Abstract
In vertebrates, the first asymmetries are established along the animal-vegetal axis during oogenesis, but the underlying molecular mechanisms are poorly understood. Bucky ball (Buc) was identified in zebrafish as a novel vertebrate-specific regulator of oocyte polarity, acting through unknown molecular interactions. Here we show that endogenous Buc protein localizes to the Balbiani body, a conserved, asymmetric structure in oocytes that requires Buc for its formation. Asymmetric distribution of Buc in oocytes precedes Balbiani body formation, defining Buc as the earliest marker of oocyte polarity in zebrafish. Through a transgenic strategy, we determined that excess Buc disrupts polarity and results in supernumerary Balbiani bodies in a 3′UTR-dependent manner, and we identified roles for the buc introns in regulating Buc activity. Analyses of mosaic ovaries indicate that oocyte pattern determines the number of animal pole-specific micropylar cells that are associated with an egg via a close-range signal or direct cell contact. We demonstrate interactions between Buc protein and buc mRNA with two conserved RNA-binding proteins (RNAbps) that are localized to the Balbiani body: RNA binding protein with multiple splice isoforms 2 (Rbpms2) and Deleted in azoospermia-like (Dazl). Buc protein and buc mRNA interact with Rbpms2; buc and dazl mRNAs interact with Dazl protein. Cumulatively, these studies indicate that oocyte polarization depends on tight regulation of buc: Buc establishes oocyte polarity through interactions with RNAbps, initiating a feedback amplification mechanism in which Buc protein recruits RNAbps that in turn recruit buc and other RNAs to the Balbiani body.
Keywords: Bucky ball, Oocyte polarity, Balbiani body, Rbpms2
INTRODUCTION
The vertebrate animal-vegetal axis is established during oogenesis, whereas the anteroposterior and dorsoventral embryonic axes arise after fertilization. Oocyte polarity is a prerequisite for determining the prospective embryonic axes and germ cell determination in some non-mammalian vertebrates. Oocyte polarity can be first distinguished histologically by the asymmetric distribution of organelles, proteins and mRNAs within the Balbiani body (Bb) (de Smedt et al., 2000; Kloc et al., 2004; Marlow, 2010; Pepling et al., 2007). The Bb is an evolutionarily conserved asymmetric structure that is present in early oocytes of all animals examined, including humans. The Bb is a transient structure assembled in primary oocytes and disassembled thereafter. In zebrafish and Xenopus the Bb is the first indicator of the vegetal pole. The relationship between the Bb and the animal-vegetal axis of mammalian oocytes is not known. Despite its conserved structure and status as the first asymmetric structure in oocytes, only one gene, bucky ball (buc), is known to be required for Bb assembly in vertebrates (Bontems et al., 2009; Dosch et al., 2004; Kloc et al., 2004; Marlow, 2010; Marlow and Mullins, 2008). In zebrafish oocytes, Bb assembly in primary oocytes [stage Ia (zygotene), Ib (diplotene of meiosis I)] requires Buc (Bontems et al., 2009; Marlow and Mullins, 2008) and its disassembly in stage II oocytes requires Magellan (Mgn; Macf1 - ZFIN), a microtubule-actin crosslinking factor (Gupta et al., 2010). Proper regulation of Bb development is essential to establish the animal-vegetal axis and deliver RNAs and proteins to the vegetal pole. Three pathways that localize RNAs are known in vertebrate oocytes: transit through the Bb pathway, utilization of the ‘late vegetal pathway’, and an animal pole transport pathway (Abrams and Mullins, 2009; Gagnon and Mowry, 2011; Kloc et al., 2001; Kloc and Etkin, 1995; Kloc and Etkin, 2005; Kloc et al., 1998; Marlow, 2010; Zhou and King, 2004). Mutations that ablate the Bb (buc) (Bontems et al., 2009; Marlow and Mullins, 2008) or block its disassembly (mgn) (Gupta et al., 2010) disrupt localization of mRNAs along the animal-vegetal axis. The resulting eggs lack animal-vegetal polarity (Bontems et al., 2009; Marlow and Mullins, 2008). In zebrafish, asymmetry is also evident in the fates of the somatic follicle cells. At the animal pole of WT oocytes a single somatic cell forms the micropyle, a channel on the eggshell required for fertilization. buc mutant eggshells have excess micropyles, which leads to polyspermy (Marlow and Mullins, 2008).
Nonsense mutations disrupting buc uncovered a role for Buc protein in promoting Bb assembly, but the regulation and function of buc during Bb assembly are not understood. Although Buc protein lacks identifiable functional domains, the dynamic localization of buc gene products in the Bb and later at the animal pole cortex (Bontems et al., 2009) suggests that localizing buc mRNA might be an important aspect of Buc regulation. Transcripts of the Xenopus homolog of the buc gene, Xvelo, localize to the oocyte vegetal pole and the relevant cis-acting elements in the Xvelo 3′UTR are known (Claussen and Pieler, 2004; Mowry and Melton, 1992). buc mRNA is not properly localized in buc mutants (Bontems et al., 2009; Marlow and Mullins, 2008), but it is not known if defective buc mRNA localization reflects a direct role of Buc protein in localizing its transcript or an indirect effect due to absence of the Bb and oocyte polarity.
RNA-binding proteins (RNAbps), which can localize RNAs and regulate their spatial and temporal translation or stability, are attractive candidate regulators of buc localization and/or Buc protein activity. Indeed, several RNAbps, or their RNAs, localize to the Bbs of zebrafish and frogs (Draper et al., 2007; Kloc et al., 2000; Kosaka et al., 2007; Kroll et al., 2002; Marlow and Mullins, 2008; Song et al., 2007; Zhao et al., 2001). Deleted in azoospermia-like (Dazl) is a conserved RNAbp required for germ cell differentiation and survival (Hashimoto et al., 2004; Houston and King, 2000; Houston et al., 1998; McNeilly et al., 2000; Ruggiu et al., 1997; Saunders et al., 2003). In Xenopus and zebrafish, dazl transcripts localize to the Bb and later remain at the vegetal pole (Bontems et al., 2009; Chang et al., 2004; Kloc et al., 2001; Kosaka et al., 2007; Maegawa et al., 1999; Marlow and Mullins, 2008).
Like Dazl, RNA binding protein with multiple splice isoforms 2 (Rbpms2; also known as Hermes) is a conserved Bb-localized RNAbp (Kosaka et al., 2007; Song et al., 2007; Zearfoss et al., 2004). Rbpms2 colocalizes with and binds germ plasm RNAs (Kosaka et al., 2007; Song et al., 2007) and has been postulated to maintain their translational repression. Rbpms2 and dazl are not localized in zebrafish buc mutants (Bontems et al., 2009; Marlow and Mullins, 2008; Nojima et al., 2010). However, it has not been determined whether Buc specifies the site of Bb assembly or participates in recruiting proteins and RNAs to the Bb via indirect or direct interaction.
Here we show that endogenous Buc protein is asymmetrically localized in oocytes at stages before formation of the Bb, where Buc later localizes; thus, Buc is the earliest marker of oocyte polarity in zebrafish. Using a transgenic approach, we found that the buc introns are required for full rescue of the egg polarity and axis defects of bucp106/p106 mutant females. As with other localized mRNAs, the buc 3′UTR harbors predicted recognition sites for RNAbps. Transgenes encoding the full-length protein and 3′UTR without introns (cbuc) cause ectopic Bb formation, whereas intron-lacking versions of buc with a truncated 3′UTR (cbuc80) disrupt animal-vegetal polarity. We show that Rbpms2 binds to buc but not other Bb-localized RNAs, such as dazl. By contrast, Dazl binds buc and dazl RNAs. Because Buc appears asymmetrically localized prior to localization of its RNA, we postulate that a mechanism involving localized translation or stabilization of Buc generates asymmetry and allows recruitment of mRNAs via interactions between Buc and RNAbps, such as Rbpms2. Our results indicate that establishing oocyte polarity in zebrafish relies on precise regulation of Buc levels and activity, possibly by a mechanism that requires buc introns. Our findings suggest that Buc initiates a positive-feedback mechanism whereby local production and/or stabilization of Buc protein allows recruitment of more buc RNA and, in turn, production of more Buc protein.
RESULTS
Asymmetric localization of Buc protein prior to Bb formation
To determine when and where endogenous Buc protein first appears during oocyte development, we generated anti-Buc antibodies. Endogenous Buc protein localized to the Bb in wild-type (WT) primary oocytes (Fig. 1A,A′,C-F; data not shown); no localized Buc protein was detected in bucp106/p106 mutant oocytes (Fig. 1B,B′) or when primary antibody was omitted (not shown). Bb localization is consistent with the essential function of Buc in forming this asymmetric oocyte structure. To investigate whether asymmetric Buc protein might precede Bb formation and provide an early marker of oocyte asymmetry, we examined earlier stages of oogenesis. We detected asymmetrically enriched perinuclear Buc protein at pre-Bb stages (Fig. 1G,G′), indicating that Buc protein and zebrafish oocytes are polarized before Bbs are detectable.
Fig. 1.
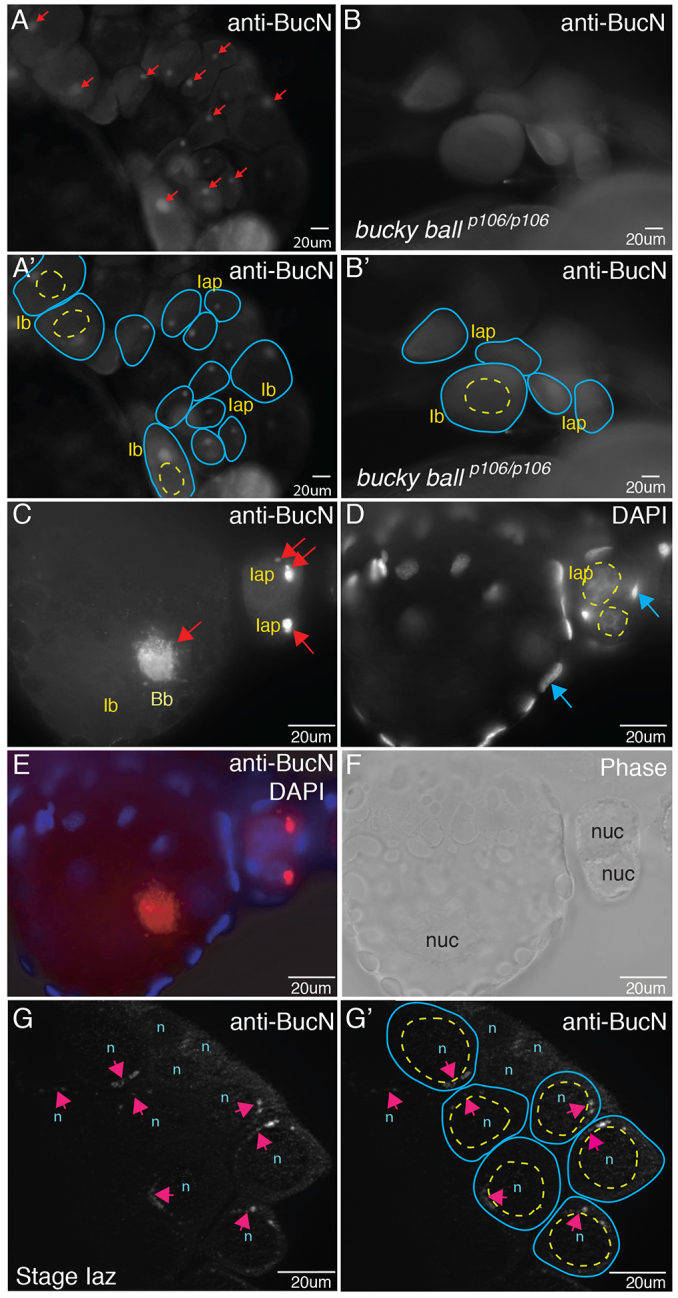
Buc protein localizes to Balbiani bodies (Bbs) and is asymmetric before Bb formation. (A,A′,C) Buc protein localization in Bbs (red arrows in A,C) of stage Iaz (zygotene), Iap (pachytene) and Ib (larger than Ia, arrested in diplotene) WT oocytes in whole-mount ovaries stained with anti-Buc antibodies. Buc protein is not detected in bucp106/p106 mutants (B,B′). (A′,B′) Tracings of the oocytes (blue lines) and their nuclei (yellow dashed lines) from A and B. (D) DAPI-labeled nuclei of oocytes (yellow dashed circles) and follicle cells (blue arrows). (E) Merge of C and D. (F) Corresponding phase image to E. (G) Perinuclear localization of Buc (pink arrows) in WT stage Iap oocytes before Bb formation. (G′) Tracing of oocytes (blue lines) and nuclei (yellow lines). n/nuc, nucleus.
Intron-containing buc transgenes rescue egg polarity phenotypes of buc mutants
Zebrafish maternal-effect mutants revealed that Buc protein is essential for Bb formation, localization of buc and other Bb mRNAs, and animal-vegetal (AnVg) axis formation (Bontems et al., 2009; Dosch et al., 2004; Marlow and Mullins, 2008; Nojima et al., 2010). To analyze the regulation and function of buc, we identified buc promoter sequences that recapitulate buc expression (supplementary material Fig. S1A,B) and determined that transgenic reporters under control of the buc promoter are expressed in early oocytes (supplementary material Fig. S1C-E).
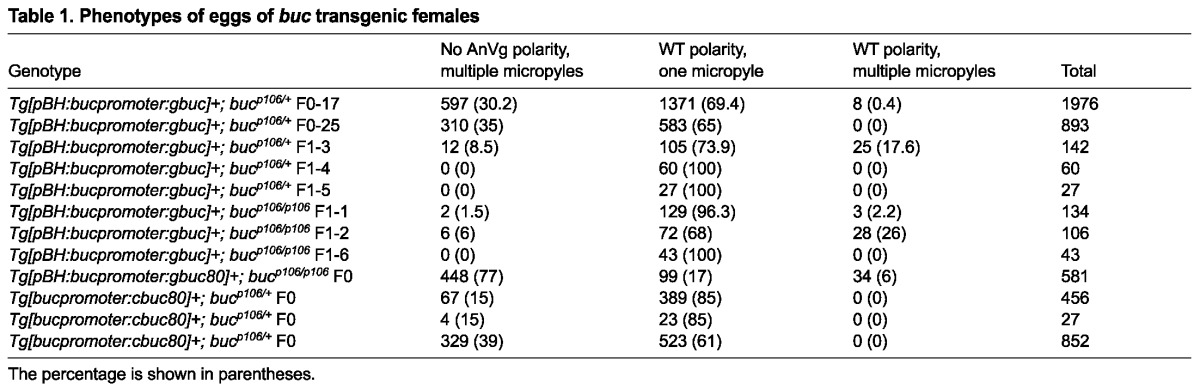
We cloned a minigene including all introns (gbuc) and verified that RNA from the transgene was properly spliced by RT-PCR, sequence analysis and expression assays at stages when endogenous buc transcripts were not detectable (supplementary material Fig. S2). To facilitate rescue and structure-function analysis in buc mutants, we generated transgenic lines expressing gbuc and various mutant derivatives in buc heterozygotes (Fig. 2A-C). Only the gbuc transgene rescued AnVg egg polarity and micropylar numbers in progeny of bucp106/p106 mutants (Table 1, Fig. 2D-I). Some embryos with rescued egg polarity had multiple micropyles (two to four on one side), indicating incomplete rescue (Fig. 2E). Sixty-three percent of rescued progeny were viable at 1 dpf and 92% of those showed normal morphology (Fig. 2F-H). As anticipated for a maternal-effect gene, only half of these rescued progeny expressed the zygotic bleeding heart reporter at 2 dpf, indicating that maternally supplied gbuc rescued egg polarity in zygotes that did not inherit the transgene (supplementary material Fig. S3). gbuc constructs with truncated 3′UTRs (gbuc80) also rescued AnVg polarity, indicating that the remaining 3′UTR sequence was sufficient to rescue egg polarity (Table 1); however, only 18% of gbuc80 rescued embryos were viable at 1 dpf and, of those, 36% were ventralized, indicating rescue was incomplete and that 3′UTR sequences may contribute to patterning (Fig. 2I; supplementary material Fig. S3).
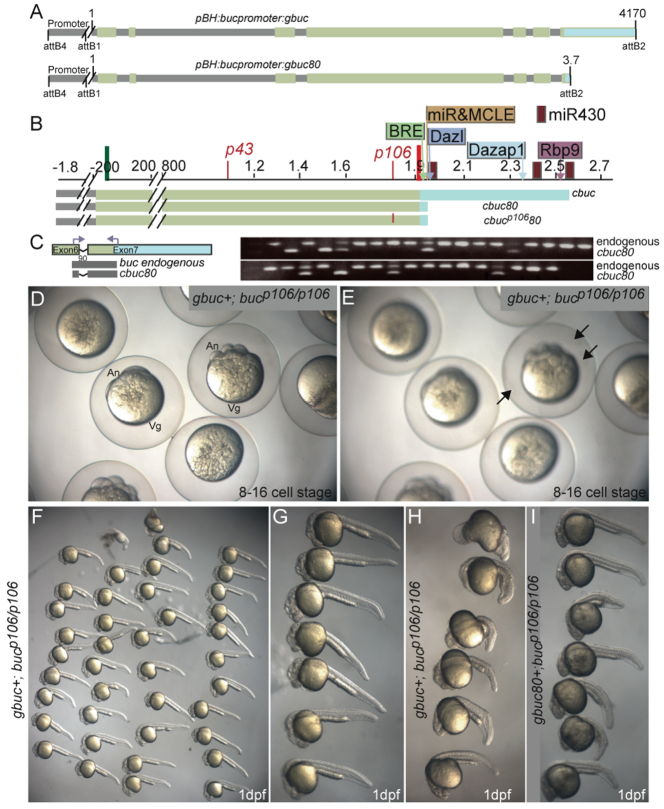
Fig. 2.
buc transgenes with introns rescue buc egg polarity phenotypes. (A,B) buc gene structure and the constructs used herein. (A) The buc promoter was used to express full-length buc or buc with a truncated 3′UTR. (B) The full-length buc ORF containing the full 3′UTR (cbuc) or a truncated buc 3′UTR (cbuc80) without introns: grey, non-coding/intron; green, exon; light blue, 3′UTR. In addition, a mutant Buc protein with the bucp106 nonsense mutation was generated (cbucp10680). (C) Schematic and genotyping assay. Products from genomic DNA are 90 bp smaller in transgenes lacking introns. Gel images of products from adult F1 progeny of cbuc80 founders. (D,E) 8- to 16-cell stage F2 progeny of a gbuc rescued mutant in different focal planes. Arrows in E indicate the excess micropyles on embryos with rescued egg polarity. (F) Clutch of gbuc rescued mutant female at 1 dpf. (G) Higher magnification of embryos from F. (H) Ventralized phenotypes of gbuc+ mutant females. (I) Rescued progeny of a gbuc80 buc mutant founder. (F-I) Rostral is left and caudal is right. (D-H) Progeny of F1 transgenic mothers. An, animal; Vg, vegetal.
Table 1.
Phenotypes of eggs of buc transgenic females
buc transgenes disrupt egg polarity
Heterozygosity for the bucp106 allele alone does not disrupt egg polarity (n>1900 eggs; 44 females) (Bontems et al., 2009; Marlow and Mullins, 2008). Females that were WT or heterozygous bucp106/+ and positive for gbuc or transgenes lacking introns (cbuc and cbuc80) produced two classes of progeny (Table 1, Fig. 3). One class exhibited normal AnVg polarity, as indicated by cytoplasm at the animal pole (Fig. 3A,C) and a single micropyle on their eggshells as observed in WT (Fig. 3D). The second class resembled buc mutants (Fig. 3B,C,E,I-K). Specifically, cytoplasm was detected around the circumference of these eggs (Fig. 3B,L,M) and their eggshells had excess numbers of micropyles (Fig. 3E). Chorion elevation and egg size were comparable to WT, indicating that these aspects of egg activation were normal (Fig. 3E,I-K). Polyspermy was evident in eggs with excess micropyles based on DAPI staining (data not shown). These data indicate that proper levels of Buc are required for normal egg polarity.
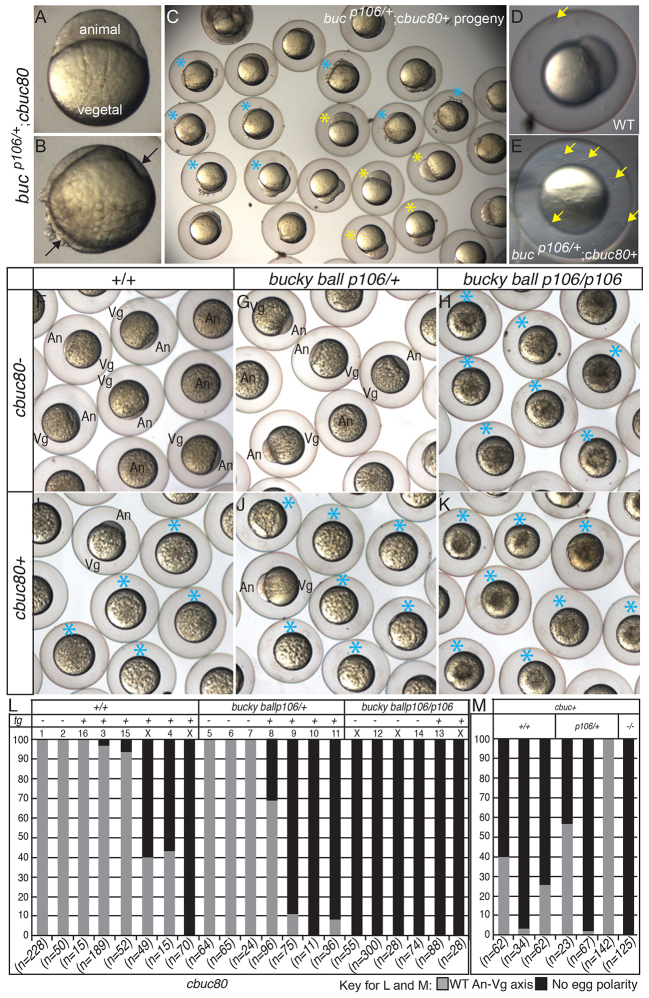
Fig. 3.
buc transgenes without introns disrupt egg polarity and follicle cell fates. (A-K) Dissecting microscope images. (A-E) The sibling in A shows normal egg polarity at high stage, whereas cytoplasm (black arrows) around the circumference of siblings in B indicates lack of egg polarity. (C) cbuc80 transgenic progeny resemble WT (yellow asterisks) or lack polarity (blue asterisks); high stage. (D) WT eggs (yellow arrow) have a single micropyle, whereas (E) there are multiple micropyles on the eggshells of progeny lacking egg polarity (n=1439 eggs), but not on eggs with polarity (n=2349). Homozygous WT (F) or buc/+ heterozygous (G) females lacking the cbuc80 transgene produce progeny with normal AnVg polarity, whereas (I,J) sibling WT and buc/+ females with cbuc80 produce progeny without AnVg polarity (blue asterisks). (H) buc mutants lack AnVg polarity (K) even when cbuc80 is present. (L) Quantification of phenotypes according to genotype and transgene status. Each bar represents individual F1 or F2 females. The numbers correspond to the gel in supplementary material Fig. S4. X, not in gel. (M) Quantification of egg phenotypes of cbuc progeny of F1 females.
Disrupting oocyte polarity causes excess micropylar cells
We conducted histological examination of the cbuc transgenic ovaries to assess oocyte polarity. Morphologically, females expressing transgenes lacking introns were normal and their ovaries were composed of oocytes of all stages. As predicted based on their eggs, cortical granule distribution and yolk accumulation in advanced stage oocytes were normal (Fig. 4A,B). However, consistent with the excess micropyles of eggs with defective polarity, we observed two populations of advanced stage oocytes, those with one somatic micropylar cell positioned at the animal pole (Fig. 4Aa) and those with multiple micropylar cells (Fig. 4Bb1,b2). The micropyle and egg polarity phenotypes were coincident in females expressing cbuc and cbuc80. No Buc protein expression was detected in the follicle cell layer of bucp106/+ females negative or positive for cbuc transgenes (supplementary material Fig. S5). Furthermore, the Tg[buc:mApple] promoter reporter showed mApple fluorescence only in oocytes (supplementary material Fig. S1C-E′′). These results indicate that buc in the germline can non-autonomously influence the otherwise ‘wild-type’ somatic cells, either by changing their fate or permitting survival of micropyle progenitors. Because the two classes of oocytes are intermixed within the same ovary in cbuc and cbuc80 transgenics, the oocyte signals that regulate micropyle numbers apparently act at short range, such that one oocyte does not affect the mycropylar cells associated with neighboring oocytes.
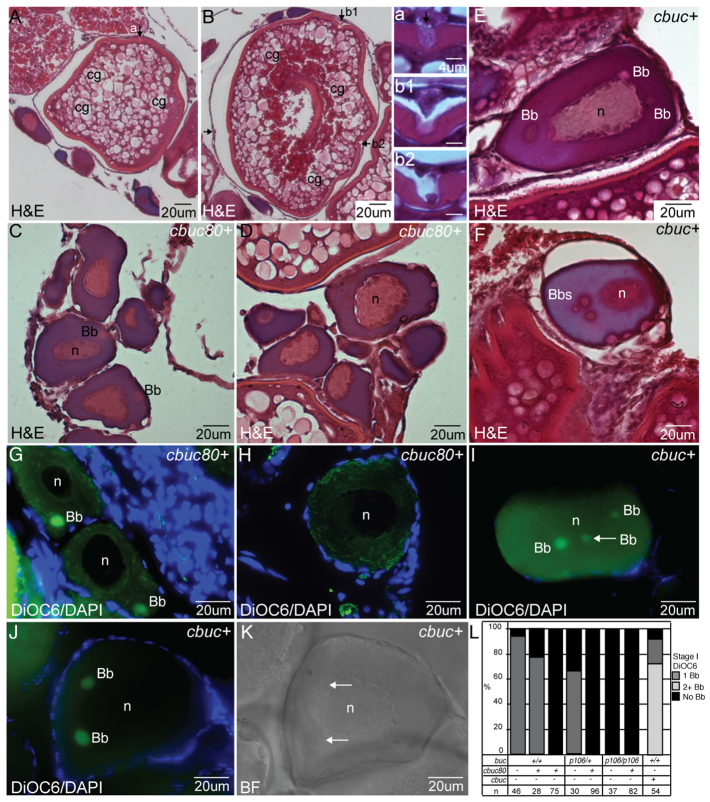
Fig. 4.
Defective Bb formation and excess polarized somatic fates in transgenics lacking introns. (A-D) Oocytes from cbuc80 transgenic founders. (A-F) Hematoxylin and Eosin (H&E)-stained F0 ovary sections reveal a normal composition of oocytes, including (A) stage III oocytes with single micropylar cells (arrow and a1), (B) stage III oocytes with multiple micropylar cells (arrows and b1 and b2), (C) stage I oocytes with Bbs and (D) stage I oocytes lacking Bbs. (E,F) Ectopic Bbs of cbuc+ F1 females. (G-J) DiOC6 staining of sectioned ovaries. cbuc80 F1 ovaries reveal primary oocytes with (G) and without (H) Bbs. (I,J) Ectopic Bbs of cbuc+ F1 females. (K) BF view of oocyte in J. Arrows indicate Bbs. (L) Quantification of Bbs from different individual F1 transgenic females labeled with DiOC6. Cg, cortical granules; n, nucleus.
Dominant phenotypes of buc transgenes
To further investigate whether cbuc transgenes disrupt Bb development like buc mutants, we examined mitochondria and endoplasmic reticulum (ER) in oocytes of cbuc transgenic females. We observed three categories of stage Ib oocytes in WT females expressing cbuc: normal, supernumerary Bbs, and those lacking Bbs (Fig. 4E,F). Moreover, mitochondria and ER were detected in the Bbs and excess Bbs of stage Ib oocytes (Fig. 4G-L) or were broadly distributed (Fig. 4H; supplementary material Fig. S6), as in buc mutants. By contrast, no ectopic Bbs were detected in cbuc80 females (Fig. 4C,D). Together, these data indicate that excess Buc disrupts polarity and, in a 3′UTR-dependent manner, causes supernumerary Bbs.
buc transgenes disrupt the localization of buc and other Bb mRNAs
To determine whether transgenes lacking introns prevented expression of endogenous buc, we used a qRT-PCR approach to distinguish endogenous buc from transgenic transcripts. As anticipated, both endogenous and cbuc80 transcripts were present in transgenic ovaries and their progeny and transgene expression correlated with the penetrance of egg polarity phenotypes (Fig. 5A). In the most strongly affected cbuc ovaries, both transcripts were reduced (Fig. 5A).
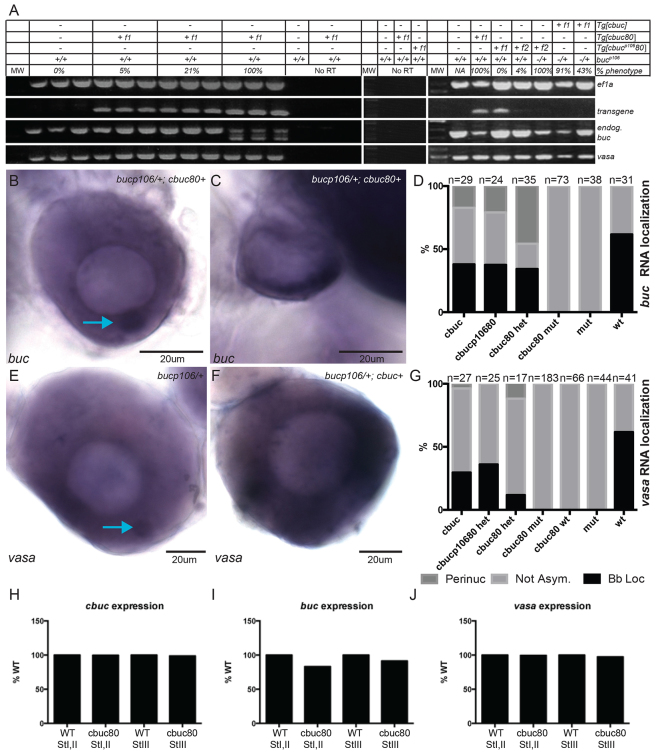
Fig. 5.
buc transgenes lacking introns disrupt RNA localization. (A) RT-PCR on ovary lysate cDNA. Transgenic and endogenous transcript expression in ovaries of F1 transgenic females expressing intron-lacking transgenes in bucp106/+ heterozygotes. (B,C) buc RNA in ovaries of cbuc80+;bucp106/+ F1 transgenic females (B) resembles WT or (C) is not asymmetrically localized. (D) Quantification of buc RNA expression patterns in stage I oocytes. (E,F) vasa expression in (E) F1 transgenic- and (F) cbuc80 F1 transgenic+ ovaries. (B,E) Blue arrows indicate the Bb. (G) Quantification of vasa expression patterns. (H-J) Relative expression of (H) cbuc80, (I) endogenous buc and (J) vasa in sorted oocytes of F1 or F2 females. (D,G) het, bucp106/+; mut, bucp106/p106; wt, buc+/+.
The cbuc80 construct contained only the first 80 bp of the 3′UTR (Fig. 2B). This truncated 3′UTR retained predicted miR-302/371-373/miR430 sites and lacked putative regulatory or protective RNAbp sites (Fig. 2B). To determine if cbuc transgenes affect RNA localization we examined buc transcript localization by whole-mount in situ hybridization using a probe that detects both endogenous and transgenic buc transcripts. Consistent with the RT-PCR analysis, buc transcripts were present in transgenic ovaries. As anticipated based on the lack of a detectable Bb, buc, vasa and other RNAs were not asymmetrically localized in cbuc80 oocytes lacking polarity (Fig. 5B-G; data not shown), a phenotype reminiscent of buc mutants (Bontems et al., 2009; Marlow and Mullins, 2008). We performed qRT-PCR on sorted oocytes and determined that cbuc80 and endogenous buc transcripts were present throughout Bb dispersal (Fig. 5H,I). These data indicate that endogenous buc mRNA was not maintained at late stages as a consequence of failure to properly localize buc transcripts, as occurs in buc mutants.
Functional Buc protein is required for dominant phenotypes
If Buc protein acts primarily to seed the assembly of a polarity complex, we reasoned that excess or mislocalized Buc protein might produce polarity phenotypes similar to buc mutants (Bontems et al., 2009; Marlow and Mullins, 2008). To determine if cbuc phenotypes were due to excess or mislocalized Buc protein, we examined Buc distribution in oocytes of various buc genotypes with and without cbuc or cbuc80. Whereas Buc protein localized to the Bb of heterozygotes lacking cbuc or cbuc80 (Fig. 6A), it was not asymmetric in heterozygotes expressing cbuc80 (Fig. 6B). Homozygous WT and heterozygous females (Fig. 6C) showed Buc protein within the Bb of nearly all primary oocytes examined. By contrast, stage Ib oocytes in WT or heterozygous bucp106/+ females expressing cbuc80 lacked asymmetric Buc protein and resembled bucp106/p106 mutant oocytes, which lacked polarity whether positive for cbuc80 or not (Fig. 6C).
Fig. 6.
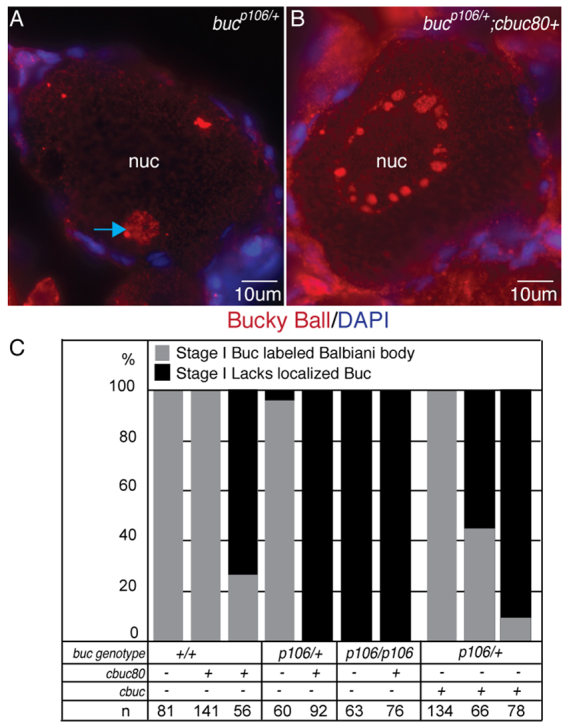
Antimorphic activity of buc transgenes lacking introns requires functional protein and disrupts endogenous Buc. (A,B) Sectioned and stained oocytes. Buc protein localizes to the Bb (blue arrow) of (A) bucp106/+;cbuc80-stage Ib oocytes, and is not asymmetric in (B) bucp106/+;cbuc80+ stage Ib oocytes. (C) Quantification of Bbs of Buc-labeled oocytes of F1 or F2 females. n, the number of oocytes examined.
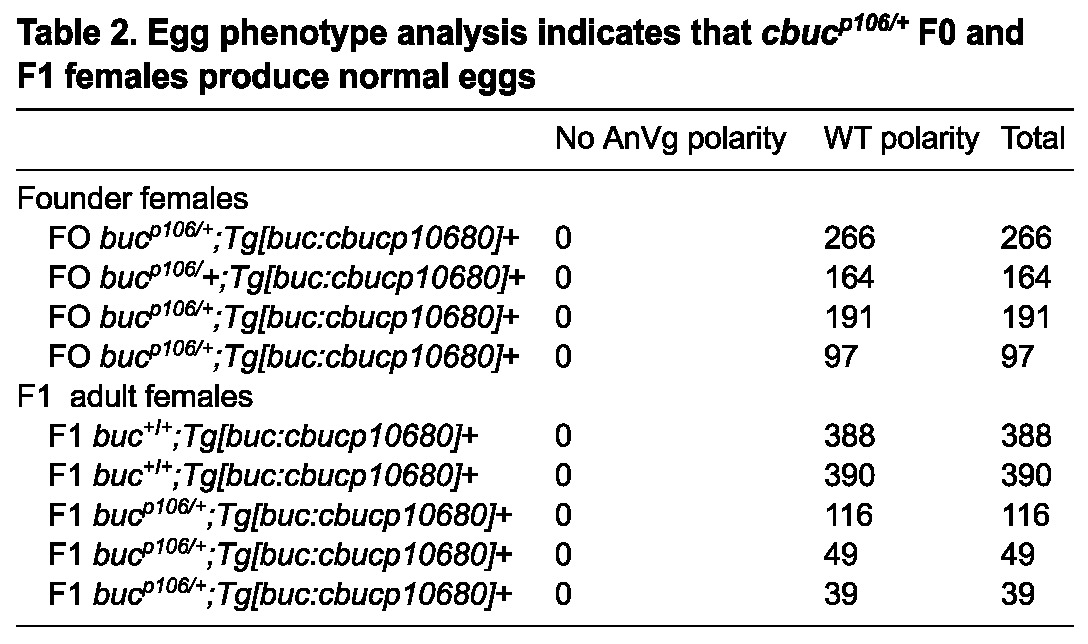
To functionally assess whether ectopic Buc protein or its mRNA disrupted polarity, we analyzed females with an analogous transgene containing the bucp106 nonsense mutant allele (cbucp10680) (Fig. 2B, Table 2). Significantly, this nonsense mutation does not cause phenotypes in bucp106/+ heterozygotes (Bontems et al., 2009; Marlow and Mullins, 2008). In contrast to females expressing cbuc80, cbucp10680 F0 and F1 transgenic females produced only progeny with normal egg polarity and single micropyles (Table 2). As for cbuc80, we analyzed cbucp10680 transcripts and found that its RNA was expressed at a comparable level (Fig. 5A). By contrast, bucp106/+ heterozygous transgenic females generated from crosses of two cbucp10680 transgenic F1s produced progeny lacking AnVg polarity (26%; n=1183 eggs, 13 females), whereas their buc+/+ sibling transgenic females infrequently produced progeny lacking egg polarity (2%; n=1021 eggs, 13 females). This indicates that higher copy numbers of cbucp10680 can disrupt AnVg polarity. Taken together, these data indicate that the dominant polarity phenotypes of cbuc80 were due to a functional coding sequence for Buc protein in the transgene.
Table 2.
Egg phenotype analysis indicates that cbucp106/+ F0 and F1 females produce normal eggs
Buc protein interacts with conserved germ plasm-associated RNAbps
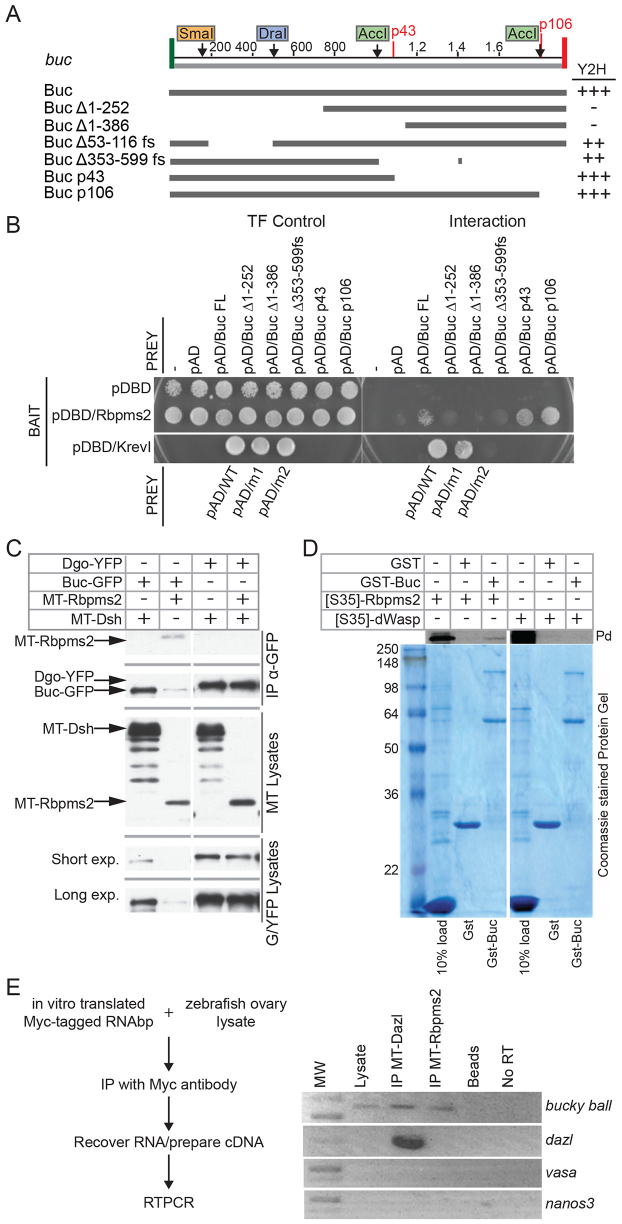
Previous studies have shown that buc mRNA localizes to the Bb and an exogenous GFP-Buc fusion expressed from RNA injected into oocytes accumulates in the Bb (Bontems et al., 2009). Here we show that endogenous Buc protein localizes to the Bb (Fig. 1) via a mechanism that is likely to involve local production or stabilization of Buc protein, because endogenous buc transcripts are not asymmetric before Bb formation or in buc mutants (Bontems et al., 2009). The lack of Bbs in buc mutants demonstrates that Buc is required to assemble this conserved aggregate of RNAs and proteins, but the mechanism of Buc action is not known. One potential mechanism is that Buc mediates Bb assembly and RNA localization by interacting with RNAbps. Rbpms2 is a conserved RNAbp that contains two RNA recognition motif (RRM) domains (Gerber et al., 1999; Kosaka et al., 2007; Song et al., 2007; Zearfoss et al., 2004). Rbpms2 protein localizes to the Bbs of oocytes in zebrafish (Kosaka et al., 2007; Marlow and Mullins, 2008) and frogs (Zearfoss et al., 2004) and is not localized in buc mutants (Marlow and Mullins, 2008). Based on their localization, we hypothesized that Buc might bind to Rbpms2, which could then, via its RNA-binding motifs, recruit or retain other Bb RNAs (such as buc mRNA). To test this possibility, we performed yeast two-hybrid (Y2H) experiments. Indeed, full-length Buc (Buc-Fl) interacted with zebrafish and human Rbpms2, but not with another RNAbp, DAZ associated protein 1 (Dazap1) (Fig. 7A,B; data not shown) (Claussen and Pieler, 2004; Kurihara et al., 2004).
Fig. 7.
Buc protein interacts with the RNAbp Rbpms2. (A) Summary of buc deletion constructs used in the yeast two-hybrid analyses in B. Buc interacts with Rbpms2 via the N-terminus of Buc. The transformation (TF) control plates select for bait (pDBD) and prey (pAD) plasmids, whereas the interaction plates select for binding between the bait and prey proteins. Control baits and preys were KrevI and a strongly interacting prey Ral/GDS (wt) and two mutants: Ral/GDSm1 (moderate/medium interaction with Krev) and Ral/GDSm2 (weak/no interaction with KrevI). (C) Rbpms2 co-immunoprecipitates with Buc in HEK293 lysates. Top panels indicate transfected plasmids. Long exposure reveals proteins with lower expression levels. (D) Pull-down assay with GST- and 35S-labeled GFP fusion proteins. (pd, pull down). GST fusion protein inputs were visualized with Coomassie Blue. (E) RNA IP experiments using in vitro synthesized Myc-tagged RNAbps. The RNAs that co-immunoprecipitated with Myc-tagged RNAbps were amplified by RT-PCR. MT-Rbpms2 immunoprecipitated buc but not nanos2, vasa or dazl. MT-Dazl associated with buc and dazl mRNAs.
To confirm the interaction observed in yeast, we transfected HEK293 cells with GFP-Buc or YFP-Diego, an unrelated bait, and Myc-Rbpms2 or Myc-Dishevelled as a control, and conducted co-immunoprecipitation (co-IP) experiments on the HEK293 cell lysates using anti-GFP antibody. We found that Rbpms2 co-immunoprecipitated with GFP-Buc, but not YFP-Diego (Fig. 7C). To independently assess binding, we conducted in vitro GST pull-down assays. We found that GST-Buc fusion protein interacted with 35S-labeled Rbpms2 (Fig. 7D). These approaches validated the interaction between Buc and Rbpms2 that we observed in the Y2H assay and indicate that Buc is likely to directly bind to Rbpms2.
We used the binding interaction between Buc and Rbpms2 to identify potential functional domains of the Buc protein. Using the Y2H assay, we mapped the Rbpms2 binding site using six partially overlapping Buc truncations covering the full protein (Fig. 7A,B; data not shown). Two truncations were N-terminal Buc deletions (BucΔ1-252 and BucΔ1-386), two carried nonsense mutations corresponding to the bucp43 and bucp106 mutant alleles (Bontems et al., 2009), and two harbored internal deletions [BucΔ53-116 frame shift (fs) and BucΔ353-599]. BucΔ1-252 and BucΔ1-386 did not interact with Rbpms2, but the Bucp43 and Bucp106 mutant proteins did. The internal deletions displayed an intermediate binding phenotype in the Y2H assays. Together, these data indicate that the Buc N-terminus mediates binding to Rbpms2 and that the internal region of the protein either augments or stabilizes this interaction. Rbpms2 has been previously reported to interact with germ plasm components in Xenopus (Song et al., 2007). Colocalization of Buc and Rbpms2 in the Bb along with germ plasm RNAs and the interaction between Buc and Rbpms2 suggest a mechanism whereby Buc interaction with Rbpms2 could recruit buc and other Bb RNAs.
Two Bb-localized RNAbps, Dazl and Rbpms2, bind buc RNA
Colocalization of Buc and Rbpms2 in the Bb along with germ plasm RNAs and the direct interaction between Buc and Rbpms2 suggest a mechanism whereby Buc interaction with Rbpms2 recruits RNAs to the Bb. To explore this hypothesis, we investigated whether Rbpms2 and another Bb RNAbp that associates with germline RNAs in primordial germ cells, Dazl (Kosaka et al., 2007; Takeda et al., 2009), bind to Bb RNAs in oocyte lysates using Myc-tagged Rbpms2 (MT-Rbpms2) and Myc-tagged Dazl (MT-Dazl) as baits (Fig. 7E). Among the ovary transcripts examined in the IP between MT-Rbpms2, we only detected buc (Fig. 7E). buc was also detected from IP with MT-Dazl. In addition to detecting buc in association with MT-Dazl, we also detected dazl, which did not bind Rbpms2 or beads alone (Fig. 7E). These results indicate that Rbpms2 and Dazl bind to some Bb mRNAs and might mediate their recruitment to the Bb.
DISCUSSION
The vertebrate AnVg axis is established during oogenesis. Oocyte polarity is a prerequisite for determining the prospective embryonic axes and setting aside the germ cell determinants in some non-mammalian vertebrates (Abrams and Mullins, 2009; Marlow, 2010; Mir and Heasman, 2008; Schier and Talbot, 2005). The Bb is an evolutionarily conserved asymmetric structure present in early oocytes of all animals examined, including humans. Our study identifies Buc protein, an essential regulator of the AnVg axis, as the earliest marker of oocyte polarity in zebrafish. Discrete localization of endogenous Buc protein and overexpression phenotypes caused by transgenes with intact open reading frames indicate that spatial or temporal regulation of Buc translation or stabilization is essential for oocyte polarity. Interestingly, we also find a non-autonomous role of the germline in limiting somatic follicle cell fates, pointing toward communication between the germline and somatic cells. Further, we identify interactions with the RNAbps Rbpms2 and Dazl and buc products. Buc protein and buc RNA bind Rbpms2, whereas buc and dazl RNAs bind to another Bb component, Dazl. Thus, interactions with RNAbps might play key roles in regulating Buc activity and establishing oocyte polarity in zebrafish.
Regulation of buc RNA is likely to generate asymmetric Buc protein localization and trigger Bb assembly
Endogenous Buc protein is localized asymmetrically before Bb assembly and later is localized to the Bb. At the stages when asymmetric perinuclear Buc protein is detected, its transcripts remain broadly distributed, indicating that local stabilization of Buc or selective translation of its mRNA, rather than global redistribution of buc RNA, is likely to initiate asymmetric Buc. Mechanisms to generate asymmetric protein localization include localized translation, local stabilization and aggregation, and active transport of a protein to a specific subcellular location (Gagnon and Mowry, 2011; Hachet and Ephrussi, 2001; Holt and Bullock, 2009; Kloc and Etkin, 2005; Kugler and Lasko, 2009; Minakhina and Steward, 2005; St Johnston, 2005; Zhou and King, 2004). If Buc protein were uniformly produced and then transported to a perinuclear position, we would expect to initially detect Buc protein throughout the oocyte followed by progressive enrichment adjacent to the nucleus. Our data argue against such a model, although we cannot exclude that Buc is present throughout the cell at levels below our detection. Moreover, asymmetric localization of Buc protein in WT and the loss of oocyte polarity caused by the buc transgenes seem more consistent with a mechanism that involves localized translation or stabilization of Buc protein. According to the localized stabilization scenario, broadly produced Buc protein would be rapidly degraded except near the nucleus where it accumulates. Alternatively, or in addition, the initially ubiquitous endogenous buc mRNA could be translationally repressed via association with RNAbps except near the nucleus, where a limiting or localized factor(s) would alleviate repression of buc RNA. Such a mechanism would explain the gain-of-function phenotypes that we observe in WT (buc+/+; bucp106/+) genotypes expressing gbuc and cbuc transgenics. This would also be consistent with the potentially dominant-negative phenotypes caused by high doses of cbucp10680, which might result from titration of a limiting repressor and premature translation of endogenous buc analogous to the mechanisms reported for oskar mRNAs harboring stop codons (Zimyanin et al., 2007). The identity of the protein(s) regulating buc translation remains to be determined. Vasa is a compelling candidate, as it is known to promote translation in the female germline of flies (Carrera et al., 2000; Lasko and Ashburner, 1988; Markussen et al., 1995; Styhler et al., 1998) and Vasa protein is perinuclear in early stage zebrafish oocytes (Knaut et al., 2000). In WT oocytes, however, Vasa protein is not localized to the Bb in zebrafish (Knaut et al., 2000), suggesting that other, yet-to-be-identified proteins would regulate Buc translation within the Bb.
Oocyte polarity and follicle cell fate
In WT oocytes there is a single animal pole where only one micropyle develops. In buc mutants, expanded or ectopic animal poles, as evidenced by multiple domains of the animal pole marker vg1 (igf2bp3 - ZFIN) (Marlow and Mullins, 2008), support development of excess micropylar cells, indicating that the animal pole environment might provide an instructive or permissive cue for micropylar cell survival or fate. Notably, we observed eggs with WT polarity and two to four micropyles on one half of the eggshell of some gbuc rescued mutant eggs. In cbuc80 and cbuc transgenic eggs without polarity, we always observed supernumerary micropyles. Similarly, all eggs from cbuc80 and cbuc transgenic females with normal polarity had only one micropyle. Concordance between egg polarity and micropyle phenotypes and the observation that follicle cells express no detectable Buc protein are consistent with a model whereby local oocyte signals regulate micropyle cell fate. The nature of the communication is not clear, but it is likely to involve a close-range signal, possibly direct cell contact, rather than a broadly diffusible signal, because oocytes with multiple micropyles can be adjacent to those with one micropylar cell.
A self-organizing mechanism to recruit RNAs to the Bb
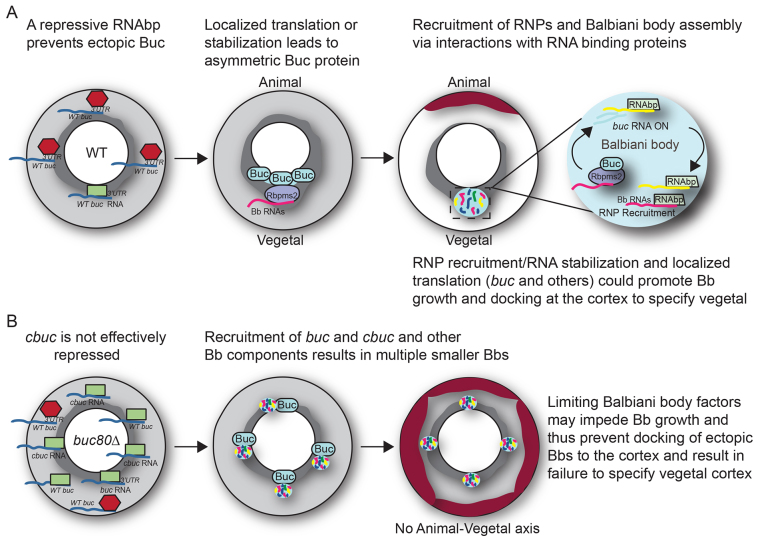
Buc protein is essential for RNA localization along the zebrafish AnVg oocyte axis, including for the earliest RNAs, which localize to the Bb near the prospective vegetal pole (Bontems et al., 2009; Marlow and Mullins, 2008; Nojima et al., 2010). Buc harbors no known RNA-binding motifs, but our analysis supports the possibility that Buc exerts its effects on RNA by serving as a scaffold and assembly factor for RNAbps. We identified a novel interaction between Buc protein and a conserved Bb-localized RNAbp Rbpms2 (Kosaka et al., 2007; Song et al., 2007). Interaction between Buc and Rbpms2 requires the N-terminus of Buc protein. Thus, our work defines a potential functional domain of Buc protein and, intriguingly, a potential mechanism by which Buc could recruit RNAs to the Bb. Our data suggest that Buc might promote oocyte polarity by interacting with RNAbps to direct the assembly of ribonucleoprotein (RNP) complexes to form the Bb (Fig. 8).
Fig. 8.
A feedback amplification model for Bb assembly. (A) Model depicting how Buc might promote oocyte polarity and Bb formation via interactions with RNAbps. (B) Potential events in cbuc transgenics. We hypothesize that endogenous buc transcripts are loaded with a repressor prior to nuclear export. Since cbuc RNA is not spliced, the repressor is not loaded and cbuc transcripts are translated ectopically or prematurely. Alternatively and independently of splicing, a limiting repressor might be overwhelmed by excess buc transcripts in transgenic oocytes. Either way, ectopic foci of Buc protein would recruit Bb components and produce the multiple small Bbs observed with DiOC6 and H&E. In cbuc80 transgenics, we hypothesize that either cbuc80 transcripts are not recruited or might not be translated as efficiently due to their lack of a 3′UTR. Consequently, local concentrations of Buc protein may not be sufficient to support the development of ectopic Bbs. Based on their smaller size, their perinuclear proximity and the eventual egg polarity defects, we hypothesize that the ectopic Bbs of cbuc transgenic oocytes do not reach the cortex, resulting in failure to specify the vegetal pole and a lack of animal-vegetal polarity.
Many Bb-localized RNAs contain a mitochondrial cloud localization element (MCLE) site, which is sufficient to direct RNAs to the Bb in Xenopus oocytes (Kosaka et al., 2007). The Xenopus MCLE includes six copies of a hexamer sequence, but zebrafish buc RNA apparently has only one copy, as is also the case for zebrafish nanos3 and dazl. Although the single hexamer sequence is present in cbuc80, this region is not sufficient for proper regulation and localization of buc. Although several RNAs, predominantly germ plasm RNAs, localize to the Bb of early oocytes, these RNAs occupy distinct cellular compartments in late stage oocytes. For example, transcripts of nanos, vasa, buc, dazl and dorsal axis regulators including syntabulin and wnt8a all localize to the Bb of stage I oocytes (Bontems et al., 2009; Draper et al., 2007; Knaut et al., 2000; Kosaka et al., 2007; Lu et al., 2011; Nojima et al., 2010), but by stage III buc transcripts are localized to the animal pole, nanos is distributed throughout the oocyte, vasa is circumferential at the cortex, and dazl and syntabulin remain at the vegetal pole. For many germ plasm RNAs examined in zebrafish oocytes, the 3′UTR is sufficient to achieve their proper localization (Kosaka et al., 2007).
Similar to the germ plasm RNAs that have been examined, patterning molecules, including wnt8a and syntabulin, localize to the Bb. syntabulin is localized by a Buc-dependent pathway. The full exon-intron structure of syntabulin is required for its proper localization and activity (Nojima et al., 2010). Like syntabulin, buc RNA localizes to the Bb (Bontems et al., 2009) and we show that full rescue of the buc mutant phenotypes requires the buc introns. Notably, incompletely rescued buc mutants are ventralized, possibly due to incomplete rescue of dorsal determinant localization in these oocytes. In Drosophila oocytes, the exon junction complex (EJC) has been proposed to regulate Gurken (TGFα) signaling between posterior follicle cells and the oocyte during axis formation, and to mediate oskar mRNA localization and translation during germ cell determination (Hachet and Ephrussi, 2001; Micklem et al., 1997; Mohr et al., 2001; Newmark and Boswell, 1994). Our finding that buc minigene constructs must have introns to rescue buc mutants, together with previous studies of syntabulin (Nojima et al., 2010), indicate that the EJC might be involved in Buc-mediated Bb development and transport.
Although it is not clear how RNAs are directed or selected for Bb localization, it is clear for those RNAs that have been examined that early localization to the Bb is prerequisite for their proper localization at later stages of oocyte development (Kosaka et al., 2007). Transport of RNA to the vegetal pole via the Bb has been proposed to involve an entrapment and expansion mechanism (Chang et al., 2004; Wilk et al., 2005). A self-organizing and amplifying Buc/Balbiani complex could facilitate robust recruitment (entrapment) of RNP complexes, including those containing buc. Localized translation of buc could drive further recruitment of RNPs and expansion of the Bb. Buc might associate with a discrete subset of RNAbps that are capable of forming multiple distinct RNPs. Alternatively, Buc could recruit all Bb-localized RNAs/RNPs, which could then be sorted within the Bb. Either mechanism would be sufficient to recruit the diversity of RNPs that are anticipated to comprise the Bb based on the unique mechanisms (3′UTR versus splicing mediated) that generate distinct and dynamic localization patterns of RNAs, including buc, that transit through the Bb in zebrafish.
A Buc-mediated feedback amplification mechanism to establish oocyte polarity
Localized Buc protein and its association with RNAbps provides a mechanism to recruit Bb RNAs, including buc. Based on the localization of Buc protein and the dominant phenotypes of the cbuc transgenes, we hypothesize that an RNAbp could specifically interact with the spliced buc mRNA to prevent ectopic translation of buc transcripts. Alternatively, or in addition, a limiting RNAbp might maintain repression of buc RNA. It is also possible that Buc accumulates via localized stabilization. Either way, after Buc protein accumulates asymmetrically adjacent to the nucleus, it can interact with its binding partners, such as Rbpms2, which could then recruit their cognate RNAs to form the Bb. Once Buc protein initiates Bb assembly, RNAs are recruited to the Bb by interaction with RNAbps that localize there, including Dazl and Rbpms2 (Kosaka et al., 2007; Song et al., 2007). Some RNAs recruited to the Bb, like vasa, may be silenced there [as Vasa protein, in contrast to its mRNA, is not a component of the Bb (Knaut et al., 2000)]. However, others, including buc and dazl, may be translationally activated within the Bb to sustain its development (Bontems et al., 2009; Kosaka et al., 2007). In the case of Buc, this feedback amplification would drive further localized production of Buc protein and expansion of the Bb. The localization and apparent abundance of Buc protein within the Bb, failure to assemble the Bb and recruit buc RNA in buc mutants (Bontems et al., 2009; Marlow and Mullins, 2008), and the protein- and 3′UTR-dependent dominant phenotypes caused by cbuc and cbuc80 transgenes, are consistent with a Buc-dependent recruitment feedback amplification mechanism acting to establish the initial asymmetry in the oocyte (Fig. 8B,C). In this model, buc mRNA is initially present throughout the oocyte, but Buc protein is only translated or stabilized in a small region. Local accumulation of Buc protein establishes the position of the Bb and recruits RNAbps that in turn recruit buc and other RNAs to the Bb. Such a feedback mechanism that recruits buc mRNA and promotes further accumulation of Buc protein and other RNAs and proteins could establish AnVg polarity and lay the foundation for the later forming embryonic axes.
MATERIALS AND METHODS
Antibodies and immunostaining
YenZym (San Francisco, CA, USA) custom antibody service was used to raise rabbit polyclonal antibodies against Buc epitopes: residues 1-15 MEGINNNSQPMGVGQ (Y1165 and Y1166) and residues 602-617 KSIHQQRPRSEYNDY (Y1163 and Y1164).
Ovaries were dissected from adults, fixed in 4% paraformaldehyde (PFA), washed in PBS, dehydrated in methanol, rinsed and stained as described (Marlow and Mullins, 2008). Primary Buc antibodies were diluted 1:500. Secondary antibodies (anti-rabbit Alexa Fluor 488 or 546; Molecular Probes) were diluted 1:500. Vectashield (Vector Labs) containing DAPI was used to label the nuclei.
Mitochondria and ER were visualized by staining with DiOC6 [Molecular Probes, D-273; 0.5 μg/ml in PBS+Tween (PBST):dimethyl sulfoxide] at room temperature followed by PBST washes (Marlow and Mullins, 2008). Images were acquired using a Zeiss Axio Observer inverted microscope equipped with Apotome and a CCD camera.
Plasmid construction, transient assays and transgenesis
The ∼2 kb buc promoter fragment was amplified from genomic DNA (primers in supplementary material Table S1) and cloned into the Gateway pCR8 entry vector (Invitrogen) and sequenced (Macrogen). Gateway adapters were added using the buc 2Kprom attB4 and buc prom attB1R primers (supplementary material Table S1) to generate the p5E-buc promoter.
The buc open reading frame (ORF) was amplified from ovary cDNA using the Invitrogen SuperScript III reverse transcriptase kit with oligo(dT) primers and the primers listed in supplementary material Table S1 to obtain the full-length (cbuc) and 3′UTR deletion (cbuc80) constructs. The amplified buc ORF was cloned into pCR8 and sequenced (Macrogen).
Rescue plasmids were cloned into pCR8 and recombined into pCS2+ derived Gateway destination vectors (Kawakami, 2005; Kawakami, 2007; Kwan et al., 2007; Villefranc et al., 2007) to generate pTolbuc:bucORFfull3′UTR (cbuc), pTolbuc:bucORF80bp3′UTR (cbuc80), pTolbuc:bucp106ORF80bpΔ3′UTR (cbucp106) and buc-intron-exon (gbuc) clones. Transgenic fish were generated by injecting 25-50 pg plasmid DNA plus 25-50 pg transposase RNA into bucp106/+ heterozygotes.
pBH-R4/R2 was generated by modifying the ‘bleeding heart’ Tol2 plasmid (pBH) from pBH-mcs(multi-cloning site) (a gift from Michael L. Nonet, Washington University St Louis) to include a Gateway-compatible attR4/attR2 cassette, namely attR4-chloramphenicol resistance-ccdB survival gene-attR2 from pTolDestR4R2 (Villefranc et al., 2007).
The buc promoter reporter construct was generated in pBH-R4/R2. The p5E-buc promoter was recombined with pME-mApple (Tol2 kit, v2.0) into pBH-R4/R2 (Invitrogen). This plasmid was injected (50 pg) along with transposase RNA (25 pg) to generate transgenic lines.
Genotyping
Genomic DNA was isolated from fin clips. Linked SSLP markers were used to genotype for buc (Bontems et al., 2009; Knapik et al., 1998).
In situ hybridization and histology
Females were anesthetized in Tricaine as described (Westerfield, 1995) and the ovaries were dissected. Whole-mount in situ hybridization was performed as described previously (Thisse and Thisse, 1998) except that hybridization was at 65°C and BM purple AP (Roche) was used. In situ probes: the buc ORF was amplified with the primers listed in supplementary material Table S1 and cloned into pCS2; vasa was described previously (Yoon et al., 1997). Images were acquired using an AxioPlan2 or AxioSkop2 microscope equipped with an AxioCam CCD camera (Zeiss) or an Olympus SZ16 fluorescent dissecting microscope and Microfire digital camera (Olympus). Images were processed in ImageJ (NIH), Adobe Photoshop and Adobe Illustrator.
For Hematoxylin and Eosin (H&E) staining, dissected ovaries were fixed in 4% PFA, washed in PBS, dehydrated in methanol, then embedded in paraffin and sectioned. Deparaffinized slides were stained in H&E, coated with Permount solution (Fisher Scientific), coverslipped, and imaged using an AxioSkop2 microscope and AxioCam CCD camera.
Oocytes were staged according to Selman et al. (Selman et al., 1993).
RT-PCR
Ovaries and other tissues (supplementary material Fig. S1A) were dissected from the specified genotypes. Oocytes were sorted according to Selman et al. (Selman et al., 1993). Trizol (Life Technologies)-extracted RNA was used for oligo(dT) cDNA preparation (using Invitrogen SuperScript III reverse transcriptase) and RT-PCR was performed using the primers listed in supplementary material Table S1.
Yeast two-hybrid assays
The ProQuest System (Invitrogen) was used for Y2H assays. Baits and preys were prepared from ovary cDNA as described above (for primers see supplementary material Table S1), cloned into pCR8, sequenced, then recombined into pDEST32 or pDEST22 vectors.
Immunoprecipitation and GST pull-downs
HEK293 cells (1×106) were transfected with 3 μg pCMV-DshMyc, pCS-YFP-Dgo (Boutros et al., 1998; Jenny et al., 2005) or pCS2-MTRbpms2 or pCS2-GFP-Buc overnight with 3:1 polyethylenimine:DNA. IP was with 1 μg of anti-Myc antibodies (9E10, Santa Cruz) (Jenny et al., 2005). Precipitated proteins were separated by SDS-PAGE, transferred to ImmobilonP (Millipore) and processed for ECL detection (GE Healthcare). Short exposures comprised 1 minute and long exposures 10-15 minutes.
GST proteins were purified as described previously (Jenny et al., 2003). 1 μg DNA was translated using the coupled in vitro transcription-translation system (Promega) with 35S. For GST pull-downs, GST fusion protein (5 μg) was bound to 15 μl GST-Sepharose (Amersham), washed, incubated with 35S-labeled proteins (5 μl) for 1 hour and analyzed as described (Jenny et al., 2003).
RNA immunoprecipitation
Ovaries were dissected, snap frozen and stored (-80°C). According to Song et al. (Song et al., 2007), ovaries were homogenized (1 ml YSS buffer) and centrifuged. The pellet and supernatant were retained. 250 μl of resuspended pellet was pre-cleared with Myc beads (30 μl; Clontech, 631208) for 1 hour at 4°C. Pre-cleared lysate was added to pCS2-MT-protein reticulocyte lysate (45 μl) plus Myc beads (30 μl) and incubated (1 hour at 4°C). Beads were washed (YSS buffer), then incubated in proteinase K lysis buffer (100 μl) and proteinase K (10 μg) (1 hour at 50°C). RNA was isolated using Trizol and precipitated (3 M sodium acetate pH 4.5 in ethanol). Precipitated RNA was used for cDNA synthesis. cDNA (0.5 μg) was used for RT-PCR analysis (primers listed in supplementary material Table S1).
Supplementary Material
Acknowledgments
We are grateful to Lilianna Solnica-Krezel, William S. Talbot and members of the F.L.M. laboratory for discussions and manuscript evaluation. We thank S. Kalinin and C. Depaolo for fish care, and the Einstein Histopathology Core for histology services.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
A.E.H., O.H. and F.L.M. performed transgenic construction, phenotypic and molecular analyses. S.R. and A.J. performed Y2H/protein interactions. A.E.H. and E.F. performed RNA IP. All authors discussed data and the manuscript. F.L.M. wrote the manuscript.
Funding
This work was supported in part by National Institutes of Health grants RO1GM089979 and start-up funds to F.L.M.; T32-GM007288 to O.H.; and RO1GM088202 to A.J. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.090449/-/DC1
References
- Abrams E. W., Mullins M. C. (2009). Early zebrafish development: it’s in the maternal genes. Curr. Opin. Genet. Dev. 19, 396–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontems F., Stein A., Marlow F., Lyautey J., Gupta T., Mullins M. C., Dosch R. (2009). Bucky ball organizes germ plasm assembly in zebrafish. Curr. Biol. 19, 414–422 [DOI] [PubMed] [Google Scholar]
- Boutros M., Paricio N., Strutt D. I., Mlodzik M. (1998). Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell 94, 109–118 [DOI] [PubMed] [Google Scholar]
- Carrera P., Johnstone O., Nakamura A., Casanova J., Jäckle H., Lasko P. (2000). VASA mediates translation through interaction with a Drosophila yIF2 homolog. Mol. Cell 5, 181–187 [DOI] [PubMed] [Google Scholar]
- Chang P., Torres J., Lewis R. A., Mowry K. L., Houliston E., King M. L. (2004). Localization of RNAs to the mitochondrial cloud in Xenopus oocytes through entrapment and association with endoplasmic reticulum. Mol. Biol. Cell 15, 4669–4681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claussen M., Pieler T. (2004). Xvelo1 uses a novel 75-nucleotide signal sequence that drives vegetal localization along the late pathway in Xenopus oocytes. Dev. Biol. 266, 270–284 [DOI] [PubMed] [Google Scholar]
- de Smedt V., Szöllösi D., Kloc M. (2000). The balbiani body: asymmetry in the mammalian oocyte. Genesis 26, 208–212 [DOI] [PubMed] [Google Scholar]
- Dosch R., Wagner D. S., Mintzer K. A., Runke G., Wiemelt A. P., Mullins M. C. (2004). Maternal control of vertebrate development before the midblastula transition: mutants from the zebrafish I. Dev. Cell 6, 771–780 [DOI] [PubMed] [Google Scholar]
- Draper B. W., McCallum C. M., Moens C. B. (2007). nanos1 is required to maintain oocyte production in adult zebrafish. Dev. Biol. 305, 589–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon J. A., Mowry K. L. (2011). Molecular motors: directing traffic during RNA localization. Crit. Rev. Biochem. Mol. Biol. 46, 229–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber W. V., Yatskievych T. A., Antin P. B., Correia K. M., Conlon R. A., Krieg P. A. (1999). The RNA-binding protein gene, hermes, is expressed at high levels in the developing heart. Mech. Dev. 80, 77–86 [DOI] [PubMed] [Google Scholar]
- Gupta T., Marlow F. L., Ferriola D., Mackiewicz K., Dapprich J., Monos D., Mullins M. C. (2010). Microtubule actin crosslinking factor 1 regulates Balbiani body function and animal-vegetal polarity of the zebrafish oocyte. PLoS Genet. 6, e1001073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachet O., Ephrussi A. (2001). Drosophila Y14 shuttles to the posterior of the oocyte and is required for oskar mRNA transport. Curr. Biol. 11, 1666–1674 [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Maegawa S., Nagai T., Yamaha E., Suzuki H., Yasuda K., Inoue K. (2004). Localized maternal factors are required for zebrafish germ cell formation. Dev. Biol. 268, 152–161 [DOI] [PubMed] [Google Scholar]
- Holt C. E., Bullock S. L. (2009). Subcellular mRNA localization in animal cells and why it matters. Science 326, 1212–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston D. W., King M. L. (2000). A critical role for Xdazl, a germ plasm-localized RNA, in the differentiation of primordial germ cells in Xenopus. Development 127, 447–456 [DOI] [PubMed] [Google Scholar]
- Houston D. W., Zhang J., Maines J. Z., Wasserman S. A., King M. L. (1998). A Xenopus DAZ-like gene encodes an RNA component of germ plasm and is a functional homologue of Drosophila boule. Development 125, 171–180 [DOI] [PubMed] [Google Scholar]
- Jenny A., Darken R. S., Wilson P. A., Mlodzik M. (2003). Prickle and Strabismus form a functional complex to generate a correct axis during planar cell polarity signaling. EMBO J. 22, 4409–4420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny A., Reynolds-Kenneally J., Das G., Burnett M., Mlodzik M. (2005). Diego and Prickle regulate Frizzled planar cell polarity signalling by competing for Dishevelled binding. Nat. Cell Biol. 7, 691–697 [DOI] [PubMed] [Google Scholar]
- Kawakami K. (2005). Transposon tools and methods in zebrafish. Dev. Dyn. 234, 244–254 [DOI] [PubMed] [Google Scholar]
- Kawakami K. (2007). Tol2: a versatile gene transfer vector in vertebrates. Genome Biol. 8 Suppl 1, S7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloc M., Etkin L. D. (1995). Two distinct pathways for the localization of RNAs at the vegetal cortex in Xenopus oocytes. Development 121, 287–297 [DOI] [PubMed] [Google Scholar]
- Kloc M., Etkin L. D. (2005). RNA localization mechanisms in oocytes. J. Cell Sci. 118, 269–282 [DOI] [PubMed] [Google Scholar]
- Kloc M., Larabell C., Chan A. P., Etkin L. D. (1998). Contribution of METRO pathway localized molecules to the organization of the germ cell lineage. Mech. Dev. 75, 81–93 [DOI] [PubMed] [Google Scholar]
- Kloc M., Bilinski S., Pui-Yee Chan A., Etkin L. D. (2000). The targeting of Xcat2 mRNA to the germinal granules depends on a cis-acting germinal granule localization element within the 3′UTR. Dev. Biol. 217, 221–229 [DOI] [PubMed] [Google Scholar]
- Kloc M., Bilinski S., Chan A. P., Allen L. H., Zearfoss N. R., Etkin L. D. (2001). RNA localization and germ cell determination in Xenopus. Int. Rev. Cytol. 203, 63–91 [DOI] [PubMed] [Google Scholar]
- Kloc M., Bilinski S., Etkin L. D. (2004). The Balbiani body and germ cell determinants: 150 years later. Curr. Top. Dev. Biol. 59, 1–36 [DOI] [PubMed] [Google Scholar]
- Knapik E. W., Goodman A., Ekker M., Chevrette M., Delgado J., Neuhauss S., Shimoda N., Driever W., Fishman M. C., Jacob H. J. (1998). A microsatellite genetic linkage map for zebrafish (Danio rerio). Nat. Genet. 18, 338–343 [DOI] [PubMed] [Google Scholar]
- Knaut H., Pelegri F., Bohmann K., Schwarz H., Nüsslein-Volhard C. (2000). Zebrafish vasa RNA but not its protein is a component of the germ plasm and segregates asymmetrically before germline specification. J. Cell Biol. 149, 875–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka K., Kawakami K., Sakamoto H., Inoue K. (2007). Spatiotemporal localization of germ plasm RNAs during zebrafish oogenesis. Mech. Dev. 124, 279–289 [DOI] [PubMed] [Google Scholar]
- Kroll T. T., Zhao W. M., Jiang C., Huber P. W. (2002). A homolog of FBP2/KSRP binds to localized mRNAs in Xenopus oocytes. Development 129, 5609–5619 [DOI] [PubMed] [Google Scholar]
- Kugler J. M., Lasko P. (2009). Localization, anchoring and translational control of oskar, gurken, bicoid and nanos mRNA during Drosophila oogenesis. Fly (Austin) 3, 15–28 [DOI] [PubMed] [Google Scholar]
- Kurihara Y., Watanabe H., Kawaguchi A., Hori T., Mishiro K., Ono M., Sawada H., Uesugi S. (2004). Dynamic changes in intranuclear and subcellular localizations of mouse Prrp/DAZAP1 during spermatogenesis: the necessity of the C-terminal proline-rich region for nuclear import and localization. Arch. Histol. Cytol. 67, 325–333 [DOI] [PubMed] [Google Scholar]
- Kwan K. M., Fujimoto E., Grabher C., Mangum B. D., Hardy M. E., Campbell D. S., Parant J. M., Yost H. J., Kanki J. P., Chien C. B. (2007). The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 236, 3088–3099 [DOI] [PubMed] [Google Scholar]
- Lasko P. F., Ashburner M. (1988). The product of the Drosophila gene vasa is very similar to eukaryotic initiation factor-4A. Nature 335, 611–617 [DOI] [PubMed] [Google Scholar]
- Lu F. I., Thisse C., Thisse B. (2011). Identification and mechanism of regulation of the zebrafish dorsal determinant. Proc. Natl. Acad. Sci. USA 108, 15876–15880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maegawa S., Yasuda K., Inoue K. (1999). Maternal mRNA localization of zebrafish DAZ-like gene. Mech. Dev. 81, 223–226 [DOI] [PubMed] [Google Scholar]
- Markussen F. H., Michon A. M., Breitwieser W., Ephrussi A. (1995). Translational control of oskar generates short OSK, the isoform that induces pole plasma assembly. Development 121, 3723–3732 [DOI] [PubMed] [Google Scholar]
- Marlow F. L. (2010). Maternal Control of Development in Vertebrates: My Mother Made Me Do It! San Rafael, CA: Morgan & Claypool Life Sciences; [PubMed] [Google Scholar]
- Marlow F. L., Mullins M. C. (2008). Bucky ball functions in Balbiani body assembly and animal-vegetal polarity in the oocyte and follicle cell layer in zebrafish. Dev. Biol. 321, 40–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeilly J. R., Saunders P. T., Taggart M., Cranfield M., Cooke H. J., McNeilly A. S. (2000). Loss of oocytes in Dazl knockout mice results in maintained ovarian steroidogenic function but altered gonadotropin secretion in adult animals. Endocrinology 141, 4284–4294 [DOI] [PubMed] [Google Scholar]
- Micklem D. R., Dasgupta R., Elliott H., Gergely F., Davidson C., Brand A., Gonzalez-Reyes A., St Johnston D. (1997). The mago nashi gene is required for the polarisation of the oocyte and the formation of perpendicular axes in Drosophila. Curr. Biol. 7, 468–478 [DOI] [PubMed] [Google Scholar]
- Minakhina S., Steward R. (2005). Axes formation and RNA localization. Curr. Opin. Genet. Dev. 15, 416–421 [DOI] [PubMed] [Google Scholar]
- Mir A., Heasman J. (2008). How the mother can help: studying maternal Wnt signaling by anti-sense-mediated depletion of maternal mRNAs and the host transfer technique. Methods Mol. Biol. 469, 417–429 [DOI] [PubMed] [Google Scholar]
- Mohr S. E., Dillon S. T., Boswell R. E. (2001). The RNA-binding protein Tsunagi interacts with Mago Nashi to establish polarity and localize oskar mRNA during Drosophila oogenesis. Genes Dev. 15, 2886–2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowry K. L., Melton D. A. (1992). Vegetal messenger RNA localization directed by a 340-nt RNA sequence element in Xenopus oocytes. Science 255, 991–994 [DOI] [PubMed] [Google Scholar]
- Newmark P. A., Boswell R. E. (1994). The mago nashi locus encodes an essential product required for germ plasm assembly in Drosophila. Development 120, 1303–1313 [DOI] [PubMed] [Google Scholar]
- Nojima H., Rothhämel S., Shimizu T., Kim C. H., Yonemura S., Marlow F. L., Hibi M. (2010). Syntabulin, a motor protein linker, controls dorsal determination. Development 137, 923–933 [DOI] [PubMed] [Google Scholar]
- Pepling M. E., Wilhelm J. E., O’Hara A. L., Gephardt G. W., Spradling A. C. (2007). Mouse oocytes within germ cell cysts and primordial follicles contain a Balbiani body. Proc. Natl. Acad. Sci. USA 104, 187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiu M., Speed R., Taggart M., McKay S. J., Kilanowski F., Saunders P., Dorin J., Cooke H. J. (1997). The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis. Nature 389, 73–77 [DOI] [PubMed] [Google Scholar]
- Saunders P. T., Turner J. M., Ruggiu M., Taggart M., Burgoyne P. S., Elliott D., Cooke H. J. (2003). Absence of mDazl produces a final block on germ cell development at meiosis. Reproduction 126, 589–597 [DOI] [PubMed] [Google Scholar]
- Schier A. F., Talbot W. S. (2005). Molecular genetics of axis formation in zebrafish. Annu. Rev. Genet. 39, 561–613 [DOI] [PubMed] [Google Scholar]
- Selman K., Wallace R., Sarka A., Qi X. (1993). Stages of oocyte development in the zebrafish, Brachydanio rerio. J. Morphol. 218, 203–224 [DOI] [PubMed] [Google Scholar]
- Song H. W., Cauffman K., Chan A. P., Zhou Y., King M. L., Etkin L. D., Kloc M. (2007). Hermes RNA-binding protein targets RNAs-encoding proteins involved in meiotic maturation, early cleavage, and germline development. Differentiation 75, 519–528 [DOI] [PubMed] [Google Scholar]
- St Johnston D. (2005). Moving messages: the intracellular localization of mRNAs. Nat. Rev. Mol. Cell Biol. 6, 363–375 [DOI] [PubMed] [Google Scholar]
- Styhler S., Nakamura A., Swan A., Suter B., Lasko P. (1998). vasa is required for GURKEN accumulation in the oocyte, and is involved in oocyte differentiation and germline cyst development. Development 125, 1569–1578 [DOI] [PubMed] [Google Scholar]
- Takeda Y., Mishima Y., Fujiwara T., Sakamoto H., Inoue K. (2009). DAZL relieves miRNA-mediated repression of germline mRNAs by controlling poly(A) tail length in zebrafish. PLoS ONE 4, e7513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse C., Thisse B. (1998). High resolution whole-mount in situ hybridization. In The Zebrafish Book (ed. Westerfield M.). Eugene, OR: University of Oregon Press; [Google Scholar]
- Villefranc J. A., Amigo J., Lawson N. D. (2007). Gateway compatible vectors for analysis of gene function in the zebrafish. Dev. Dyn. 236, 3077–3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. (1995). The Zebrafish Book. Eugene, OR: University of Oregon Press; [Google Scholar]
- Wilk K., Bilinski S., Dougherty M. T., Kloc M. (2005). Delivery of germinal granules and localized RNAs via the messenger transport organizer pathway to the vegetal cortex of Xenopus oocytes occurs through directional expansion of the mitochondrial cloud. Int. J. Dev. Biol. 49, 17–21 [DOI] [PubMed] [Google Scholar]
- Yoon C., Kawakami K., Hopkins N. (1997). Zebrafish vasa homologue RNA is localized to the cleavage planes of 2- and 4-cell-stage embryos and is expressed in the primordial germ cells. Development 124, 3157–3165 [DOI] [PubMed] [Google Scholar]
- Zearfoss N. R., Chan A. P., Wu C. F., Kloc M., Etkin L. D. (2004). Hermes is a localized factor regulating cleavage of vegetal blastomeres in Xenopus laevis. Dev. Biol. 267, 60–71 [DOI] [PubMed] [Google Scholar]
- Zhao W. M., Jiang C., Kroll T. T., Huber P. W. (2001). A proline-rich protein binds to the localization element of Xenopus Vg1 mRNA and to ligands involved in actin polymerization. EMBO J. 20, 2315–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., King M. L. (2004). Sending RNAs into the future: RNA localization and germ cell fate. IUBMB Life 56, 19–27 [DOI] [PubMed] [Google Scholar]
- Zimyanin V., Lowe N., St Johnston D. (2007). An oskar-dependent positive feedback loop maintains the polarity of the Drosophila oocyte. Curr. Biol. 17, 353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.