Abstract
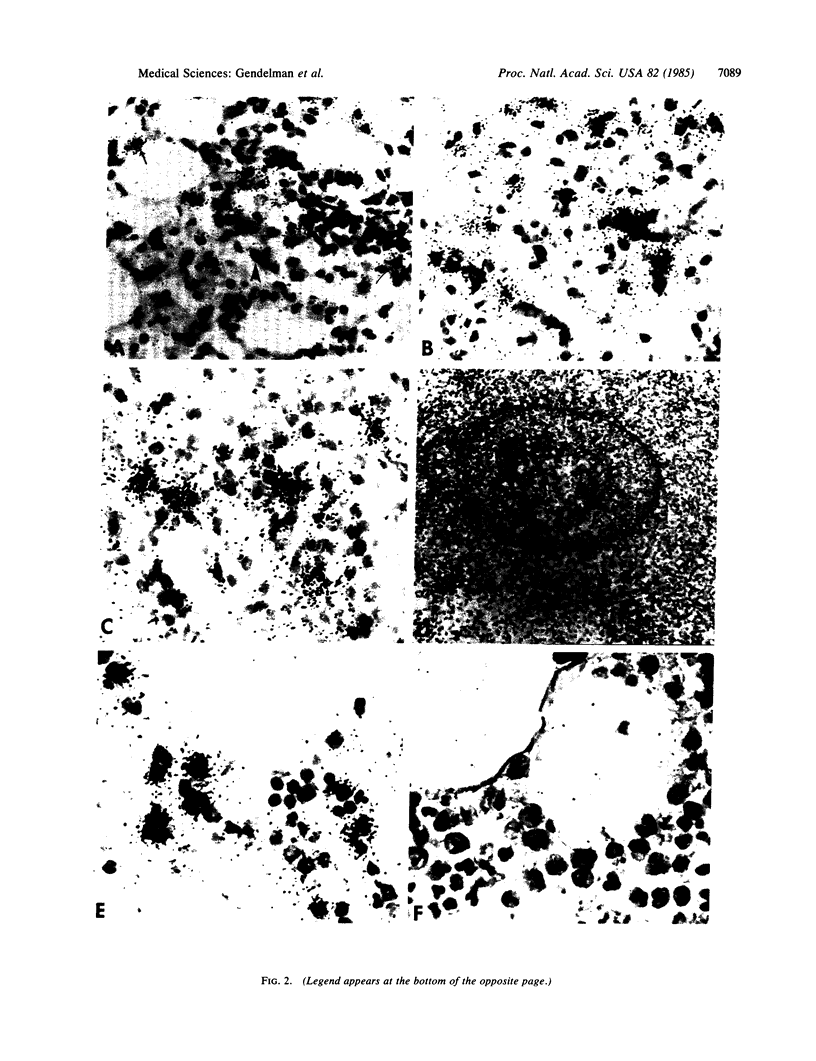
Lentiviruses, as exemplified by visna virus of sheep, are nononcogenic retroviruses that cause slowly progressive diseases after prolonged periods of incubation. Earlier studies on visna have shown that the long incubation period of the disease is associated with constant production of minimal quantities of virus in tissues, whereas virus could be obtained by culturing monocytes and macrophages from explants of lymphatic tissues and inflamed organs. In this study the role of macrophages in lentivirus infection was explored using two sheep that were intrabronchially inoculated with virus. When sections of paraffin-embedded tissue, processed by a recently described technique which combines immunocytochemistry for the identification of macrophages and in situ hybridization for identification of viral nucleic acid, were examined, we found that virus replication is associated almost exclusively with infection in selected populations of macrophages in the interalveolar region of the alveoli, in inflammatory exudate cells in the lung, in lymph nodes, and in the spleen. Although large numbers of alveolar macrophages had viral RNA, few of these cells produced virus. While this minimally productive type of viral replication provides an explanation for the slow pace of the infection, restricted replication in terminally differentiated, short-lived macrophages does not explain persistent virus replication in the animal. With the discovery of clusters of infected macrophage precursors in the bone marrow, a mechanism for persistence was found. The macrophage precursor cells provide an important missing link in the virus-target-cell circuit and may be the reservoir of latently infected cells which perpetuate lentivirus infections in both animals and humans.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brahic M., Haase A. T., Cash E. Simultaneous in situ detection of viral RNA and antigens. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5445–5448. doi: 10.1073/pnas.81.17.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahic M., Stowring L., Ventura P., Haase A. T. Gene expression in visna virus infection in sheep. Nature. 1981 Jul 16;292(5820):240–242. doi: 10.1038/292240a0. [DOI] [PubMed] [Google Scholar]
- Cork L. C., Narayan O. The pathogenesis of viral leukoencephalomyelitis-arthritis of goats. I. Persistent viral infection with progressive pathologic changes. Lab Invest. 1980 Jun;42(6):596–602. [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Geballe A. P., Ventura P., Stowring L., Haase A. T. Quantitative analysis of visna virus replication in vivo. Virology. 1985 Feb;141(1):148–154. doi: 10.1016/0042-6822(85)90191-6. [DOI] [PubMed] [Google Scholar]
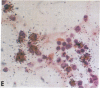
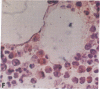
- Gendelman H. E., Moench T. R., Narayan O., Griffin D. E., Clements J. E. A double labeling technique for performing immunocytochemistry and in situ hybridization in virus infected cell cultures and tissues. J Virol Methods. 1985 Jun;11(2):93–103. doi: 10.1016/0166-0934(85)90033-3. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Moench T. R., Narayan O., Griffin D. E. Selection of a fixative for identifying T cell subsets, B cells, and macrophages in paraffin-embedded mouse spleen. J Immunol Methods. 1983 Dec 16;65(1-2):137–145. doi: 10.1016/0022-1759(83)90310-1. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Kennedy-Stoskopf S., Clements J. E., Pezeshkpour G. H. Slow virus-macrophage interactions. Characterization of a transformed cell line of sheep alveolar macrophages that express a marker for susceptibility to ovine-caprine lentivirus infections. Lab Invest. 1984 Nov;51(5):547–555. [PubMed] [Google Scholar]
- Gillies S. D., Folsom V., Tonegawa S. Cell type-specific enhancer element associated with a mouse MHC gene, E beta. Nature. 1984 Aug 16;310(5978):594–597. doi: 10.1038/310594a0. [DOI] [PubMed] [Google Scholar]
- Gonda M. A., Wong-Staal F., Gallo R. C., Clements J. E., Narayan O., Gilden R. V. Sequence homology and morphologic similarity of HTLV-III and visna virus, a pathogenic lentivirus. Science. 1985 Jan 11;227(4683):173–177. doi: 10.1126/science.2981428. [DOI] [PubMed] [Google Scholar]
- Gorecki M., Rozenblatt S. Cloning of DNA complementary to the measles virus mRNA encoding nucleocapsid protein. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3686–3690. doi: 10.1073/pnas.77.6.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase A. T., Stowring L., Narayan P., Griffin D., Price D. Slow persistent infection caused by visna virus: role of host restriction. Science. 1977 Jan 14;195(4274):175–177. doi: 10.1126/science.188133. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Gillam I. C., Delaney A. D., Tener G. M. Acetylation of chromosome squashes of Drosophila melanogaster decreases the background in autoradiographs from hybridization with [125I]-labeled RNA. J Histochem Cytochem. 1978 Aug;26(8):677–679. doi: 10.1177/26.8.99471. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J. The nature of the host range restriction of SV40 and polyoma viruses in embryonal carcinoma cells. Curr Top Microbiol Immunol. 1982;101:1–30. doi: 10.1007/978-3-642-68654-2_1. [DOI] [PubMed] [Google Scholar]
- Moench T. R., Gendelman H. E., Clements J. E., Narayan O., Griffin D. E. Efficiency of in situ hybridization as a function of probe size and fixation technique. J Virol Methods. 1985 Jun;11(2):119–130. doi: 10.1016/0166-0934(85)90035-7. [DOI] [PubMed] [Google Scholar]
- Molineaux S., Clements J. E. Molecular cloning of unintegrated visna viral DNA and characterization of frequent deletions in the 3' terminus. Gene. 1983 Aug;23(2):137–148. doi: 10.1016/0378-1119(83)90045-8. [DOI] [PubMed] [Google Scholar]
- Narayan O., Cork L. C. Lentiviral diseases of sheep and goats: chronic pneumonia leukoencephalomyelitis and arthritis. Rev Infect Dis. 1985 Jan-Feb;7(1):89–98. doi: 10.1093/clinids/7.1.89. [DOI] [PubMed] [Google Scholar]
- Narayan O., Griffin D. E., Silverstein A. M. Slow virus infection: replication and mechanisms of persistence of visna virus in sheep. J Infect Dis. 1977 May;135(5):800–806. doi: 10.1093/infdis/135.5.800. [DOI] [PubMed] [Google Scholar]
- Narayan O., Kennedy-Stoskopf S., Sheffer D., Griffin D. E., Clements J. E. Activation of caprine arthritis-encephalitis virus expression during maturation of monocytes to macrophages. Infect Immun. 1983 Jul;41(1):67–73. doi: 10.1128/iai.41.1.67-73.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan O., Wolinsky J. S., Clements J. E., Strandberg J. D., Griffin D. E., Cork L. C. Slow virus replication: the role of macrophages in the persistence and expression of visna viruses of sheep and goats. J Gen Virol. 1982 Apr;59(Pt 2):345–356. doi: 10.1099/0022-1317-59-2-345. [DOI] [PubMed] [Google Scholar]
- Nathanson N., Panitch H., Palsson P. A., Petursson G., Georgsson G. Pathogenesis of visna. II. Effect of immunosuppression upon early central nervous system lesions. Lab Invest. 1976 Nov;35(5):444–451. [PubMed] [Google Scholar]
- Oliver R. E., Gorham J. R., Parish S. F., Hadlow W. J., Narayan O. Ovine progressive pneumonia: pathologic and virologic studies on the naturally occurring disease. Am J Vet Res. 1981 Sep;42(9):1554–1559. [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Quintrell N., Medeiros E., Bishop J. M., Nowinski R. C., Sarkar N. H. Transcription of mouse mammary tumor virus genes in tissues from high and low tumor incidence mouse strains. J Mol Biol. 1973 Oct 5;79(4):663–679. doi: 10.1016/0022-2836(73)90070-3. [DOI] [PubMed] [Google Scholar]
- Westin E. H., Gallo R. C., Arya S. K., Eva A., Souza L. M., Baluda M. A., Aaronson S. A., Wong-Staal F. Differential expression of the amv gene in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2194–2198. doi: 10.1073/pnas.79.7.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]