Abstract
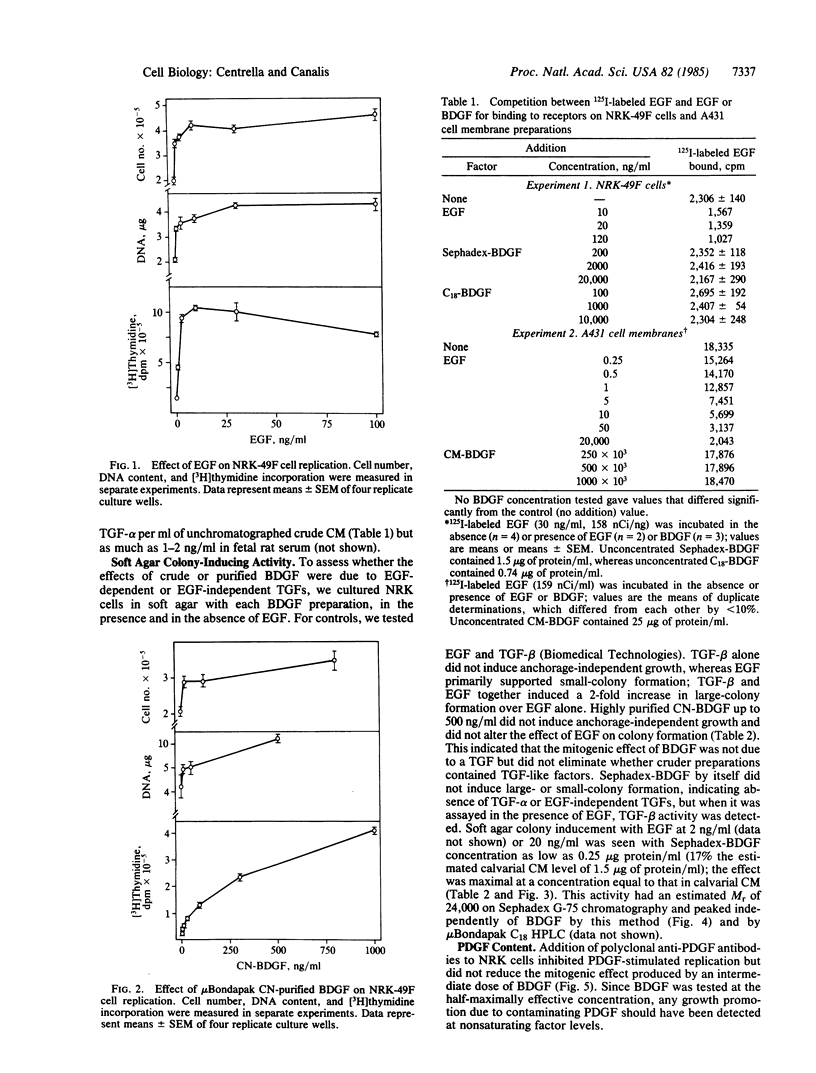
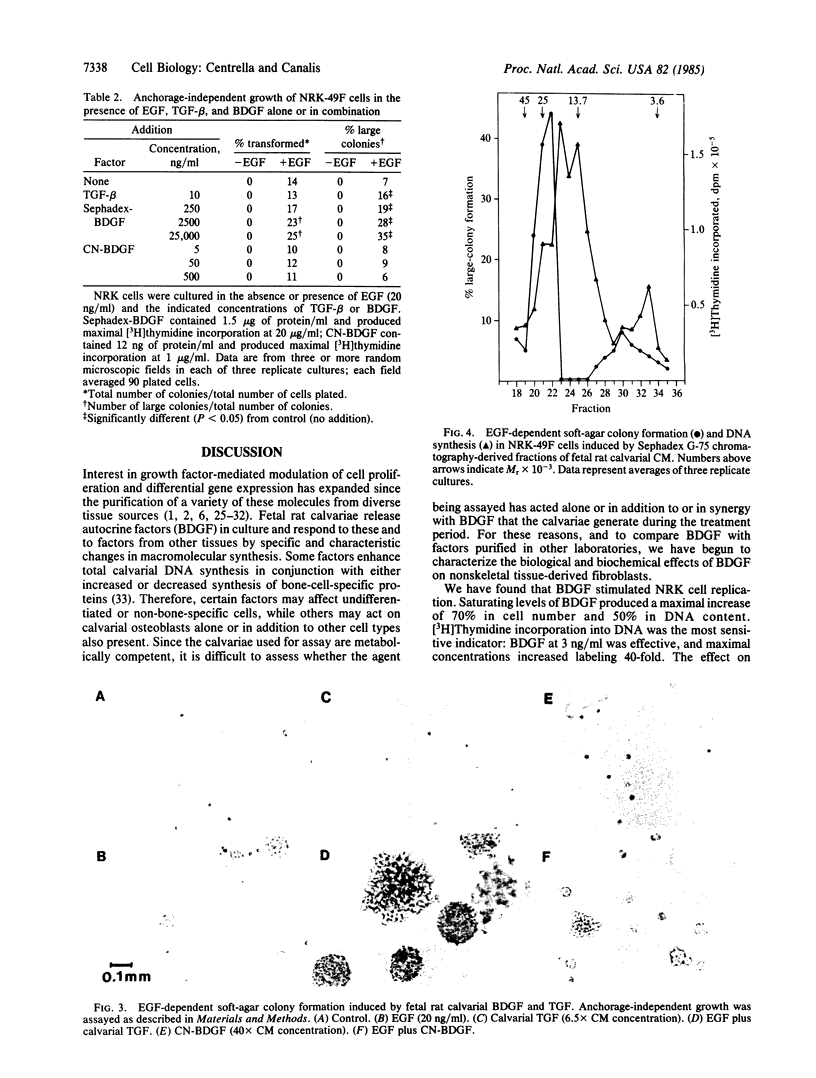
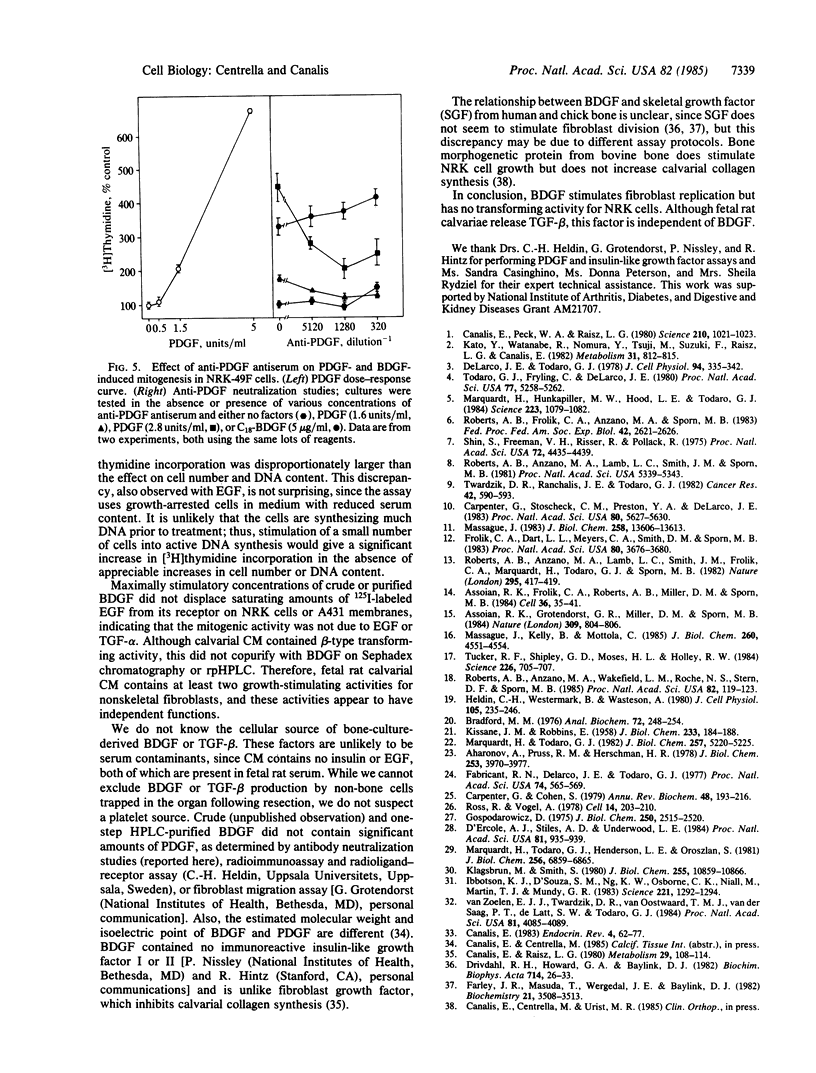
Conditioned medium recovered from fetal rat calvarial cultures contains an autocrine factor termed bone-derived growth factor (BDGF); this factor has been purified by acid extraction, gel-permeation chromatography, and two reversed-phase HPLC steps and examined for mitogenicity on normal rat kidney fibroblasts (NRK, clone 49F). HPLC-purified BDGF caused a dose-related increase in cell number, DNA content, and [3H]thymidine incorporation into acid-insoluble material. Since highly purified BDGF appeared less mitogenic than cruder preparations, the latter were tested for additional growth factors, with particular attention to those required for anchorage-independent colony formation in soft agar. BDGF did not displace 125I-labeled epidermal growth factor (EGF) in a radioligand-receptor assay, indicating the absence of EGF and transforming growth factor alpha (TGF-alpha). Without EGF, no BDGF preparation induced NRK cells to form soft agar colonies. However, calvarial conditioned medium contained a factor which, like TGF-beta, induced large soft-agar colonies in the presence of EGF; this TGF-beta-like factor did not copurify with BDGF. Polyclonal antibodies against platelet-derived growth factor did not neutralize the effects of BDGF on NRK cells. BDGF is a potent mitogen for nonskeletal-tissue-derived fibroblasts. Although crude BDGF preparations do contain TGF-beta, BDGF is distinct from this factor and others necessary for NRK cell transformation to anchorage-independent growth.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharonov A., Pruss R. M., Herschman H. R. Epidermal growth factor. Relationship between receptor regulation and mitogenesis in 3T3 cells. J Biol Chem. 1978 Jun 10;253(11):3970–3977. [PubMed] [Google Scholar]
- Assoian R. K., Frolik C. A., Roberts A. B., Miller D. M., Sporn M. B. Transforming growth factor-beta controls receptor levels for epidermal growth factor in NRK fibroblasts. Cell. 1984 Jan;36(1):35–41. doi: 10.1016/0092-8674(84)90071-0. [DOI] [PubMed] [Google Scholar]
- Assoian R. K., Grotendorst G. R., Miller D. M., Sporn M. B. Cellular transformation by coordinated action of three peptide growth factors from human platelets. 1984 Jun 28-Jul 4Nature. 309(5971):804–806. doi: 10.1038/309804a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Canalis E., Peck W. A., Raisz L. G. Stimulation of DNA and collagen synthesis by autologous growth factor in cultured fetal rat calvaria. Science. 1980 Nov 28;210(4473):1021–1023. doi: 10.1126/science.7434011. [DOI] [PubMed] [Google Scholar]
- Canalis E., Raisz L. G. Effect of fibroblast growth factor on cultured fetal rat calvaria. Metabolism. 1980 Feb;29(2):108–114. doi: 10.1016/0026-0495(80)90133-x. [DOI] [PubMed] [Google Scholar]
- Canalis E. The hormonal and local regulation of bone formation. Endocr Rev. 1983 Winter;4(1):62–77. doi: 10.1210/edrv-4-1-62. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Stoscheck C. M., Preston Y. A., DeLarco J. E. Antibodies to the epidermal growth factor receptor block the biological activities of sarcoma growth factor. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5627–5630. doi: 10.1073/pnas.80.18.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ercole A. J., Stiles A. D., Underwood L. E. Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proc Natl Acad Sci U S A. 1984 Feb;81(3):935–939. doi: 10.1073/pnas.81.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drivdahl R. H., Howard G. A., Baylink D. J. Extracts of bone contain a potent regulator of bone formation. Biochim Biophys Acta. 1982 Jan 12;714(1):26–33. doi: 10.1016/0304-4165(82)90123-4. [DOI] [PubMed] [Google Scholar]
- Fabricant R. N., De Larco J. E., Todaro G. J. Nerve growth factor receptors on human melanoma cells in culture. Proc Natl Acad Sci U S A. 1977 Feb;74(2):565–569. doi: 10.1073/pnas.74.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley J. R., Masuda T., Wergedal J. E., Baylink D. J. Human skeletal growth factor: characterization of the mitogenic effect on bone cells in vitro. Biochemistry. 1982 Jul 6;21(14):3508–3513. doi: 10.1021/bi00257a038. [DOI] [PubMed] [Google Scholar]
- Frolik C. A., Dart L. L., Meyers C. A., Smith D. M., Sporn M. B. Purification and initial characterization of a type beta transforming growth factor from human placenta. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3676–3680. doi: 10.1073/pnas.80.12.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D. Purification of a fibroblast growth factor from bovine pituitary. J Biol Chem. 1975 Apr 10;250(7):2515–2520. [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Chemical and biological properties of a growth factor from human-cultured osteosarcoma cells: resemblance with platelet-derived growth factor. J Cell Physiol. 1980 Nov;105(2):235–246. doi: 10.1002/jcp.1041050207. [DOI] [PubMed] [Google Scholar]
- Ibbotson K. J., D'Souza S. M., Ng K. W., Osborne C. K., Niall M., Martin T. J., Mundy G. R. Tumor-derived growth factor increases bone resorption in a tumor associated with humoral hypercalcemia of malignancy. Science. 1983 Sep 23;221(4617):1292–1294. doi: 10.1126/science.6577602. [DOI] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Kato Y., Watanabe R., Nomura Y., Tsuji M., Suzuki F., Raisz L. G., Canalis E. Effect of bone-derived growth factor on DNA, RNA, and proteoglycan synthesis in cultures of rabbit costal chondrocytes. Metabolism. 1982 Aug;31(8):812–815. doi: 10.1016/0026-0495(82)90080-4. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., Smith S. Purification of a cartilage-derived growth factor. J Biol Chem. 1980 Nov 25;255(22):10859–10866. [PubMed] [Google Scholar]
- Marquardt H., Hunkapiller M. W., Hood L. E., Todaro G. J. Rat transforming growth factor type 1: structure and relation to epidermal growth factor. Science. 1984 Mar 9;223(4640):1079–1082. doi: 10.1126/science.6320373. [DOI] [PubMed] [Google Scholar]
- Marquardt H., Todaro G. J., Henderson L. E., Oroszlan S. Purification and primary structure of a polypeptide with multiplication-stimulating activity from rat liver cell cultures. Homology with human insulin-like growth factor II. J Biol Chem. 1981 Jul 10;256(13):6859–6865. [PubMed] [Google Scholar]
- Marquardt H., Todaro G. J. Human transforming growth factor. Production by a melanoma cell line, purification, and initial characterization. J Biol Chem. 1982 May 10;257(9):5220–5225. [PubMed] [Google Scholar]
- Massagué J. Epidermal growth factor-like transforming growth factor. I. Isolation, chemical characterization, and potentiation by other transforming factors from feline sarcoma virus-transformed rat cells. J Biol Chem. 1983 Nov 25;258(22):13606–13613. [PubMed] [Google Scholar]
- Massagué J., Kelly B., Mottola C. Stimulation by insulin-like growth factors is required for cellular transformation by type beta transforming growth factor. J Biol Chem. 1985 Apr 25;260(8):4551–4554. [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Lamb L. C., Smith J. M., Frolik C. A., Marquardt H., Todaro G. J., Sporn M. B. Isolation from murine sarcoma cells of novel transforming growth factors potentiated by EGF. Nature. 1982 Feb 4;295(5848):417–419. doi: 10.1038/295417a0. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Lamb L. C., Smith J. M., Sporn M. B. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Wakefield L. M., Roche N. S., Stern D. F., Sporn M. B. Type beta transforming growth factor: a bifunctional regulator of cellular growth. Proc Natl Acad Sci U S A. 1985 Jan;82(1):119–123. doi: 10.1073/pnas.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Frolik C. A., Anzano M. A., Sporn M. B. Transforming growth factors from neoplastic and nonneoplastic tissues. Fed Proc. 1983 Jun;42(9):2621–2626. [PubMed] [Google Scholar]
- Ross R., Vogel A. The platelet-derived growth factor. Cell. 1978 Jun;14(2):203–210. doi: 10.1016/0092-8674(78)90107-1. [DOI] [PubMed] [Google Scholar]
- Shin S. I., Freedman V. H., Risser R., Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4435–4439. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Fryling C., De Larco J. E. Transforming growth factors produced by certain human tumor cells: polypeptides that interact with epidermal growth factor receptors. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5258–5262. doi: 10.1073/pnas.77.9.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker R. F., Shipley G. D., Moses H. L., Holley R. W. Growth inhibitor from BSC-1 cells closely related to platelet type beta transforming growth factor. Science. 1984 Nov 9;226(4675):705–707. doi: 10.1126/science.6093254. [DOI] [PubMed] [Google Scholar]
- Twardzik D. R., Ranchalis J. E., Todaro G. J. Mouse embryonic transforming growth factors related to those isolated from tumor cells. Cancer Res. 1982 Feb;42(2):590–593. [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Epithelioid and fibroblastic rat kidney cell clones: epidermal growth factor (EGF) receptors and the effect of mouse sarcoma virus transformation. J Cell Physiol. 1978 Mar;94(3):335–342. doi: 10.1002/jcp.1040940311. [DOI] [PubMed] [Google Scholar]
- van Zoelen E. J., Twardzik D. R., van Oostwaard T. M., van der Saag P. T., de Laat S. W., Todaro G. J. Neuroblastoma cells produce transforming growth factors during exponential growth in a defined hormone-free medium. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4085–4089. doi: 10.1073/pnas.81.13.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]



