Abstract
Interleukin 17A-secreting T-helper 17 cells are pathogenic in inflammatory kidney diseases, but their intra-renal regulation is poorly understood. Mouse unilateral ureteral obstruction was examined to better define T-helper 17 cell dynamics during interstitial inflammation. Cell sub-types were analyzed by multi-color flow cytometry, by cell sorting and by their effects on in vitro-generated T-helper 17 cells. Interleukin 17A expression progressively increased in obstructed kidneys and was localised to CCR6+CCR4+/−CD4+ T-cells. Numbers of CCR6+CD4+ T-cells increased >10-fold by 72 hours, were enriched for interleukin 17A production and were highly proliferative by in vivo bromodeoxyuridine labelling. Secreted products from leukocytes of obstructed kidneys enhanced interleukin 17A production by in vitro-generated T-helper 17 cells. The T-helper 17-enhancing activity was identified as interleukin-1 produced by renal dendritic cells and monocytes. The in vivo validity of these findings was confirmed in mice lacking interleulin-1-receptor and in mice treated with a recombinant interleukin-1 receptor antagonist which exhibited reduced intra-renal T-helper 17 activity compared to control animals. Thus, the inflamed kidney accumulates CCR6+ T-helper 17 cells that undergo activation and proliferation. Production of interleukin 1 family cytokines by resident dendritic cells and infiltrating monocytes enhances intra-renal T-helper 17 activation in acute kidney injury.
Keywords: Obstructive nephropathy, Lymphocytes, Inflammation, Cytokines, Chemokine Receptor
Introduction
Acute kidney injury (AKI) is characterized by increased interstitial lymphocytes, mononuclear phagocytes and neutrophils.1, 2 Obstruction and ischemia represent 30-50 % of AKI and frequently progress to chronic kidney disease3-5. During AKI and glomerulonephritis, CD4+ T-helper (Th) cells participate in renal inflammation and fibrosis.6-10 Naïve Th cells differentiate into functionally distinct subsets distinguished by signature effector cytokines.11, 12 T-helper type 1 (Th1) cells produce interferon-gamma (IFN-γ) and are linked with tissue-destructive inflammation whereas Th2 cells secrete interleukins (IL)-4, -5 and -13 and, under some conditions, are anti-inflammatory.11, 12 Recently some disease processes, originally believed to be Th1-mediated, were shown to be partly or predominantly driven by a pro-inflammatory CD4+ T cell subtype, Th17, that is characterised by production of IL-17-family cytokines.13-15 Interleukin-17A, the best characterized and most potent of these, stimulates neutrophil migration to sites of inflammation and amplifies effects of other pro-inflammatory cytokines on fibroblasts, epithelial and mesangial cells13, 16, 17. IL-17A-producing cells are pathogenic in Crohn's disease, multiple sclerosis, rheumatoid arthritis,15 glomerulonephritis9, 18, 19 and AKI.20, 21
Th17 cells differ from Th1 and Th2 cells by cytokine profile, chemokine receptor expression and mechanisms of differentiation and activation.14 In man, Th17 cells express CCR2, CCR4, CCR6 and CXCR422, 23 while, in mouse, they preferentially express CCR6, CCR4 and CCR7.24 Although Th17 cells express some trafficking receptors in a tissue-specific manner, CCR6 appears to be uniformly expressed.23 Th17 differentiation is incompletely understood but TGF-β, IL-6, IL-21 and IL-23 are implicated in both man and mouse.15, 25-27 IL-23 promotes the expansion of established Th17 populations but does not induce Th17 differentiation in naïve T-cell precursors.28 IL-1 family cytokines are also critically required for early Th17 cell programming and for Th17 cell-mediated autoimmunity14, 29. In synergy with IL-6 and IL-23, IL-1 regulates Th17 cell differentiation and maintains IL-17A expression in Th17 effectors.30 IL-1 also regulates activation of previously-differentiated (effector-memory) Th17 cells during tissue-specific inflammation and autoimmunity31 – a process that may be of specific relevance to Th17 cell involvement in renal inflammation.
Recently published studies have demonstrated the pathogenic significance of IL-17A-producing cells in diverse renal diseases.17, 18, 21, 32-39 Drawing upon our prior observations demonstrating the presence of renal effector-memory Th17 cells in mouse UUO20, the present study sought to define molecular species and their cellular sources that underlie the presence of activated Th17 cells in the obstructed kidney, the key phenotypic characteristics of these cells, especially as they relate to inflammatory processes, and the functional significance of the identified pathway accounting for the Th17 cell accumulation in the obstructed kidney.
Results
IL-17A is increasingly expressed in the renal cortex following UUO and is localised to CD4+ T-cells
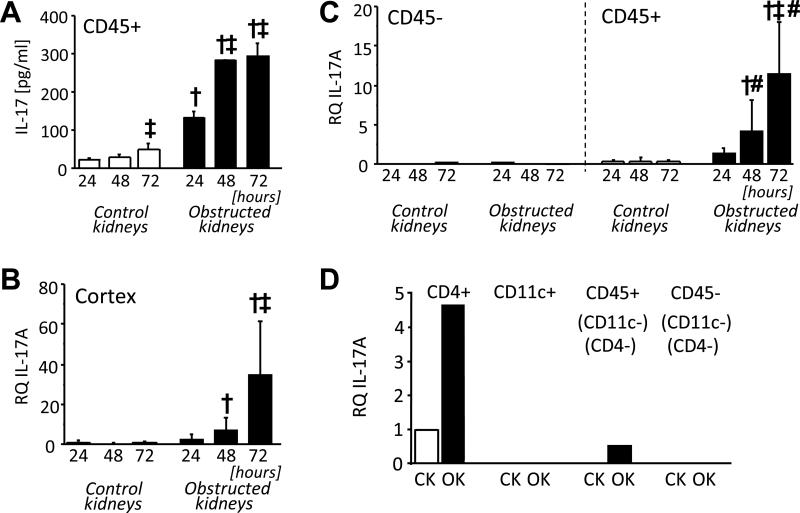
Total leukocytes (CD45-enriched cells), prepared from mouse kidneys at 24, 48 and 72 hours following UUO and stimulated with a low concentration of anti-CD3 antibody, produced IL-17A that was of higher concentration from cells of obstructed compared with non-obstructed (control) kidneys and increased progressively over time (Figure 1A). The largest increment in anti-CD3-inducible IL-17A occurred between 24 and 48 hours. Cortical tissue from individual kidneys was subjected to quantitative RT-PCR (qRT-PCR) (Figure 1B) demonstrating a progressive increase in IL-17A mRNA in obstructed kidneys between 24 and 72 hours confirming in situ IL-17A expression following UUO. Quantitative RT-PCR of magnetic bead-separated CD45+ and CD45− cells from kidney digests indicated that IL-17A mRNA was confined to the CD45+ leukocyte-enriched fractions (Figure 1C). Fluorescence-activated cell sorting (FACS) of 72-hour kidney digests into 4 individual fractions based on expression of CD45, the Th marker CD4 and the dendritic cell (DC) marker CD11c demonstrated that IL-17A mRNA was localised to the CD4+ fraction of obstructed kidneys (Figure 1D). Thus, consistent with our previous findings,20 a subset of T-cells within obstructed but not control kidneys are primed to secrete IL-17A in high amounts following low-level T-cell receptor stimulation. Furthermore, a progressive increase in intra-renal expression of IL-17A occurs within 72 hours of UUO and is localised to CD4+ leukocytes.
Figure 1. IL-17A expression in obstructed kidneys.
Mice were subjected to UUO by left ureteral ligation for 24, 48 and 72 hours (n = 3-5 per group). A. Production of IL-17A in culture supernatant of CD45-enriched cells from obstructed and non-obstructed (control) kidneys following 24 hour culture with low-dose anti-CD3 stimulation. B. Relative quantity (RQ) of IL-17A mRNA in whole cortex of obstructed and control kidneys measured by quantitative RT-PCR. C Relative quantity (RQ) of IL-17A mRNA in CD45-depleted (CD45−) and CD45-enriched (CD45+) cells of obstructed and control kidneys measured by quantitative RT-PCR. D. Relative quantity (RQ) of IL-17A mRNA in FACS-purified cell populations from control (CK) and obstructed kidneys (OK) prepared 72 hours after UUO. Sorted cell populations were: CD4+ (CD4 T-cells), CD11c+ (Dendritic Cells) CD45+ (CD4−, CD11c−) (All other leukocytes) and CD45− (CD4−, CD11c−) (Non-leukocyte kidney cells). Results are presented as means ± SD. † p<0.05 compared to equivalent result for control kidneys; ‡ p<0.05 compared to 24 hour obstructed kidneys; # p<0.05 compared to equivalent result for CD45− cells.
Renal Th17 cells preferentially express CCR6 and undergo progressive accumulation and proliferation in obstructed kidneys
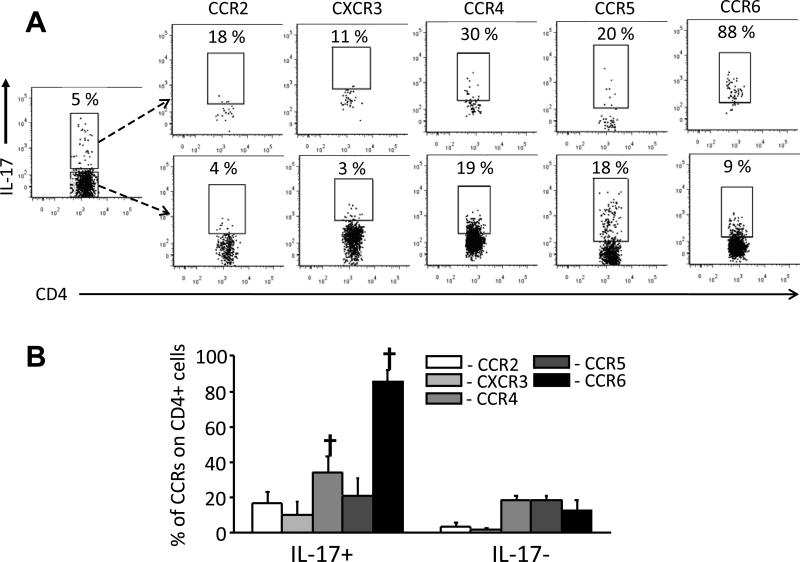
Chemokine receptor expression was examined as a means to identify T-cell subpopulations enriched for Th17 activity. Combined surface and intracellular staining of anti-CD3-stimulated cells of 72-hour obstructed kidney cells was analysed by multi-colour flow cytometry. Cells were surface-stained for CD45, CD4 and one of several chemokine receptors (CCR2, CCR4, CCR5, CCR6, CXCR3) then intracellularly stained for IL-17A (Figure 2A and 2B). IL-17A+CD4+ cells were most readily distinguishable from IL-17A−CD4+ cells by frequency of CCR6 expression (>88% vs. <9% in this experiment, one of 3 performed). CCR4 expression was also more frequent on IL-17A+CD4+ cells. Combined CD4/CCR6/CCR4 staining indicated that IL-17A+ cells constituted 30% of CCR6+CCR4− and 23% of CCR6+CCR4+ CD4+ T-cells but were rare among the CCR6− subpopulations (Figure 3A). IL-17A staining level was highest among the CCR6+CCR4− cells. Quantitative RT-PCR of FACS-purified CD4+CCR6+ and CD4+CCR6− cells from 72-hour obstructed and control kidneys confirmed that IL-17A mRNA was most readily detectable in CD4+CCR6+ cells (Figure 3B and Supplemental Figure S1). Importantly, whereas CD4+/CCR6+ cells were present within control kidneys and could be successfully purified, IL-17A mRNA was undetectable in these cells.
Figure 2. Chemokine receptor expression of IL-17+ and IL-17− CD4+ T-cells from obstructed kidney.
CD45-enriched cells from 72-hour obstructed kidneys were cultured for 24 hours with low-dose anti-CD3 stimulation then surface stained for CD45, CD4 and one of several cytokine receptors (CCR2, CXCR3, CCR4, CCR5, CCR6) followed by intracellular staining for IL-17A and analysis by flow cytometry. A. Representative dot plots showing frequency of chemokine receptor expression on IL-17A+ and IL-17A− subpopulations following gating on CD45+ CD4+ cells. B. Graphical representation of results expressed as Means ± SD for frequencies of chemokine receptor expression on IL-17A+ CD4+ and IL-17A− CD4+ cells obstructed. † = p<0.05 compared to equivalent result for IL-17A− CD4+ cells.
Figure 3. CD4+CCR6+ T cells are the predominant source of IL-17A in obstructed kidneys.
A. Representative dots plots showing the frequencies of IL-17A intracellular staining among 4 populations of CD45+CD4+ T-cells from 72-hour obstructed kidney: CCR6−CCR4+, CCR6−CCR4−, CCR6+CCR4− and CCR6+CCR4+. B. Relative quantity (RQ) of IL-17A mRNA in 4 FACS-purified cell populations from control (CK) and obstructed (OK) kidneys 72 hours after UUO: CD4+CCR6+, CD4+CCR6−, CD4−CCR6− (all CD45+) and CD45− (see Supplemental Figure S1 for illustration of sorting strategy and purity).
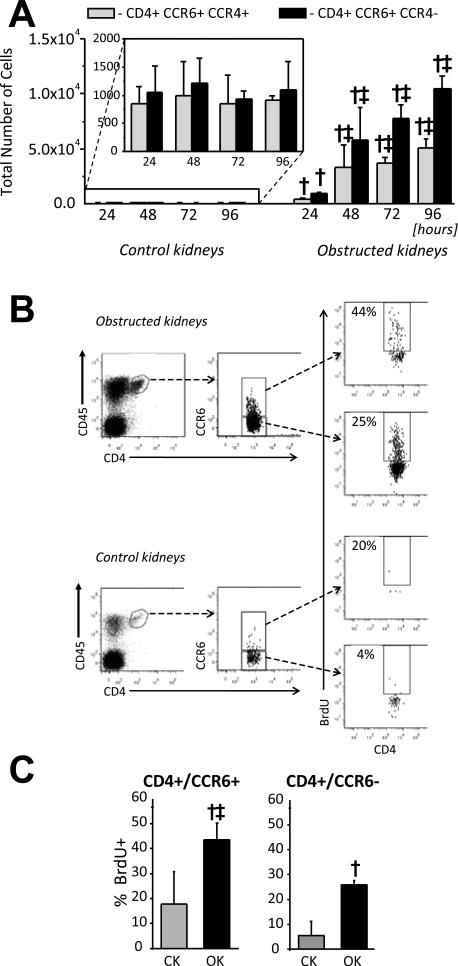
Subsequently, CCR6 expression (± CCR4) was used to analyse the dynamics of Th17 cells and other CD4+ T-cells within obstructed kidneys. Total CD4+CCR6+CCR4+ and CD4+CCR6+CCR4− cell numbers were compared for individual obstructed and control kidneys at 24, 48, 72 and 96 hours post-UUO (Figure 4A). The numbers of both increased early (24 hrs) in obstructed kidneys and continued to increase, albeit at a slower rate, up to 96 hours. The proliferative activity of CCR6+ and CCR6− Th cells accumulating with obstructed kidneys was compared by in vivo bromodeoxyuridine (BrdU) labelling for 72 hours after UUO (Figure 4B and 4C). BrdU incorporation was detected in greater proportions of both CD4+ T-cell subsets in obstructed compared with control kidneys. However, the proportion of BrdU+ cells among the CD4+CCR6+ subset of obstructed kidneys was almost twice that of CD4+CCR6− cells, indicating a greater rate of proliferation.
Figure 4. Accumulation and proliferation of CCR6+ Th cells in obstructed kidneys.
A. Calculated total numbers of CCR6+CCR4+CD4+ and CCR6+CCR4−CD4+ cells in control and obstructed kidneys between 24 and 96 hours following UUO based on viable cell counts and flow cytometry of whole-kidney digests. † = p<0.05 compared to equivalent result for control kidneys; ‡ = p<0.05 compared to equivalent result at 24 hours. B. Kidney cells suspensions prepared following 72 hours of UUO and continuous BrdU ingestion via drinking water were surface stained for CD45, CD4 and CCR6 followed by intracellular staining for BrdU. Representative dot plots are shown illustrating the frequency of BrdU+ cells among the CCR6+ and CCR6− CD4+ T-cells from obstructed and control kidneys. C. Graphical representation of analysis illustrated in B. Results are expressed as Means ± SD of the percent BrdU+ among CCR6+CD4+ and CCR6−CD4+ T-cells for individually analysed control (CK) and obstructed (OK) kidneys (n = 5). † = p<0.05 compared to equivalent result for control kidneys; ‡ = p<0.05 compared to equivalent result for CD4+CCR6− cells).
Renal leukocyte populations secrete Th17 activating factors following UUO
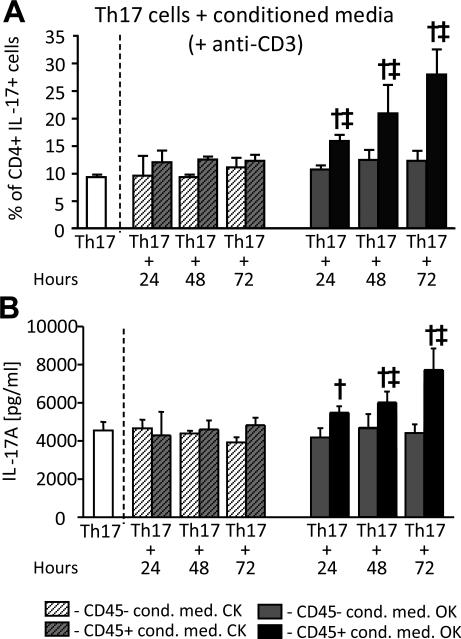
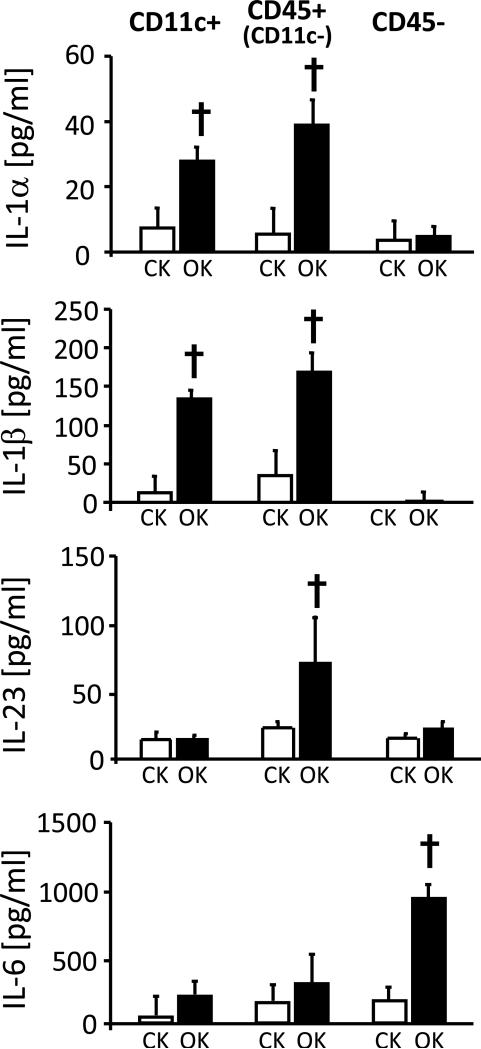
As we had previously observed that liposomal clodronate administration prior to UUO resulted in loss of intra-renal CD4+ T-cells priming for IL-17A production20, we hypothesised that intra-renal Th17 cell activity following UUO is promoted by one or more factors produced locally by cells of the mononuclear phagocyte system. To investigate further, conditioned media (CM) were prepared from CD45+ and CD45− cells of obstructed and control kidneys 24, 48 and 72 hours post-UUO and were added to in vitro-generated Th17 cells during re-stimulation with anti-CD3 antibody. IL-17A production was quantified intra-cellularly by flow cytometry (Figure 5A and Supplemental Figure S2) and in supernatants by ELISA (Figure 5B). The results indicated that CM from CD45+ cells of obstructed kidneys promoted IL-17A production by CD4+ T cells. This IL-17A enhancing effect on IL-17A secretion was progressively greater for medium from CD45+ cells prepared following 24, 48 and 72 hours of UUO (5A,5B) and was also observed in the absence of TCR stimulation (Supplemental Figure S3). The effect was absent for CM from all other fractions. These observations confirmed the production by intra-renal leukocytes of one or more soluble factors capable of enhancing Th17 cell activation. The expression of four candidate mediators – IL-1α, IL-1β, IL-23 and IL-6 – was analysed by ELISA in CM of CD11c+ (DC-enriched), CD45+CD11c− (Non-DC leukocytes) and CD45− (non-leukocyte) cells of obstructed and control cells 72 hours post-UUO (Figure 6). Increased secretion of IL-1α and IL-1β was observed for both DC-enriched and DC-depleted leukocytes from obstructed kidneys. Increased production of IL-23 at this time-point was observed for DC-depleted leukocytes alone and increased IL-6 was predominantly confined to non-leukocyte renal cells. Consistent results were also obtained by qRT-PCR carried out on the same cell populations analysed without in vitro culture (Supplemental Figure S4A). Moreover, addition of CM from CD11c+ cells from obstructed kidneys to in vitro-generated Th17 induced a similar enhancing effect on IL-17A production to that observed for total CD45+ cells (Supplemental Figure S4B and S4C).
Figure 5. Leukocytes from obstructed kidney release Th17 cell activating factors.
In vitro-generated mouse Th17 cells were re-stimulated by overnight culture with 0.1 μg/ml anti-CD3 antibody in the absence (white columns) or presence (all other columns) of conditioned media from CD45-depleted (CD45−) or CD45-enriched (CD45+) cells of control (CK) or obstructed (OK) kidneys following 24, 48 and 72 hours of UUO. IL-17A production by the re-stimulated Th17 cells under all conditions was measured by intra-cellular flow cytometry expressed as % IL-17+ among the total CD4+ cells (A, see Supplemental Figure S2 for examples of staining) and by assay of supernatants for IL-17A concentration expressed as pg/ml (B). All results are presented as Mean ± SD of 3 individual samples. † = p<0.05 compared to equivalent control kidney conditioned medium addition, ‡ = p<0.05 compared to equivalent CD45− conditioned medium addition.
Figure 6. Secretion of candidate Th17 activation factors by leukocyte and non-leukocyte cell populations of obstructed kidneys.
Cytokine (IL-1α, IL-1β, IL-23 and IL-6) concentrations in culture supernatants of cell populations from control (CK) and obstructed (OK) kidneys prepared following 72 hours of UUO by magnetic column separations and placed in culture overnight. Cell populations were DC-enriched (CD11c+), Non-DC leukocytes (CD45+CD11c−) and non-leukocyte renal cells (CD45−). Results for are expressed as means ± SD pg/ml for three individual supernatants from each group of sorted cells. † = p<0.05 compared with equivalent control kidney sample.
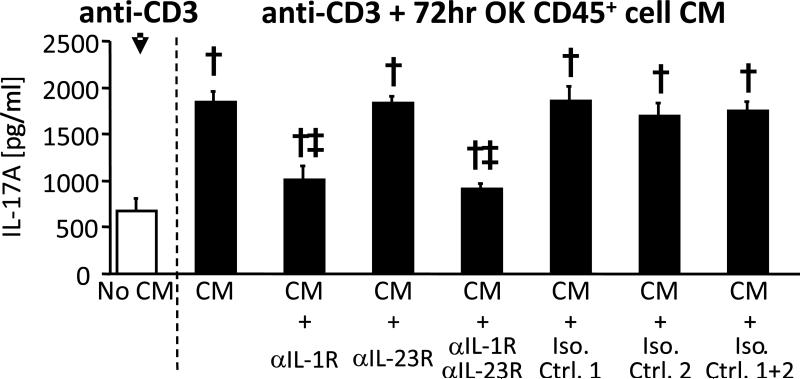
IL-1 is responsible for intra-renal Th17-enhancing activity following UUO
The contribution of IL-1α/β and IL-23 to the Th17-enhancing effect of CM from CD45+ cells of obstructed kidney was examined using blocking antibodies against the receptors for IL-1α/β and IL-23 (Figure 7). The induction of IL-17A production by CM of CD45+ cells from obstructed kidney was abolished by blockade of IL-1 receptor (IL-1RI/CD121a) but not by blockade of IL-23 receptor (IL-23R). To confirm the role of IL-1 in intra-renal Th17 activation, UUO was carried out for 72 hours in IL-1RI knockout (IL-1RI-KO) and wild-type (WT) mice with Th17 activity analysed by intra-cellular staining (Figure 8A-C) and ELISA (Figure 8D) of anti-CD3-stimulated cells from obstructed kidneys. Intra-renal Th1 responses were similarly compared using assays for IFN-γ. The results revealed reduced Th17 activity in obstructed kidneys of IL-1RI-KO animals in the form of reduced mean fluorescence intensity (MFI) of anti-IL-17A staining among the IL-17A+ cells (Figure 8C) and reduced concentration of IL-17A in culture supernatants (Figure 8D). In contrast, obstructed kidneys from IL-1RI-KO animals had increased Th1 activity (8A-D). The obstructed and control kidneys of IL-1RI-KO mice did not have reduced frequency of CD4+ or CD4+CCR6+ T-cells (Supplemental Figure S5). The results suggested that signalling through the IL-1RI is unnecessary for intra-renal accumulation of CD4+CCR6+ cells following UUO but regulates the proportion of Th cells that are competent for IL-17A secretion.
Figure 7. Increased IL-17A production by in vitro generated Th17 cells is inhibited by IL-1R1 blockade.
IL-17A concentrations in culture supernatants from in vitro-generated Th17 cells stimulated for 24 hours with 0.1 μg/ml anti-CD3 in absence (white column) or presence (all other columns) of conditioned medium (CM) from total leukocytes (CD45+) cells of 72 hour obstructed kidneys without and with addition of blocking antibodies or relevant isotype control antibodies (Iso. Ctrl.). Blocking antibodies were: anti-IL-1R1 (2.5 μg/ml) and/or anti-IL-23R (5 μg/ml). Results are expressed as Means ± SD pg/ml for triplicate wells of each condition. † = p<0.05 compared to No CM control sample; ‡ = p<0.05 compared to result for relevant isotype control.
Figure 8. Diminished renal Th17 activity in obstructed kidneys of IL-R1-deficient mice.
Wild type and IL-1RI knockout (KO) mice were subjected to UUO for 72 hours. Whole kidney cell suspensions (A-C) and total CD45+ leukocytes (D) from obstructed kidneys were stimulated overnight with 1.0 μg/ml anti-CD3 antibody. A. Examples of flow cytometry analysis of IL-17A and IFN-γ intracellular staining of CD45+CD4+ The percent of analyzed cells with positive staining for CD45 (left plots), IL-17A (middle plots) and IFN-γ (right plots) are shown. B. Graphical representation of intracellular cytometry analyses for IL-17A and IFN-γ expressed as Means ± SD of the percent of CD45+/CD4+ T-cells that were cytokine-positive for individual obstructed kidneys (n = 5 per group). C. Graphical representation of intracellular cytometry analyses for IL-17A and IFN-γ expressed as Means ± SD of the mean fluorescence intensity (MFI) of intra-cellular cytokine staining among the cytokine-positive CD45+CD4+ T-cells for individual obstructed kidneys. D IL-17A and IFN-γ ELISA results from culture supernatants of anti-CD3-stimulated total CD45+ leukocytes from 72 hour control and obstructed kidneys of IL-1R-1 wild-type and IL-1R1 knockout mice. Results are expressed as Means ± SD pg/ml for triplicate samples from each condition. † = p<0.05 compared to equivalent result for WT.
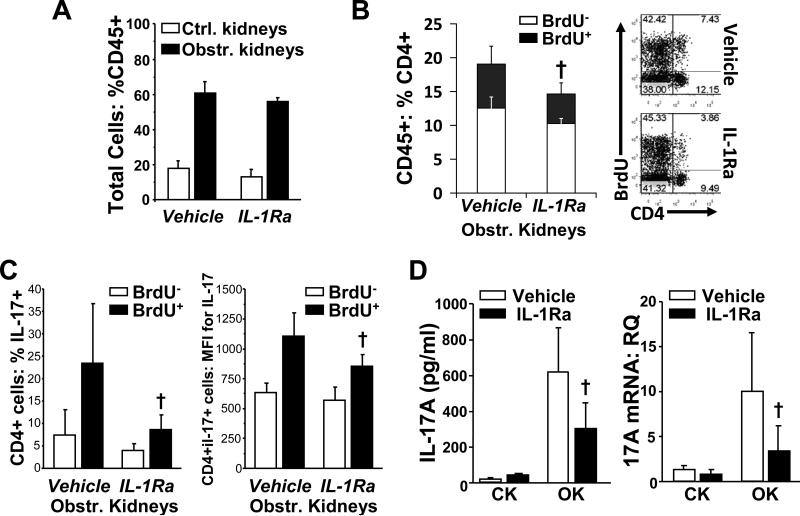
To elucidate the contribution of IL-1 to both proliferation and activation of intra-renal Th17 cells in genetically normal animals, groups of mice were administered the recombinant IL-1R1 antagonist, anakinra, (IL-1Ra) or vehicle for 72 hours post-UUO along with continuous in vivo BrdU labelling. IL-1Ra administration did not result in an overall reduction in proportionate accumulation of CD45+ leukocytes in obstructed kidneys (Figure 9A) or in the proportion of intra-renal CD45+ that were BrdU+ (data not shown). Nonetheless, the proportion of CD4+ T-cells among the CD45+ cells was reduced in obstructed kidneys of IL-1Ra-treated mice – primarily due to reduced proportion of CD4+BrdU+ cells (Figure 9B). Intracellular staining of anti-CD3-stimulated cells from obstructed kidneys demonstrated that, for BrdU+, but not BrdU− CD4+ T-cells, IL-1Ra administration was associated with reductions in the proportion that were IL-17A+ as well in the MFI of anti-IL-17A staining among the IL-17A+ cells (Figure 9C). Reduced Th17 activity in obstructed kidneys of IL-1Ra-treated mice was also demonstrated by IL-17A ELISA of anti-CD3-stimulated culture supernatants and by qRT-PCR of renal cortical tissue for IL-17A mRNA (Figure 9D). In contrast to results for IL-1R1-KO mice, IL-1Ra therapy was not associated with evidence of enhanced intra-renal Th1 activity (Supplemental Figure S6A). Analyses of splenic T-cells indicated that there was no extra-renal effect of IL-1Ra on proliferation rate of CD4+ T-cells or on anti-CD3-stimulated production of IL-17A and IFNγ (Supplemental Figure S6B and S6C). Histological analysis of the kidneys from this experiment demonstrated a modest protective effect of IL-1Ra therapy against tubular dilatation and atrophy but not against interstitial inflammatory cell infiltration (Supplemental Figure 7). In keeping with Jones et al,40 this observation indicated that IL-1 family cytokines and their associated enhancement of intra-renal Th17 activity are not the predominant drivers of renal damage in this model.
Figure 9. Diminished proliferation and activation of renal Th17 cells in obstructed kidneys of mice treated with IL-1Ra.
Groups of mice (n = 6) subjected to UUO for 72 hours were continuously exposed to BrdU via drinking water and received daily i.p. injections of vehicle or of IL-1Ra. A. Proportions of leukocytes (CD45+) among total cell suspensions from non-obstructed control kidneys and obstructed kidneys of the two groups by flow cytometric analysis. B. Left: Proportions of non-proliferative (BrdU−) and proliferative (BrdU+) CD4+ T-cells among the CD45+ leukocytes from obstructed kidneys of the two groups by combined surface and intracellular flow cytometric analysis. Right: Examples of flow cytometric dot plots used to co-analyze CD4 and BrdU staining following gating on CD45+ cells of obstructed kidneys. C. Magnetic column-separated CD45+ cells from obstructed kidney were stimulated overnight with low-dose anti-CD3 antibody and were analyzed by surface and intracellular flow cytometry for the proportions of IL-17A+ cells (left) and the mean fluorescence intensity of IL-17A staining (right) among the BrdU− and BrdU+ CD4+ T-cells. D. Culture supernatants from anti-CD3-stimulated, CD45+ cells of CK and OK from the two groups were analyzed by ELISA for IL-17A production (left). Whole renal cortex samples of CK and OK from the two groups were analyzed by qRT-PCR for relative quantity (RQ) of IL-17A mRNA (right). All results are presented as mean ± SD. † = p < 0.05 compared to equivalent result for vehicle-treated group.
Th17-enhancing IL-1 is produced by DCs and monocytes in obstructed kidney
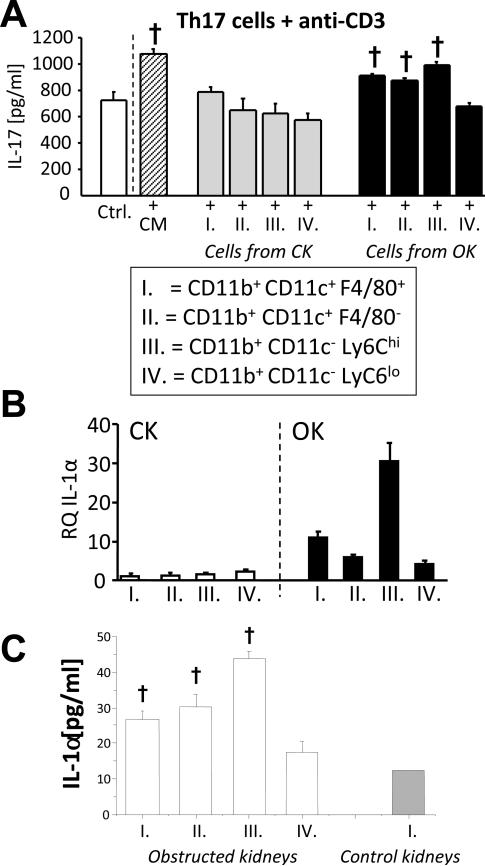
To more clearly identify cellular sources of Th17-enhancing activity following UUO, four individual myeloid cell populations were sorted to high purity by FACS from 72-hour obstructed and control kidneys based on surface expression of CD11b, CD11c, F4/80 and Ly6C. These populations were designated: I. F4/80+ DCs (CD11b+CD11c+F4/80+Ly6C−). II. F4/80− DCs (CD11b+CD11c+F4/80−Ly6C−). III. Inflammatory monocytes (CD11b+CD11c−Ly6Chi). IV. Macrophages (CD11b+CD11c−Ly6Clo) (Supplemental Figure S8). Co-culture of F4/80+ DCs, F4/80− DCs and inflammatory monocytes (but not macrophages) from obstructed kidneys with re-stimulated, in vitro-generated Th17 cells resulted in enhanced IL-17A production (Figure 10A). Of interest, CM from total CD45+ cells and from the sorted myeloid cell subpopulations did not enhance IFN-γ production by re-stimulated in vitro-generated Th1 cells (Supplemental Figure S9). Quantitative RT-PCR analysis and ELISA of culture supernatants from the sorted populations confirmed elevated IL-1α expression by inflammatory monocytes and both F4/80+ and F4/80− DCs (Figure 10B & 10C). A similar expression pattern was observed for IL-23, whereas IL-6 expression was found to be highest in macrophages purified from obstructed kidney (Supplemental Figure S10). Taken together, the results indicate that IL-1α and/or IL-1β represent important intra-renal Th17 enhancing factors and are produced within obstructed kidney both by resident (DCs) and infiltrating (monocytes) myeloid cell populations.
Figure 10. Release of Th17 activation factors by multiple renal myeloid cell populations following UUO.
A. IL-17A ELISA results for supernatants of in vitro generated Th17 cells that were stimulated for 24 hours with anti-CD3 in the presence of control medium (Ctrl.) or conditioned medium from total CD45+ leukocytes of 72 hour obstructed kidneys (CM) or were co-cultured with 4 different myeloid cell populations FACS-purified from control (CK) and obstructed (OK) kidneys 72 hours after UUO (n = 5). The surface marker characteristics of the 4 myeloid cell populations are listed in the box. Staining and purity of sorted cells are presented in Supplemental Figure S6 † = p<0.05 compared to Ctrl. B. Quantitative RT-PCR result showing relative quantity (RQ) of IL-1α mRNA in FACS-purified cell populations expressed as fold expression relative to population I from control kidneys. C IL-1α ELISA result for supernatants of populations I – IV (obstructed kidneys) and population I (control kidneys) placed in culture in equal numbers for 24 hours following FACS purification. † = p<0.05 compared to result for control kidney population I.
Discussion
To our knowledge, the present study is the first to identify the basis for activation of Th17 cells within the injured kidney, namely, locally-produced IL-1 originating from DCs and monocytes. Moreover, using mice deficient in IL-1R1 and administration of IL-1Ra, we demonstrate the non-redundant role of IL-1 as a mediator of Th17 cell activation and proliferation in vivo following UUO. As such, our studies provide novel insights regarding the nature of the processes that enable the accumulation and activation of Th17 cells in the injured kidney.
In recent years, important roles for Th17 cells, their dominant secreted product, IL-17A, and the factors responsible for their differentiation and activation have been demonstrated in a range of kidney diseases.18, 19 Increased intra-renal leukocyte expression of IL-17A has been reported in human and rodent transplant rejection, lupus nephritis and crescentic glomerulonephritis as well as in human nephrotic syndrome and rodent ischemia-reperfusion injury (IRI).17, 21, 32-36, 39, 41, 42 Deficiency or blockade of IL-17A ameliorates the severity of kidney disease in rodent models of IRI, nephrotoxic serum nephritis (NTN) and anti-myeloperoxidase glomerulonephritis,17, 21, 37 while the presence and/or frequency of intra-renal IL-17-producing lymphocytes correlates with disease severity in human lupus nephritis and chronic transplant rejection.36, 39
Of interest, this recent literature indicates that the potential cellular sources for pathogenic IL-17A within the kidney extend beyond conventional CD4+ T-cells to include neutrophils,21 CD4−CD8− (double-negative) T-cells33, 34 and γδ-T-cells.43 Nevertheless, our own prior observations in UUO20 and those of others in glomerulonephritis17, 44, 45 and transplant rejection39 confirm that diverse forms of progressive renal immune/inflammatory disease are associated with interstitial accumulation of bona fide Th17 cells expressing markers of effector-memory and activated phenotypes. In the current study, we confirm that UUO produces a progressive increase in IL-17A expression within obstructed renal cortex over 72 hours and, using magnetic cell separation and high-level purification by FACS, we sequentially localise IL-17A mRNA to CD45+ leukocytes, CD4+ T-cells and, finally, the CCR6+CD4+ T-cell sub-fraction. The observation indicates a degree of intrinsic activation of Th17 cells as detectable IL-17A transcript was absent in equivalent fractions from non-obstructed kidneys. Given the presumed non-antigen-specific nature of obstruction-associated inflammatory injury, this finding is in keeping with so-called “bystander activation” of effector-memory Th17 cells although TCR specificity for renal auto-antigens remains an intriguing possibility.46, 47 In keeping with the recent findings of Turner et al. in NTN,45 we find that intra-renal Th17 cells in UUO are predominantly CCR6+. At 72 hours, up to one third of CD4+CCR6+ cells were capable of rapid IL-17A production upon low-level TCR stimulation. Notably, the number of CD4+CCR6+ Th cells increased over 10-fold in obstructed kidneys between 24 and 96 hours following UUO with a high proportion of these having incorporated BrdU by 72 hours. Although this does not formally identify the site of T-cell proliferation, the lower rate of BrdU incorporation in the equivalent subset from the non-obstructed kidneys provides evidence that intra-renal proliferation is also an important mechanism of Th17 cell accumulation in acute kidney injury.
Recently, a role has emerged for IL-23 in the differentiation and activation of pathogenic Th17 cells and other IL-17A-producing leukocytes in rodent models of glomerulonephritis and IRI and in human ANCA-associated vasculitis.17, 21, 32, 38, 48 In other autoimmune and inflammatory conditions, IL-1 has been identified as an important local mediator of pathogenic Th17 cell activation.29, 43 In the current study, we demonstrate the secretion of soluble Th17-enhancing activity by cells from obstructed kidneys. This activity was localised initially to CD45+ intra-renal leukocytes (both CD11c+ and CD11c−) and next to F4/80+ DCs, F4/80− DCs and Ly6Chi (inflammatory) monocytes though not to Ly6Clo macrophages. Interestingly, while increased expression of both IL-1α/β and IL-23 were observed in DCs and monocytes from obstructed kidneys, IL-1 family cytokines, acting via IL-1RI, were found to be entirely responsible for this in vitro Th17-activating effect. The in vivo relevance of this finding was validated in IL-1R1-deficient mice in which Th17 cell activity was reduced in obstructed kidneys compared to wild-type animals despite comparable, or greater, accumulation of CD4+CCR6+ T-cells. Similarly, mice treated with IL-1Ra following UUO demonstrated reduced intra-renal Th17 activity. In this experiment, concomitant BrdU labelling indicated that IL-1R blockade reduced Th17 activity among proliferative (BrdU+) Th cells from obstructed kidneys. In contrast, BrdU incorporation and IL-17A production by splenic Th cells was unaffected. Thus, we convincingly show that both activation and proliferation of intra-renal Th17 cells are positively regulated by IL-1. While this does not rule out a role for IL-23 in other aspects of the triggering of Th17 cells during intra-renal inflammation, it does emphasise the significance of IL-1 in specifically promoting Th17 cell activation and expansion at sites of localised “sterile” inflammation.49, 50
Given the growing interest in clinical application of IL-1 antagonists and blocking antibodies to inflammatory diseases,50, 51 the potential to target pathogenic renal Th17 responses by this strategy merits consideration. Notably, Timoshanko et al. observed attenuation of experimental glomerulonephritis in mice lacking IL-1R1, IL-1α or IL-1β and Furuichi et al demonstrated reduced early severity of renal ischemia reperfusion injury in IL-1α/β-deficient mice as well as exacerbated injury in mice genetically lacking IL-1Ra.52, 53 In UUO, Jones et al. observed reduced intra-renal pro-fibrotic activity at 7 (but not 14) days in IL-1R1-deficient compared to wild-type mice although T-cell infiltration and Th activity was not analyzed in this study.40 In our own experiments using IL-1Ra during the initial 72 hours following UUO, we observed only a mild protective effect against tubular dilatation and atrophy by histological analysis with no overt reduction in the extent of interstitial cellular infiltrates. Furthermore, despite the inhibition of Th17 activity, neutrophil infiltration of obstructed kidneys at this time-point was not reduced when analyzed by flow cytometry (data not shown). Thus, while UUO has proven to be of value as a model system in which to evaluate the dynamics and activation factors for intra-renal Th17 cells during kidney injury, our data suggests that IL-1 blockade and its associated inhibition of localised Th17 activity may be of limited benefit in obstructive renal injury. As Kitching and Holdsworth have recently highlighted, cross-regulation of Th17 and Th1 cells during inflammation could result in augmentation of harmful Th1 responses following specific Th17 targeting.18 However, while we observed increased numbers of CD4+IFNγ+ T-cells in obstructed kidneys of IL-1R1-KO compared to WT mice, this phenomenon was not reproduced in IL1Ra-treated mice in which a trend toward reduced Th1 activity in obstructed kidneys was observed in the form of lower anti-CD3-stimulated IFNγ production (see Supplemental Figure S6A). In this experiment it was not possible to accurately co-stain for intracellular BrdU and IFNγ (data not shown). Thus, while our findings clearly document the enhancing effect of IL-1 on intra-renal Th17 activation and proliferation, its role in regulating intra-renal Th1 (as well as other Th subsets) remains unclear. In the broader sense, the lack of robust benefit of IL-1 blockade and intra-renal Th17 suppression in UUO likely reflects the general observation that therapeutic approaches for inflammatory diseases based on targeting of a single cytokine/chemokine have been less successful than a multi-pronged approach is employed. In contrast, antigen-driven, immune-mediated kidney diseases in which Th17 cells have been shown to play a major pathogenic role (e.g. ANCA-associated vasculitis and crescentic glomerulonephritis) may prove to be more responsive to IL-1 blockade either alone or in combination with inhibition of other Th17-differentiation and activation factors such as IL-23, IL-6 and TGFβ1. It should also be noted that Th17 cells represent only one discreet target for locally-produced IL-1 within the kidney and, as shown by Timoshanko et al in chimeric mice, the responses of non-immune, resident renal cells to IL-1 contribute significantly to TNF production and glomerular injury in a model of crescentic glomerulonephritis.52 In summary, we have employed the UUO model as an “incubator” of localised Th17 cell accumulation within the inflamed kidney. Our findings favour the following conclusions: (a) Effector-memory CD4+ T-cells pre-programmed for IL-17A production accumulate in substantial numbers within injured kidney through chemokine receptor-specific recruitment and localised proliferation. (b) Even without antigenic priming, acute renal inflammation induces local activation of Th17 cells of which IL-1, produced by DCs and inflammatory monocytes, is a key mediator. Our findings will be of value in better defining the dynamics and pathogenic properties of intra-renal Th17 cells, their dependence upon activation factors secreted locally within the kidney by cells of the mononuclear phagocyte system and the targets available for inhibiting their effector functions during kidney disease.
Methods
Experimental animals
Eight to 12-week old female C57BL/6 (B6) mice were purchased from Harlan Laboratories UK (Bicester, UK). Mice genetically deficient in IL-1R1 on a B6 background, originally from Jackson Laboratories, Bar Harbor, ME, were bred on-site. Animals were housed in specific pathogen-free facilities. Procedures were carried out under licence from the Irish Department of Health and Children and approved by the NUI Galway Animal Ethics Committee.
Reagents
Antibodies, culture media, buffer solutions, ELISA reagents and other materials used in the study are detailed in Supplemental Methods.
Cell Cultures
Cells were cultured in 96-well round-bottom plates at 106 cells/ml with other additions as described for individual experiments. For ELISA of secreted products, supernatants were withdrawn at 24 hours. For surface and intracellular staining of cultured cells, Brefeldin A (GolgiPlug® 1μl/ml, BD Biosciences) was added for 8 hours prior to analysis. For preparation of conditioned media, magnetic column-purified cells were placed in culture at 106 cells/ml for 24 hours following which media were withdrawn and frozen at −20°C prior to being used at 1:1 ratio with fresh medium as described for individual experiments.
Unilateral ureteral obstruction (UUO) and preparation of kidney cell digests (see also Supplemental Methods)
Mouse UUO with preparation of cell suspensions between 24 and 96 hours later by collagenase/DNase digestion was conducted as previously described.20,54 In some experiments, mice received a bolus of BrdU intra-peritoneally at the time of surgery followed by continuous exposure to BrdU-containing water for 72 hours. In some experiments, groups of mice also received the IL-1R antagonist (IL-1Ra), anakinra, 100 mg/kg i.p. in 250 μl of PBS or PBS alone by intraperitoneal injection at 0, 24, and 48 hours following UUO.
Flow cytometry, fluorescence-activated cell sorting (FACS) and magnetic column separation of kidney cells
Kidney cells suspended in 100 μl aliquots of FACS buffer were incubated with 3-5 fluorochrome-labelled and/or biotinylated antibodies for 30 minutes at 4°C followed by washing in FACS buffer and, if necessary, incubation with fluorochrome-labelled streptavidin for 30 minutes at 4°C and were re-suspended in 4% paraformaldehyde in PBS. Intracellular staining was carried out using the Cytofix/Cytoperm® and BrdU detection kits (BD Pharmingen) according to manufacturer's instructions. Analysis as performed using a BD Biosciences FACSCanto® flow cytometer and FlowJo® software (TreeStar Inc., Olten, Switzerland).
For FACS, cell suspensions from 3-5 kidneys were re-suspended in FACS sorting buffer with fluorochrome- and biotin-labelled monoclonal antibodies for 30 minutes at 4°C then were washed, filtered through 40 μm mesh and sorted using a BD Biosciences FACSAriaII® sorter with purity determined by post-sort analysis.
Magnetic column separations of kidney cell suspensions were carried out in MACS buffer by manufacturer-recommended protocols using MS columns and an OctoMACS® separator (Miltenyi Biotec Inc., Auburn, CA, USA). For individual experiments, positive and/or negative column fractions were retained, washed in MACS buffer and used for culture and/or qRT-PCR. In some experiments cell suspensions were first subjected to anti-CD11c magnetic column separation then the negative fractions was subjected to anti-CD45 magnetic column separation to sequentially prepare CD11c+, CD45+CD11c− and CD45− cell fractions.
Cultures with in vitro-generated Th17 and Th1 cells
Th17- and Th1-skewed mouse CD4+ T-cell cultures were generated over 10 days from splenocytes of healthy adult mice using standard protocols (see Supplemental Methods for details). For re-stimulation experiments, CD4+ T-cells were re-purified from differentiation cultures by magnetic column separation then were plated in fresh culture medium 106 cells/ml with or without anti-CD3 (1.0 or 0.1 μg/ml) for 24 hours. In individual experiments, conditioned or control media were added at 1:1 ratio with fresh medium. In some experiments, previously optimized concentrations of blocking antibodies or relevant isotype control antibodies were also added. In others, FACS-purified cells from non-obstructed and obstructed kidneys were added at 1:10 ratio. After 24 hours, concentrations of IL-17A and IFN-γ were measured in culture supernatants by ELISA.
RNA preparation and quantitative (Real Time) RT-PCR (see also Supplemental Methods)
Total RNA was isolated from whole kidney or sorted cell populations using RNeasy Mini or Micro kits (Qiagen Inc., Valencia, CA). RNA was converted to cDNA using High Capacity cDNA Reverse Transcription Kit with RNase Inhibitor (Applied Biosystems, Carlsbad, CA). Equal amounts of cDNA were analyzed by Real-Time quantitative PCR using TaqMan® Master Mix and commercial primer/probe sets for mouse GAPDH, IL-1α, IL-1β, IL-17A, IL-6 and IL-23(p19) on a StepOne Plus® Real Time PCR System (all from Applied Biosystems). Relative quantifications were performed using the comparative CT method with normalization to GAPDH and results expressed as fold difference relative to a relevant control sample.
Statistical analysis
Individual experiments were carried out between 2 and 6 times to ensure reproducibility. For culture experiments, between 3 and 6 replicates were initiated and individually analyzed for each condition. Data are presented as Means ± SD. Statistical comparisons between individual groups were performed by unpaired, two-tailed t-tests using Microcal Origin™ V6.0 software (Northampton, MA) with significance assigned at p <0.05.
Supplementary Material
Acknowledgments
Sources of Support: This study was supported by Science Foundation Ireland under grant numbers SFI PI 06/IN.1/B652 (MG), SFI 09/SRC/B1794 (MG and RC), by a Science Foundation Ireland Stoke's Professorship (RC), by NIH Grant DK68545 (MG and KN) and by a project grant from the Health Research Board of Ireland (CS).
Footnotes
Disclosure
KM is a co-founder and minority shareholder in Opsona Therapeutics, a Start-up company involved in the development of anti-inflammatory therapeutics.
The study was presented, in part, at the American Society of Nephrology's Renal Week 2010 Meeting in Denver, CO in November 2010.
References
- 1.Bajwa A, Kinsey G, Okusa M. Immune mechanisms and novel pharmacological therapies of acute kidney injury. Curr Drug Targets. 2009;10:1–196-1204. doi: 10.2174/138945009789753174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedewald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int. 2004;66:4–86-491. doi: 10.1111/j.1523-1755.2004.761_3.x. [DOI] [PubMed] [Google Scholar]
- 3.Schrier RW, Wang W, Poole B, et al. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest. 2004;114:5–-14. doi: 10.1172/JCI22353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Venkatachalam MA, Griffin KA, Lan R, et al. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol - Renal Physiol. 2010;298:F–1078-F1094. doi: 10.1152/ajprenal.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 2009;75:1–145-1152. doi: 10.1038/ki.2009.86. [DOI] [PubMed] [Google Scholar]
- 6.Ysebaert DK, De Greef KE, De Beuf A, et al. T cells as mediators in renal ischemia/reperfusion injury. Kidney Int. 2004;66:4–91-496. doi: 10.1111/j.1523-1755.2004.761_4.x. [DOI] [PubMed] [Google Scholar]
- 7.Tapmeier TT, Fearn A, Brown K, et al. Pivotal role of CD4+ T cells in renal fibrosis following ureteric obstruction. Kidney Int. 2010;78:3–51-362. doi: 10.1038/ki.2010.177. [DOI] [PubMed] [Google Scholar]
- 8.Burne MJ, Daniels F, El Ghandour A, et al. Identification of the CD4+ T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest. 2001;108:1–283-1290. doi: 10.1172/JCI12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Summers SA, Steinmetz OM, Li M, et al. Th1 and Th17 Cells Induce Proliferative Glomerulonephritis. J Am Soc Nephrol. 2009;20:2–518-2524. doi: 10.1681/ASN.2009030337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heymann F, Meyer-Schwesinger C, Hamilton-Williams EE, et al. Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury. J Clin Invest. 2009;119:1–286-1297. doi: 10.1172/JCI38399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mucida D, Cheroutre H, Sidonia F, et al. The many face-lifts of CD4 T helper cells. Adv Immunol. 2010;107:1–39-52. doi: 10.1016/B978-0-12-381300-8.00005-8. [DOI] [PubMed] [Google Scholar]
- 12.Farrar JD, Asnagli H, Murphy KM. T helper subset development: roles of instruction, selection, and transcription. J Clin Invest. 2002;109:4–31-435. doi: 10.1172/JCI15093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moseley TA, Haudenschild DR, Rose L, et al. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:1–55-174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 14.Mills KHG. Induction, function and regulation of IL-17-producing T cells. Eur J Immunol. 2008;38:2–636-2649. doi: 10.1002/eji.200838535. [DOI] [PubMed] [Google Scholar]
- 15.Bettelli E, Oukka M, Kuchroo VK. TH-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:3–45-350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 16.Woltman AM, De Haij S, Boonstra JG, et al. Interleukin-17 and CD40-Ligand synergistically enhance cytokine and chemokine production by renal epithelial cells. J Am Soc Nephrol. 2000;11:2–044-2055. doi: 10.1681/ASN.V11112044. [DOI] [PubMed] [Google Scholar]
- 17.Paust H-J, Turner J-E, Steinmetz OM, et al. The IL-23/Th17 axis contributes to renal injury in experimental glomerulonephritis. J Am Soc Nephrol. 2009;20:9–69-979. doi: 10.1681/ASN.2008050556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitching AR, Holdsworth SR. The emergence of Th17 cells as effectors of renal injury. J Am Soc Nephrol. 2011;22:2–35-238. doi: 10.1681/ASN.2010050536. [DOI] [PubMed] [Google Scholar]
- 19.Turner J-E, Paust H-J, Steinmetz OM, et al. The Th17 immune response in renal inflammation. Kidney Int. 2010;77:1–070-1075. doi: 10.1038/ki.2010.102. [DOI] [PubMed] [Google Scholar]
- 20.Dong X, Bachman LA, Miller MN, et al. Dendritic cells facilitate accumulation of IL-17 T cells in the kidney following acute renal obstruction. Kidney Int. 2008;74:1–294-1309. doi: 10.1038/ki.2008.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Huang L, Vergis AL, et al. IL-17 produced by neutrophils regulates IFN-γ mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest. 2010;120:3–31-342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kryczek I, Banerjee M, Cheng P, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1–141-1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Acosta-Rodriguez EV, Rivino L, Geginat J, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:6–39-646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 24.Nakae S, Iwakura Y, Suto H, et al. Phenotypic differences between Th1 and Th17 cells and negative regulation of Th1 cell differentiation by IL-17. J Leukoc Biol. 2007;81:1–258-1268. doi: 10.1189/jlb.1006610. [DOI] [PubMed] [Google Scholar]
- 25.Acosta-Rodriguez E, Napolitani G, Lanzavecchia A, et al. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:9–42 - 949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 26.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:2–81-286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Manel N, Unutmaz D, Littman D. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORγt. Nat Immunol. 2008;9:6–41 - 649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volpe E, Servant N, Zollinger R, et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:6–50 - 657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 29.Sutton C, Brereton C, Keogh B, et al. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1–685-1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung Y, Chang SH, Martinez GJ, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signalling. Immunity. 2009;30:5–76-587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H, Rohowsky-Kochan C. Regulation of IL-17 in human CCR6+ effector memory T cells. J Immunol. 2008;180:7–948-7957. doi: 10.4049/jimmunol.180.12.7948. [DOI] [PubMed] [Google Scholar]
- 32.Ooi JD, Phoon RKS, Holdsworth SR, et al. IL-23, not IL-12, directs autoimmunity to the Goodpasture antigen. J Am Soc Nephrol. 2009;20:9–80-989. doi: 10.1681/ASN.2008080891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Z, Kyttaris VC, Tsokos GC. The role of IL-23/IL-17 axis in lupus nephritis. J Immunol. 2009;183:3–160-3169. doi: 10.4049/jimmunol.0900385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crispín JC, Oukka M, Bayliss G, et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181:8–761-8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shao X, Yang X, Zhao X, et al. The prevalence of Th17 cells and FOXP3 regulate T cells (Treg) in children with primary nephrotic syndrome. Ped Nephrol. 2009;24:1–683-1690. doi: 10.1007/s00467-009-1194-x. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Ito S, Chino Y, et al. Laser microdissection-based analysis of cytokine balance in the kidneys of patients with lupus nephritis. Clin Exp Immunol. 2010;159:1–-10. doi: 10.1111/j.1365-2249.2009.04031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gan P-Y, Steinmetz OM, Tan DSY, et al. Th17 Cells promote autoimmune anti-myeloperoxidase glomerulonephritis. J Am Soc Nephrol. 2010;21:9–25-931. doi: 10.1681/ASN.2009070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nogueira E, Hamour S, Sawant D, et al. Serum IL-17 and IL-23 levels and autoantigen-specific Th17 cells are elevated in patients with ANCA-associated vasculitis. Nephrol Dial Transplant. 2010;25:2–209-2217. doi: 10.1093/ndt/gfp783. [DOI] [PubMed] [Google Scholar]
- 39.Deteix C, Attuil-Audenis V, Duthey A, et al. Intragraft Th17 infiltrate promotes lymphoid neogenesis and hastens clinical chronic rejection. J Immunol. 2010;184:5–344-5351. doi: 10.4049/jimmunol.0902999. [DOI] [PubMed] [Google Scholar]
- 40.Jones LK, O'Sullivan KM, Semple T, et al. IL-1RI deficiency ameliorates early experimental renal interstitial fibrosis. Nephrol Dial Transplant. 2009;24:3–024-3032. doi: 10.1093/ndt/gfp214. [DOI] [PubMed] [Google Scholar]
- 41.Hsieh HG, Loong CC, Lui WY, et al. IL-17 expression as a possible predictive parameter for subclinical renal allograft rejection. Transplant Int. 2001;14:2–87-298. doi: 10.1007/s001470100344. [DOI] [PubMed] [Google Scholar]
- 42.Van Kooten C, Boonstra JG, Paape ME, et al. Interleukin-17 activates human renal epithelial cells in vitro and is expressed during renal allograft rejection. J Am Soc Nephrol. 1998;9:1–526-1534. doi: 10.1681/ASN.V981526. [DOI] [PubMed] [Google Scholar]
- 43.Sutton CE, Lalor SJ, Sweeney CM, et al. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:3–31-341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Steinmetz OM, Turner J-E, Paust H-J, et al. CXCR3 mediates renal Th1 and Th17 immune response in murine lupus nephritis. J Immunol. 2009;183:4–693-4704. doi: 10.4049/jimmunol.0802626. [DOI] [PubMed] [Google Scholar]
- 45.Turner J-E, Paust H-J, Steinmetz OM, et al. CCR6 recruits regulatory T cells and Th17 cells to the kidney in glomerulonephritis. J Am Soc Nephrol. 2010;21:9–74-985. doi: 10.1681/ASN.2009070741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Macconi D, Chiabrando C, Schiarea S, et al. Proteasomal processing of albumin by renal dendritic cells generates antigenic peptides. J Am Soc Nephrol. 2009;20:1–23-130. doi: 10.1681/ASN.2007111233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rees A. Cross dendritic cells anger T cells after kidney injury. J Am Soc Nephrol. 2009;20:3–-5. doi: 10.1681/ASN.2008111200. [DOI] [PubMed] [Google Scholar]
- 48.Edgerton C, Crispín JC, Moratz CM, et al. IL-17 producing CD4+ T cells mediate accelerated ischemia/reperfusion-induced injury in autoimmunity-prone mice. Clin Immunol. 2009;130:3–13-321. doi: 10.1016/j.clim.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10:8–9-102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 50.Mills KHG, Dunne A. Immune modulation: IL-1, master mediator or initiator of inflammation. Nat Med. 2009;15:1–363-1364. doi: 10.1038/nm1209-1363. [DOI] [PubMed] [Google Scholar]
- 51.Dinarello CA. Blocking IL-1 in systemic inflammation. J Exp Med. 2005;201:1–355-1359. doi: 10.1084/jem.20050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Timoshanko JR, Kitching AR, Iwakura Y, et al. Contributions of IL-α and IL-β to crescentic glomerulonephritis in mice. J Am Soc Nephrol. 2004;15:9–10-918. doi: 10.1097/01.asn.0000115704.86897.f4. [DOI] [PubMed] [Google Scholar]
- 53.Furuichi K, Wada T, Iwata Y, et al. Interleukin-1-dependent sequential chemokine expression and inflammatory cell infiltration in ischemia-reperfusion injury. Crit Care Med. 2006;34:2–447-2455. doi: 10.1097/01.CCM.0000233878.36340.10. [DOI] [PubMed] [Google Scholar]
- 54.Dong X, Swaminathan S, Bachman LA, et al. Antigen presentation by dendritic cells in renal lymph nodes is linked to systemic and local injury to the kidney. Kidney Int. 2005;68:1–096-1108. doi: 10.1111/j.1523-1755.2005.00502.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.