasman J., Woodland H. R., Zorn A. M. (2006). Global analysis of the transcriptional network controlling Xenopus endoderm formation. Development 133, 1955–1966 [DOI] [PubMed] [Google Scholar]
- Spence J. R., Lauf R., Shroyer N. F. (2011a). Vertebrate intestinal endoderm development. Dev. Dyn. 240, 501–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence J. R., Mayhew C. N., Rankin S. A., Kuhar M. F., Vallance J. E., Tolle K., Hoskins E. E., Kalinichenko V. V., Wells S. I., Zorn A. M., et al. (2011b). Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford D., Prince V. E. (2002). Retinoic acid signaling is required for a critical early step in zebrafish pancreatic development. Curr. Biol. 12, 1215–1220 [DOI] [PubMed] [Google Scholar]
- Sumi T., Tsuneyoshi N., Nakatsuji N., Suemori H. (2008). Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical Wnt/beta-catenin, Activin/Nodal and BMP signaling. Development 135, 2969–2979 [DOI] [PubMed] [Google Scholar]
- Tada S., Era T., Furusawa C., Sakurai H., Nishikawa S., Kinoshita M., Nakao K., Chiba T., Nishikawa S. (2005). Characterization of mesendoderm: a diverging point of the definitive endoderm and mesoderm in embryonic stem cell differentiation culture. Development 132, 4363–4374 [DOI] [PubMed] [Google Scholar]
- Tai C. C., Sala F. G., Ford H. R., Wang K. S., Li C., Minoo P., Grikscheit T. C., Bellusci S. (2009). Wnt5a knock-out mouse as a new model of anorectal malformation. J. Surg. Res. 156, 278–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe T., Sekine K., Enomura M., Koike H., Kimura M., Ogaeri T., Zhang R. R., Ueno Y., Zheng Y. W., Koike N., et al. (2013). Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499, 481–484 [DOI] [PubMed] [Google Scholar]
- Thomason R. T., Bader D. M., Winters N. I. (2012). Comprehensive timeline of mesodermal development in the quail small intestine. Dev. Dyn. 241, 1678–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiso N., Filippi A., Pauls S., Bortolussi M., Argenton F. (2002). BMP signalling regulates anteroposterior endoderm patterning in zebrafish. Mech. Dev. 118, 29–37 [DOI] [PubMed] [Google Scholar]
- Tremblay K. D., Hoodless P. A., Bikoff E. K., Robertson E. J. (2000). Formation of the definitive endoderm in mouse is a Smad2-dependent process. Development 127, 3079–3090 [DOI] [PubMed] [Google Scholar]
- Trier J. S., Moxey P. C. (1979). Morphogenesis of the small intestine during fetal development. Ciba Found. Symp. 1979, 3–29 [DOI] [PubMed] [Google Scholar]
- Turner J. R. (2009). Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809 [DOI] [PubMed] [Google Scholar]
- Ueda T., Yamada T., Hokuto D., Koyama F., Kasuda S., Kanehiro H., Nakajima Y. (2010). Generation of functional gut-like organ from mouse induced pluripotent stem cells. Biochem. Biophys. Res. Commun. 391, 38–42 [DOI] [PubMed] [Google Scholar]
- Walton K. D., Kolterud A., Czerwinski M. J., Bell M. J., Prakash A., Kushwaha J., Grosse A. S., Schnell S., Gumucio D. L. (2012). Hedgehog-responsive mesenchymal clusters direct patterning and emergence of intestinal villi. Proc. Natl. Acad. Sci. USA 109, 15817–15822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Cortina G., Wu S. V., Tran R., Cho J.-H., Tsai M.-J., Bailey T. J., Jamrich M., Ament M. E., Treem W. R., et al. (2006a). Mutant neurogenin-3 in congenital malabsorptive diarrhea. N. Engl. J. Med. 355, 270–280 [DOI] [PubMed] [Google Scholar]
- Wang Z., Dollé P., Cardoso W. V., Niederreither K. (2006b). Retinoic acid regulates morphogenesis and patterning of posterior foregut derivatives. Dev. Biol. 297, 433–445 [DOI] [PubMed] [Google Scholar]
- Wang F., Scoville D., He X. C., Mahe M. M., Box A., Perry J. M., Smith N. R., Lei N. Y., Davies P. S., Fuller M. K., et al. (2013). Isolation and characterization of intestinal stem cells based on surface marker combinations and colony-formation assay. Gastroenterology 145, 383, e1-e21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J. M., Melton D. A. (2000). Early mouse endoderm is patterned by soluble factors from adjacent germ layers. Development 127, 1563–1572 [DOI] [PubMed] [Google Scholar]
- Yamaguchi T. P., Bradley A., McMahon A. P., Jones S. (1999). A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 126, 1211–1223 [DOI] [PubMed] [Google Scholar]
- Yang X., Dormann D., Münsterberg A. E., Weijer C. J. (2002). Cell movement patterns during gastrulation in the chick are controlled by positive and negative chemotaxis mediated by FGF4 and FGF8. Dev. Cell 3, 425–437 [DOI] [PubMed] [Google Scholar]
- Yui S., Nakamura T., Sato T., Nemoto Y., Mizutani T., Zheng X., Ichinose S., Nagaishi T., Okamoto R., Tsuchiya K., Clevers H., Watanabe M. (2012). Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5(+) stem cell. Nat. Med. 18, 618–623 [DOI] [PubMed] [Google Scholar]
- Zhou W.-J., Geng Z. H., Spence J. R., Geng J.-G. (2013). Induction of intestinal stem cells by R-spondin 1 and Slit2 augments chemoradioprotection. Nature 501, 107–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn A. M., Wells J. M. (2007). Molecular basis of vertebrate endoderm development. Int. Rev. Cytol. 259, 49–111 [DOI] [PubMed] [Google Scholar]
- Zorn A. M., Wells J. M. (2009). Vertebrate endoderm development and organ formation. Annu. Rev. Cell Dev. Biol. 25, 221–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn A. M., Butler K., Gurdon J. B. (1999). Anterior endomesoderm specification in Xenopus by Wnt/beta-catenin and TGF-beta signalling pathways. Dev. Biol. 209, 282–297 [DOI] [PubMed] [Google Scholar]