Abstract
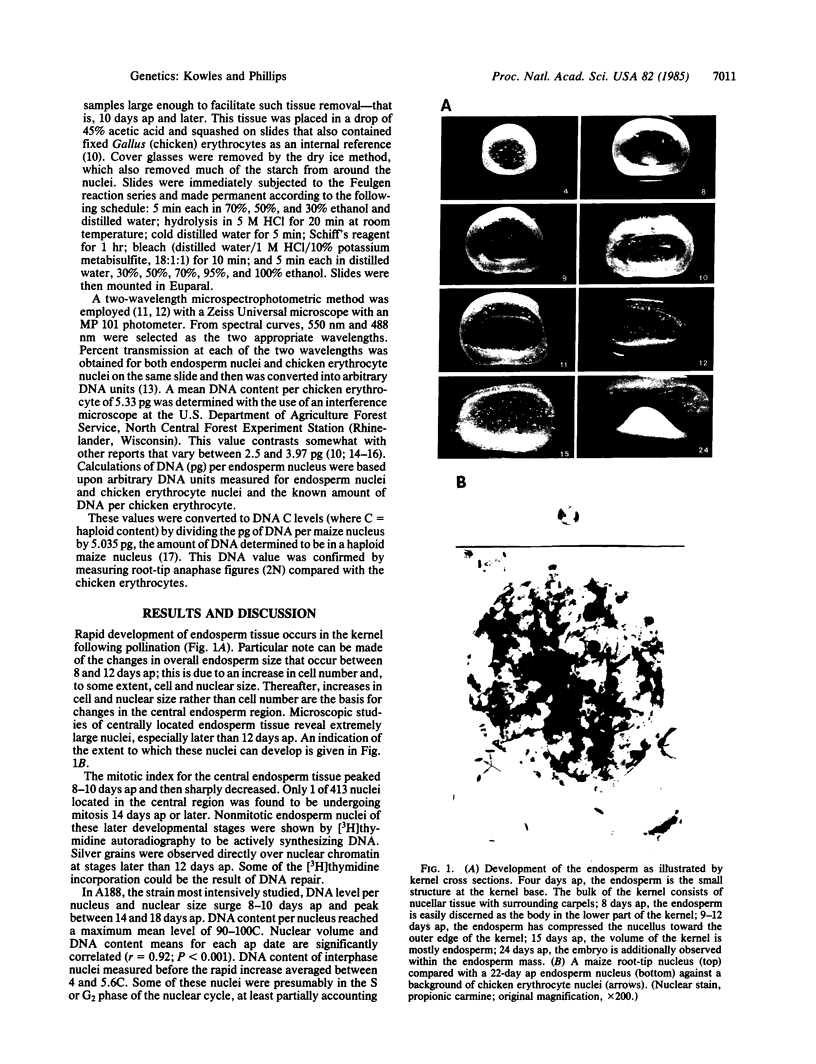
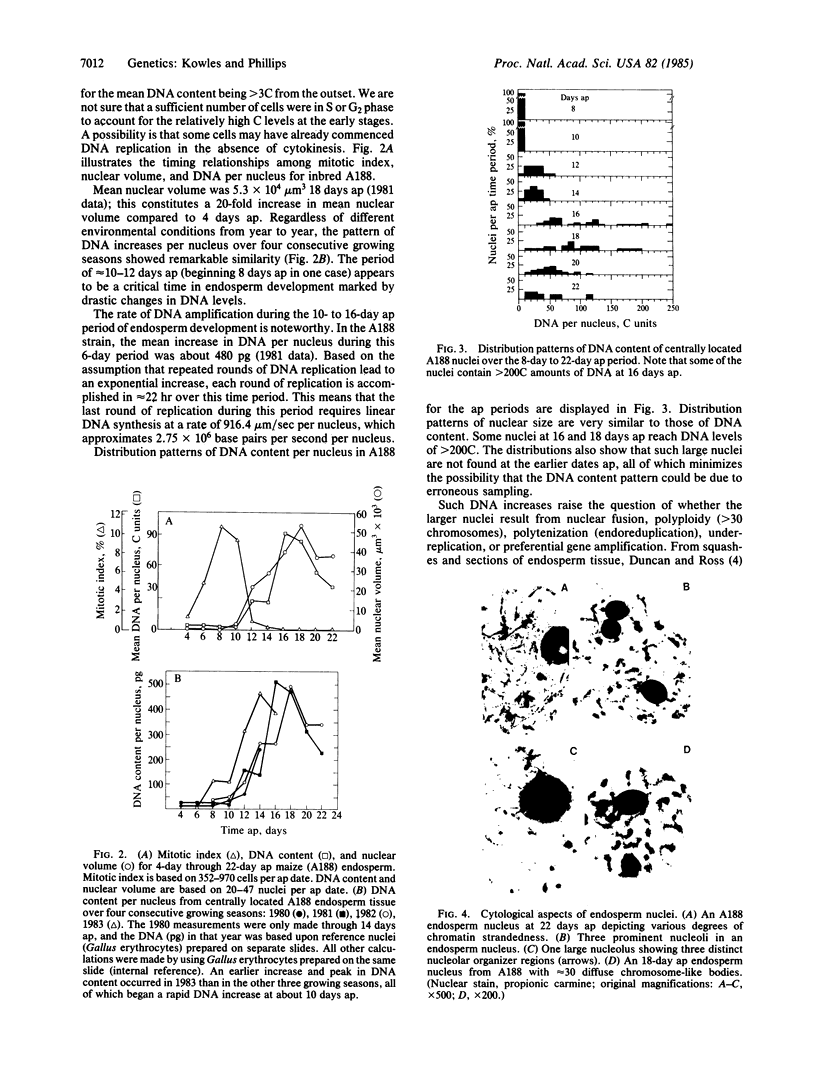
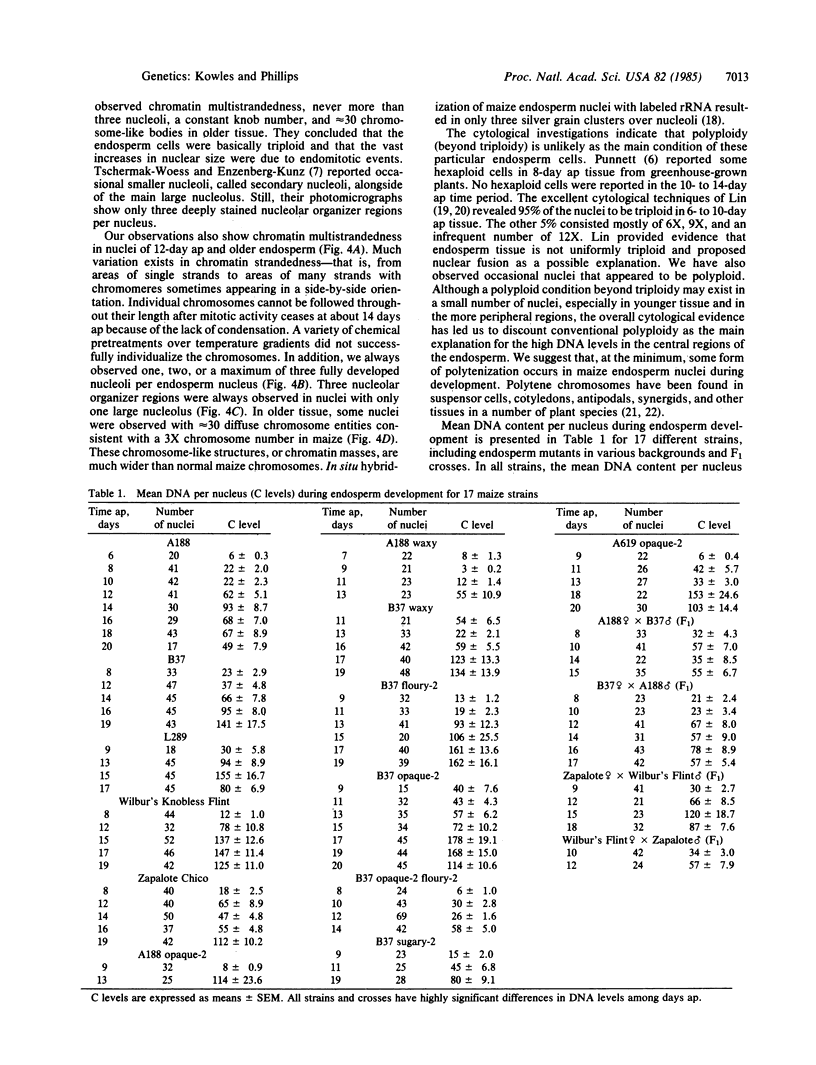
Increased DNA levels in centrally located endosperm nuclei are shown to be related to endosperm development in Zea mays. Mitotic activity sharply decreases in endosperm cells 10-12 days after pollination. At this time nuclear size and DNA content per nucleus (where C = haploid content) sharply increase until peak levels are reached at about 14-18 days after pollination. Mean DNA content per endosperm nucleus in strain A188 was shown by Feulgen cytophotometry to increase to about 90C by this peak stage, with the pattern being remarkably consistent over four consecutive growing seasons. Some individual nuclei achieved levels of >200C. Most other strains compared during one growing season averaged even higher peak levels of DNA per nucleus than did A188. Individual nuclei in those strains reached levels as high as 690C. A decrease in DNA level was observed in older endosperms with most strains. Endosperm mutant strains did not show a significant reduction in DNA. Opaque-2 mutants in several backgrounds achieved higher levels of DNA per nucleus. DNA levels from F1 endosperms did not indicate heterosis. Regardless of differences in DNA content, the pattern of DNA increasing as development proceeds followed by a DNA decrease was observed for most strains. Cytological studies reveal much variation in chromatin strandedness, a maximum of three nucleoli, a maximum of three nucleolar organizer regions, and ≈30 diffuse chromatin masses in older endosperm tissue. A form of DNA amplification, perhaps polytenization, appears to be occurring during endosperm development.
Keywords: chromatin, polyploidy, polytenization, replication, cytophotometry
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DUNCAN R. E., ROSS J. G. The nucleus in differentiation and development. III. Nuclei of maize endosperm. J Hered. 1950 Oct;41(10):259–268. doi: 10.1093/oxfordjournals.jhered.a106054. [DOI] [PubMed] [Google Scholar]
- Dhillon S. S., Miksche J. P. DNA, RNA, protein and heterochromatin changes during embryo development and germination of soybean (Glycine max L.). Histochem J. 1983 Jan;15(1):21–37. doi: 10.1007/BF01006069. [DOI] [PubMed] [Google Scholar]
- MENDELSOHN M. L. The two-wavelength method of microspectrophotometry. I. A microspectrophotometer and tests on model systems. J Biophys Biochem Cytol. 1958 Jul 25;4(4):407–414. doi: 10.1083/jcb.4.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENDELSOHN M. L. The two-wavelength method of microspectrophotometry. II. A set of tables to facilitate the calculations. J Biophys Biochem Cytol. 1958 Jul 25;4(4):415–424. doi: 10.1083/jcb.4.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips R. L., Weber D. F., Kleese R. A., Wang S. S. The Nucleolus Organizer Region of Maize (ZEA MAYS L.): Tests for Ribosomal Gene Compensation or Magnification. Genetics. 1974 Jun;77(2):285–297. doi: 10.1093/genetics/77.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWIFT H. The constancy of desoxyribose nucleic acid in plant nuclei. Proc Natl Acad Sci U S A. 1950 Nov;36(11):643–654. doi: 10.1073/pnas.36.11.643. [DOI] [PMC free article] [PubMed] [Google Scholar]